Introduction
Cancer is a leading cause of mortality worldwide and
the number of cancer cases and deaths is projected to continue
rising, with the current estimation at 17 million deaths due to
cancer per year (1). Colon, lung,
breast, liver and stomach cancer have the highest mortality rates.
Specifically, colorectal cancer (CRC) is the leading cause of
mortality among women and the third leading cause among men in
Japan, as of 2011, and it continues to increase (2). Surgical resection of a primary tumor
and regional lymph nodes is important for CRC treatment, and the
pathological staging, represented as T and N factors, depends on
tumor invasion and lymph node metastasis, respectively, in the
surgically-resected specimen. There are several reports regarding
CRC prognosis and pathological factors, and N factor (lymph node
metastasis) and the number of the nodes are reported to be one of
the most important prognostic factors (3,4). It is
necessary and essential to evaluate the total number of resected
lymph nodes and metastases to determine the adjuvant chemotherapy
regimen (4). In relation to the
surgically-resected lymph nodes in CRC, the National Comprehensive
Cancer network recommends the examination of at least 12 lymph
nodes for reliable CRC staging (5).
Various methods to determine sufficient number of
surgically-resected lymph nodes, such as fat clearance (6,7),
Schwartz (8) and GEWF solution
(9,10), have been reported. However, a caveat
of these methods is that it requires a significant amount of time
(1–9 days) to clear the fat from the resected mesentery in which
there are lymph nodes to be evaluated. There is presently no
consensus on a convenient and effective method to detect the lymph
nodes. According to the NCCN and ESMO guidelines, the Japanese
Society for Cancer of the Colon and Rectum also recommends
identifying at least 12 lymph nodes for appropriate CRC staging
(5,11). The general and traditional method is
to manually search for lymph nodes by touching then fixing with
formalin. Although the surgeon can efficiently evaluate the lymph
nodes and metastases, the palpation method is still very
time-consuming. In the present study, we present a simple and
effective method, called as the fat-dissociation method, which
dissociates the mesenteric fat therefore reducing the volume and
leading to clear visualization of these lymph nodes. This method
