Introduction
Implementing scientific evidence into clinical
practice requires an active translational process consisting of
several consecutive elements, the so-called ‘research-to-practice
pipeline’ (1). The quality of the
pipeline will determine the size of any information leak. A
considerable number of studies emphasize the often inadequate
translation of evidence-based guidelines into clinical practice
(2,3).
The aim of the ‘National Second-Opinion Project on
Testicular Cancer’ initiated in Germany in 2006 was to optimize the
flow of evidence into clinical practice by sealing the leaky
pipeline. Another aim was to maximize the availability of
cutting-edge clinical knowledge regarding testicular cancer
management by establishing a nationwide second-opinion network run
by 31 selected colleagues acting as second-opinion givers. Short
reaction times and immediate incorporation of new research results
were intended to increase the pipeline flow rate and overcome
barriers to translating evidence into clinical practice (4,5).
In a previous interim analysis of 642 cases, we
demonstrated the high demand for second opinions before primary
therapy in Germany (6). In the
present study, we report the results of the ‘National
Second-Opinion Project on Testicular Cancer’ after a period of 5
years, including data from the first 2 years of follow-up. The
project is based on the hypothesis that patients benefit from
second opinions offered systematically before the initiation of
treatment after orchiectomy.
Materials and methods
In 2006, a modular web-based interactive database
program was developed by the German Testicular Cancer Study Group
(GTCSG) in cooperation with the DOCxcellence Co., Berlin, Germany.
The system was examined by the Data Protection Commissioner of the
State of Berlin and found to be in accordance with the law. It also
received security clearance for nationwide application. Patients
must provide their informed consent to their personal data being
recorded and used by the system.
The system is available to all urologists in Germany
free of charge, regardless of whether they work in clinical
departments or private practices (http://www.zm-hodentumor.de). It provides a second
opinion for therapy planning after the primary diagnosis and
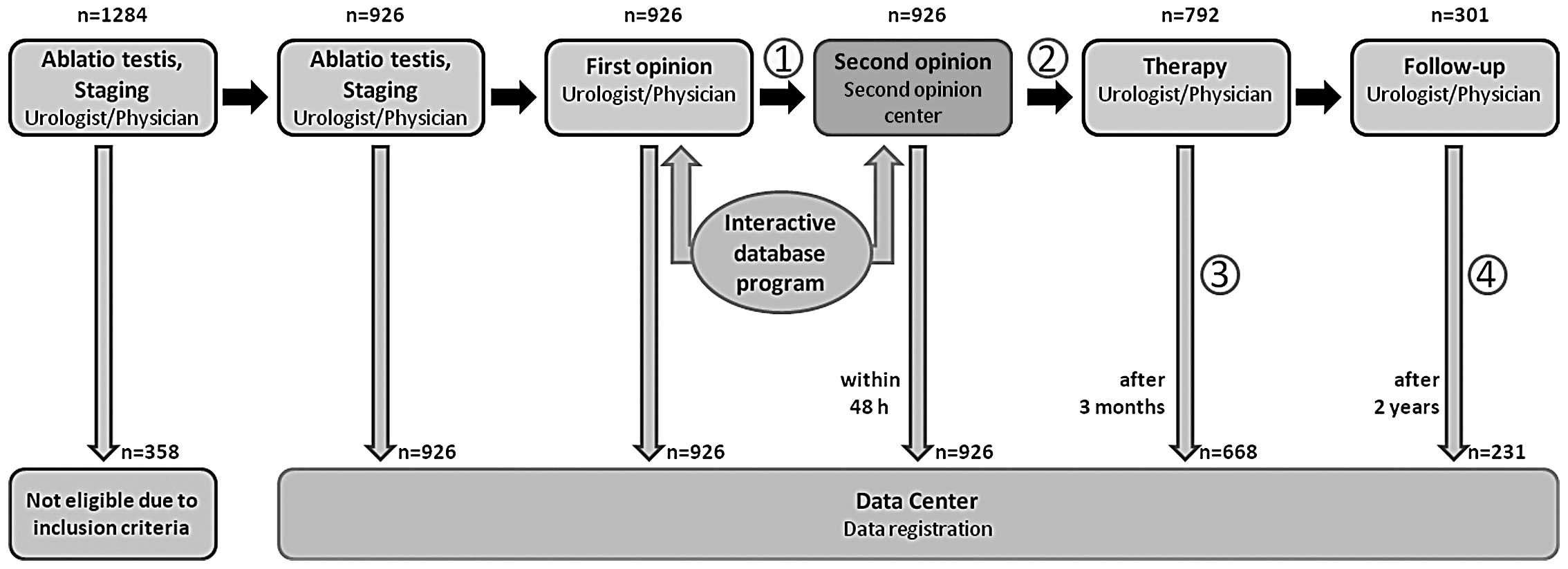
staging of germ cell cancer. The system functions as recently
described (Fig. 1) (6). During the study period, 31 clinical
departments functioned as second-opinion centers in Germany and
Austria (7). Multidimensional
criteria were used to appoint them. The main selection criterion
was an active role in developing the treatment guidelines for germ
cell cancer (8,9). Centers were also required to provide
proof of clinical and research activities in the field of germ cell
cancer.
The first endpoint of the present investigation was
the rate of discordance between the first and second opinion (i.e.,
between the therapy planned by the inquiring urologist and that
recommended by the second-opinion giver). Another endpoint was the
degree of compliance with the recommended treatment. A third
endpoint was the treatment outcome as measured by the
progression-free survival (PFS) rate.
Statistical analysis
For this interim analysis, the anonymized patient
data were evaluated with SPSS v.11.0 by the Dross Institute of
Statistics at the Free University of Berlin. The advice seekers may
choose 1 of 15 different treatment options as their first opinion,
including ‘Findings are not conclusive enough for a definite
recommendation’ and ‘Others: free text.’
The second opinions were multiple-choice answers
relating to the same 15 categories. Second-opinion centers may then
recommend up to four alternative treatment options and make
free-text comments. The initially planned treatment was considered
to be concordant with the second opinion if it coincided with at
least one recommendation made by the second-opinion center.
Discordant cases were further differentiated as follows: i) first
opinion favors more extensive treatment, i.e. ‘overtreatment’
according to guidelines; ii) first opinion favors less extensive
treatment, i.e. ‘undertreatment’ according to guidelines; iii)
discordance with no substantial difference in the scope of
therapy.
In these cases, the treatment scope of alternative
options was assessed in relation to the clinical tumor stage for
which it was recommended by the guidelines (8,9).
Significance was determined by Pearson’s χ2 test
(two-sided). Standardized residuals for cells in the
cross-classification table were used for content-related
interpretation of a significant result. Significance was also
determined using a Monte Carlo simulation with 10,000 trials and a
99% confidence interval.
Results
A total of 1,284 requests were submitted by 350
urologists/physicians to the 31 second-opinion centers from
November 2006 to October 2011. The present interim analysis only
took into account the requests from colleagues working in private
practice or hospital departments but not at one of the 31
institutions serving as second-opinion centers; the latter were
excluded as unsuitable for assessing the additional benefit of a
second-opinion network, since they would have had access to an
expert opinion anyway. At the time of the interim analysis, 926
cases met this inclusion criterion and were thus eligible. Patient
characteristics are given in Table
I.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variables | n |
|---|
| Total second-opinion
requests | 1,284 |
| Requests from
colleagues in private practice and hospital departments | 926a |
|
Answers/second-opinion giver (range,
0–199) | 29.9±51.1 |
| Average patient age
in years | 37.0±11.3 |
| Histological finding
at time of diagnosis of germ cell tumor | |
| Seminoma | 424 (45.8%) |
| Average age in
years | 40.9±10.7 |
| Non-seminoma | 454 (49.0%) |
| Average age in
years | 31.8±11.3 |
| Unclear whether
seminoma or non-seminoma | 48 (5.2%) |
The 3-month follow-up deadline was reached in 792
cases at the time of the analysis. The applied therapy may be
ascertained in 668/792 cases, which corresponds to a response rate
of 84.3%. The 2-year follow-up has thus far been completed in 301
patients, and aftercare information was provided for 236 of them,
corresponding to a response rate of 78.4%.