allows for faster and easier identification of lymph nodes compared
to the conventional palpation method, specifically the count and
condition of the extracted lymph nodes.
Materials and methods
Clinical tissue samples
The present study included 19 patients who underwent
surgery for CRC at Osaka Medical Center for Cancer and
Cardiovascular Diseases (OMCCCD) from November to December 2013.
This study was performed after written informed consent had been
obtained in accordance with our institutional ethics guidelines
approved by the OMCCCD Ethics Committee. The resected tumor and
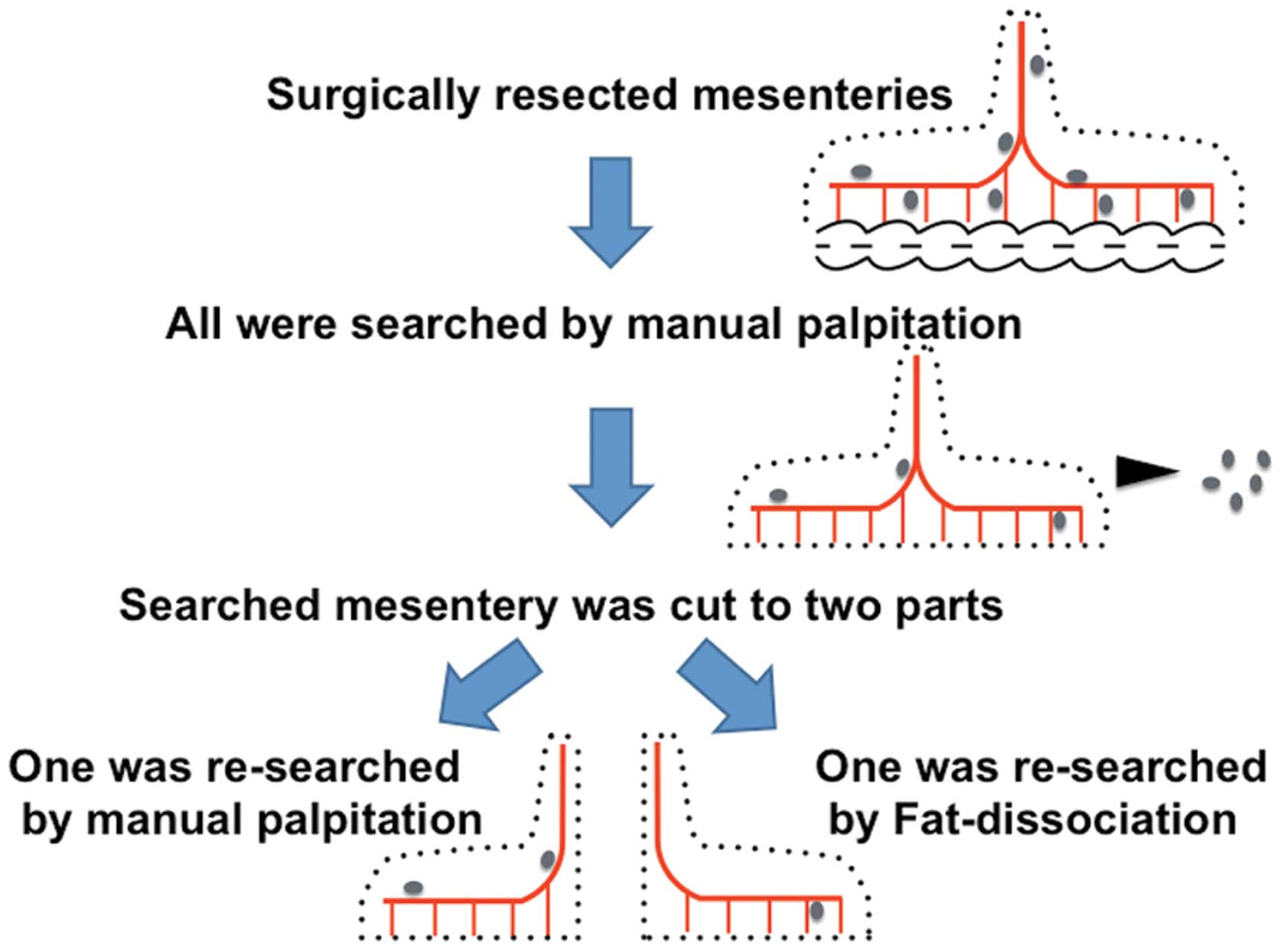
surrounding tissue were examined. The general course to examine the
resected specimen is as follows: the mesentery is carefully
separated from the primary tumor lesion and the surgeons explore
the lymph nodes in the mesentery, which takes 30 min to 1 h. In the
present study, we also performed the general procedure above, and
re-examined the lymph nodes remaining in the mesentery that had
already been explored (Fig. 1).
After the conventional method, namely, searching for lymph nodes in
the mesentery by a surgeon palpating manually, the remaining
mesenteric specimen was cut into two parts of equal mass, and one
part was re-searched to examine the remaining lymph nodes by two
other surgeons palpating manually for at least 10 min. The other
piece of mesenteric specimen was examined for lymph nodes by using
the new method of dissociating the mesenteric fat to visualize the
lymph nodes clearly. All picked up lymph nodes were treated with
standard formalin fixation and evaluated by using hematoxylin and
eosin staining.
Dissociation of mesenteric tissue
The mesenteric tissue was dissociated by using the
following solution: 0.5–1 mg/ml collagenase (C6885; Sigma-Aldrich,
St. Louis, MO, USA) with up to 0.25 % trypsin (25200072; Life
Technologies, Carlsbad, CA, USA). We examined the dilution effect
in DPBS (14190250; Life Technologies) or DMEM (D5796;
Sigma-Aldrich), but there was no significant difference; finally,
the solution was diluted in DPBS. Regarding the volume of the
solution added to the specimen, we investigated several conditions
and finally 1 ml solution was added to a 3-g piece of mesentery.
The components were mixed at 100 rpm using a rotator for 10 min at
several temperatures (Fig. 2).