The first-opinion therapy suggested by the advice
seeker was concordant with the second-opinion therapy recommended
by the centers in only 58.0% of the cases. Discordance between
first and second opinions was found in 39.5%, while deviation from
recommendations remained unclear in the remaining cases (2.5%). The
discordance rate increased significantly with tumor stage
(Pearson’s χ2 test; <0.001; Table II), the difference being most
obvious between stages I and IIa/IIb. Discordance was not
associated with histology (seminomas and non-seminomas; Pearson’s
χ2 test, p=0.442).
 | Table IIConcordance between the first and
second opinion in relation to tumor stage (n=926). |
Table II
Concordance between the first and
second opinion in relation to tumor stage (n=926).
| First and second
opinion | |
|---|
|
| |
|---|
| Clinical tumor
stage(categorized) | Concordant n (%) | Discordant: second
opinion more extensive therapy n (%) | Discordant: no clear
difference in the scope of therapy n (%) | Discordant: first
opinion more extensive therapy n (%) | Concordance status
not clear n (%) | Total n (%) |
|---|
| I | 357 (66.0) | 26 (4.8) | 87 (16.1) | 65 (12.0) | 6 (1.1) | 541 (100) |
| IIa, IIb | 78 (44.3) | 15 (8.0) | 55 (31.3) | 21 (11.9) | 8 (4.5) | 177 (100) |
| IIc, IIIa, IIIb,
IIIca | 83 (48.8) | 15 (8.8) | 49 (28.8) | 16 (9.4) | 7 (4.1) | 170 (100) |
| Unknown | 19 (48.7) | 1 (2.6) | 15 (41.0) | 1 (2.6) | 2 (5.1) | 38 (100) |
| Total | 537 (58.0) | 57 (6.2) | 206 (22.2) | 103 (11.1) | 23 (2.5) | 926 (100) |
In the case of a discordant second opinion, the
scope of the first-opinion therapy was assessed to determine
whether it involved over- or undertreatment. The second-opinion
treatment was less extensive in 28.1% and more extensive in 15.6%
of the discordant cases than that originally planned. In another
56.3% of the cases, there was no substantial difference in the
scope of the treatment between first and second-opinion.
In 30.8% of the cases (206/668), the applied therapy
was not in accordance with either the first or the second opinion.
The remaining 462 cases showed a clear tendency towards compliance
with the second rather than the first opinion (85.3 vs. 14.7%).
Two-year PFS data were available for 188 patients at
the time of the interim analysis. A total of 18 relapses or
progressive tumors were reported. This corresponded to a 2-year PFS
of 90.4% for the total patient population. The 2-year PFS
stratified by tumor stage was 95.2% (118/124) for stage I, 92.0%
(23/25) for stage IIa–IIb and 75% (24/32) for stage ≥IIc (Table III). Relapse or progression of the
tumor was diagnosed by routine imaging procedures in 15/18 cases
(83.3%), by biopsy (with previous imaging) in 6/18 cases (33.3%)
and by elevated tumor markers in only 4/18 cases (22.2%).
 | Table IIITwo-year progression-free survival in
relation to tumor stage (n=188). |
Table III
Two-year progression-free survival in
relation to tumor stage (n=188).
| Clinical tumor stage
(categorized) | Progression-free at
2-year follow-up | Total n (%) |
|---|
|
|---|
| Yes n (%) | No n (%) |
|---|
| Ia, Ib, Is | 118 (95.2) | 6 (4.8) | 124 (100) |
| IIa, IIb | 23 (92.0) | 2 (8.0) | 25 (100) |
| IIc, IIa, IIIb,
IIIc | 24 (75.0) | 8 (25.0) | 32 (100) |
| Unknown | 5 (71.4) | 2 (28.6) | 7 (100) |
| Total | 170 (90.4) | 18 (9.6) | 188a (100) |
Discussion
A multidisciplinary set of quality indicators has
recently been developed for the treatment of testicular cancer
patients (10). Here, the concept
of treatment quality is extended to include indicators beyond
survival. The incorporation of new research results and guideline
conformity play a crucial role in this context. The question as to
what procedure conforms to the guidelines can be scrutinized
systematically or even individually in cases where doubts arise
regarding treatment decisions. The problem is that doubts do not
always arise when they should, and then the inappropriate approach
is taken with firm conviction. This suggests that guideline
conformity should be systematically checked by a second-opinion
system, since the research- or guideline-to-practice pipeline has
often proved to be leaky in the past (2,3).
The establishment of the national second-opinion
network for testicular germ cell cancer by the German Testicular
Cancer Study Group (GTCSG) in 2006 (6) represented a worldwide unique approach
for actively propagating and implementing guideline
recommendations. Given the decentralized health care system in
Germany with treatment of only a very small average annual number
of testicular tumors per institution, this network was intended to
promote the delivery of cutting-edge therapy to a large number of
testicular cancer patients.
In 2011, approximately every 8th newly diagnosed
case of testicular cancer in Germany received a second opinion from
our service (n=465/3,950; 11.8%) (11). Given the segmental structure of the
German health care system, which is reflected in many countries
worldwide, this was a substantial quota. It suggests that
physicians do actually appreciate being able to request a second
opinion, provided that: (i) it does not take much time, and (ii) it
does not involve referring the patient to another physician.
This high acceptance rate demonstrates the high
level of responsibility taken by the participating advice seekers,
since there were no financial incentives for the additional time
and effort. They were only committed to ensuring that their
patients received the best primary therapy available. The same was
true for the participating physicians in the second-opinion
centers, who provided their second opinion very quickly (median
reaction time of only 10.64±16.99 h) and free of charge.
A 2006 study showed that publication of the GTCSG
guidelines significantly influenced the management of testicular
cancer patients at a university treatment center in 1999 (12). Our data reveal a 40% discrepancy
between the first and second opinion, which probably reflects an up
to 40% deviation of primary caregivers from guideline
recommendations. Furthermore, patterns of care studies have shown
that compliance with recommended imaging procedures decreases
during follow-up in testicular cancer patients (13).
Of note, nearly one third of the applied therapies
were not in accordance with either the second opinion or the
initially planned therapy. Limited implementation definitely
accounts for an attenuated effect of the second opinions. However,
imprecise data input of the second-opinion seekers due to the
relatively long 3-month interval between the initiation of
treatment and its registration by our data center may have also
played a role in the relatively high rate of ‘third opinions’.
Another factor may be the often suboptimal adherence to medical
advice among testicular cancer patients (14).
Today, testicular cancer patients have an overall
cure rate of more than 95% (11).
For this reason and owing to the comparatively young age of onset,
considerable attention is paid to treatment-related morbidity in
these patients. In contrast to other malignancies, the relapse rate
here is not indicative of treatment quality, since overtreatment,
for example, may increase toxicity and long-term morbidity without
affecting the relapse rate.
Thus, the aim of treatment is, on the one hand, not
to jeopardize the excellent cure rate of the disease by
undertreatment, and, on the other hand, to avoid the morbidity
associated with overtreatment. The second-opinion project is
committed to achieving this goal.
Approximately every 6th second opinion (n=160/926;
17.3%) prompted a relevant change of therapy, which confirms the
findings of our first interim report (6). Thus, with reference to all cases
submitted, over- or undertreatment is avoided in 80 German patients
per year by following the second-opinion recommendations. Another
103 patients per year receive optimized treatment due to minor
changes if adjusted therapeutic strategies with no relevant changes
in scope are also taken into account.
The current analysis provides first follow-up data
on the testicular cancer patients treated in the second-opinion
network; 18 of 188 patients (9.6%) had relapse or progression of
the disease. Stratified by both the total patient population and
the tumor stage, the 2-year PFS did not differ from that of
patients receiving second-opinion treatment in clinical trials
(15,16). A correlation of the clinical relapse
rate with the second-opinion conformity of the applied therapy will
be validated in a future analysis as soon as more 2-year follow-up
data are available.
Our results are limited by some conceptual
shortcomings in the present second-opinion project. For example,
the relatively high number of second-opinion givers may raise
concerns about second-opinion quality being diluted by too many
participating physicians. The basic idea was to distribute
second-opinion requests evenly among the consultants in order to
guarantee a short reaction time. However, this goal was not
achieved: the distribution turned out to be relatively uneven (n=0
to n=199; Table I). Indeed, only 21
of 31 second-opinion givers had more than one second-opinion
request to answer during the study period. It thus seems expedient
to rethink the procedure for selecting second-opinion givers in the
future.
Exchange with experts from other fields (e.g.
radiation oncology, medical oncology, nuclear medicine) was
maintained by the second-opinion giver dependent on each individual
case, but not carried out systematically. The latter would have
been desirable in the decision-making process, reflecting another
weakness of the project.
Two-year PFS was used to measure treatment quality.
We are aware that, in the face of a changing treatment landscape
with a trend toward avoiding late toxicities of cancer therapy,
this parameter does not cover all the most important aspects of
cutting-edge testicular cancer treatment. Unfortunately,
therapy-associated morbidities were beyond the scope of our
project, and the follow-up period is still too short to give them
adequate consideration.
Nevertheless, an unexpected finding is the high
level of project participation in the strongly finance-oriented
German health care system. The ideal aim of making optimal
treatment decisions by discussing cases with specialized centers
can be achieved in a nearly conflict-free fashion by the
established system. The physicians seeking advice did not run the
risk of being discredited or losing their patients by referral to
(more) qualified colleagues. Moreover, the time required was so
small that it posed no obstacle. All this helps to explain the
large number of highly-motivated project participants.
In conclusion, the ‘National Second-Opinion Project
for Testicular Cancer’ demonstrates for the first time a new way to
improve the ‘research-to-practice pipeline’. The possibility of
nearly obstacle-free online communication among physicians with no
financial disadvantage or loss of authority is deemed to have
improved the quality of care delivered to testicular cancer
patients in Germany. The ‘National Second-Opinion Project for
Testicular Cancer’ should therefore serve as a model for
establishing further second-opinion networks for other health care
systems or diseases.
Acknowledgements
The authors thank the Deutsche Krebshilfe e.V.
(German Cancer Aid) for their financial support. We acknowledge
Daniel Schuster and Ralf Schuchardt from the DOCxcellence GmbH,
Berlin, Germany, for developing and maintaining the Internet-based
database system. Further, we cordially thank Joanne Weirowski for
translating the manuscript.
References
|
1
|
Glasziou P and Haynes B: The paths from
research to improved health outcomes. Evid Based Nurs. 8:36–38.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rawlins MD: NICE work - providing guidance
to the British National Health Service. N Engl J Med.
351:1383–1385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raine R, Sanderson C and Black N:
Developing clinical guidelines: a challenge to current methods.
BMJ. 331:631–633. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McNeil BJ: Shattuck Lecture - Hidden
barriers to improvement in the quality of care. N Engl J Med.
345:1612–1620. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Julian DG: Translation of clinical trials
into clinical practice. J Intern Med. 255:309–316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schrader M, Weissbach L, Hartmann M, et
al: Burden or relief: do second-opinion centers influence the
quality of care delivered to patients with testicular germ cell
cancer? Eur Urol. 57:867–872. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
ZMZ-Ärzte-Projekt Zweitmeinung Hodentumor.
http://zm-hodentumor.de/.
(In German).
|
|
8
|
Krege S, Beyer J, Souchon R, et al:
European consensus conference on diagnosis and treatment of germ
cell cancer: a report of the second meeting of the European Germ
Cell Cancer Consensus group (EGCCCG): part I. Eur Urol. 53:478–496.
2008. View Article : Google Scholar
|
|
9
|
Krege S, Beyer J, Souchon R, et al:
European consensus conference on diagnosis and treatment of germ
cell cancer: a report of the second meeting of the European Germ
Cell Cancer Consensus Group (EGCCCG): part II. Eur Urol.
53:497–513. 2008. View Article : Google Scholar
|
|
10
|
Vlayen J, Vrijens F, Devriese S, Beirens
K, Van Eycken E and Stordeur S: Quality indicators for testicular
cancer: a population-based study. Eur J Cancer. 48:1133–1140. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robert Koch-Institut und die Gesellschaft
der epidemiologischen Krebsregister in Deutschland e.V. (Hrsg).
Krebs in Deutschland 2007/2008. 8. Ausgabe. Journal. 2012.(in
German).
|
|
12
|
Schrader AJ, Ohlmann CH, Rossmanith S,
Hofmann R and Heidenreich A: Impact of evidence-based
interdisciplinary guidelines on testis cancer management. Cancer.
106:313–319. 2006. View Article : Google Scholar
|
|
13
|
Yu HY, Madison RA, Setodji CM and Saigal
CS: Quality of surveillance for stage I testis cancer in the
community. J Clin Oncol. 27:4327–4332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moynihan C, Norman AR, Barbachano Y, et
al: Prospective study of factors predicting adherence to medical
advice in men with testicular cancer. J Clin Oncol. 27:2144–2150.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berrino F, De Angelis R, Sant M, et al:
Survival for eight major cancers and all cancers combined for
European adults diagnosed in 1995–99 results of the EUROCARE-4
study. Lancet Oncol. 8:773–783. 2007.PubMed/NCBI
|
|
16
|
Rosen A, Jayram G, Drazer M and Eggener
SE: Global trends in testicular cancer incidence and mortality. Eur
Urol. 60:374–379. 2011. View Article : Google Scholar : PubMed/NCBI
|