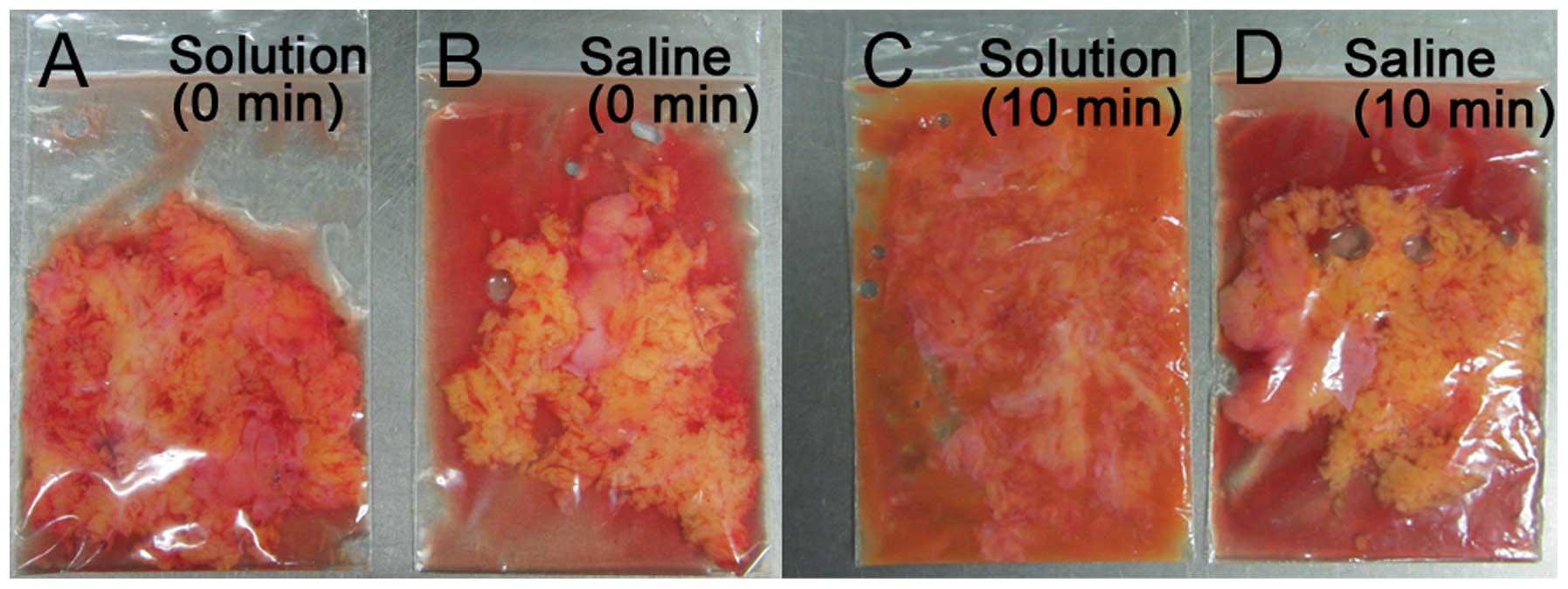
Next, the mesentery was placed on a paper to collect the
dissociated fat tissue, followed by grinding on the paper. The
lymph nodes were then easily identified since the area around the
vessels was clear of fat, hence the naming of this new methodology
as the fat-dissociation method (Fig.
3).
Statistical analysis
For continuous variables, data are expressed as
median (range). The relationship between the lymph node count by
re-palpation and the fat-dissociation method was analyzed using the
Wilcoxon rank-sum and signed-rank tests. They were analyzed using
JMP software (version 11.0; SAS Institute, Cary, NC, USA).
Differences with p-value <0.05 were considered statistically
significant.
Results
First, several conditions of fat-dissociation were
examined whether the mesenteric fat were dissociated (Table I). We tested various concentrations
and mixtures of reagents to establish the ideal and practical
concentration, incubation time, and temperature. When only
collagenase or trypsin was used, the dissociation effect was
moderate. The most effective and applicable condition (and the
condition used in this report) was 1 mg/ml collagenase and 0.25 %
trypsin for 10 min at 40°C.
 | Table IConditions of the fat-dissociation
method. |
Table I
Conditions of the fat-dissociation
method.
| Collagenase
(mg/ml) | Trypsin (%) | Incubation time
(min) | Temp. (°C) | Dissociationa |
|---|
| 0 | 0.25 | 120 | 40 | 2 |
| 0.5 | 0.05 | 30 | 22 | 1 |
| 0.5 | 0.125 | 45 | 37 | 3 |
| 0.5 | 0.125 | 7200b | 22 | 3 |
| 1 | 0 | 30 | 22 | 2 |
| 1 | 0.25 | 360 | 22 | 3 |
| 1 | 0.25 | 15 | 37 | 3 |
| 1 | 0.25 | 10 | 40 | 3 |
The defined lymph node dissection was performed for
each CRC case according to the JSCCR guidelines (12). A total of 20 specimens derived from
19 CRC cases were evaluated on the number of lymph nodes in the
first and second lymph node regions by pathological examination
according to the Japanese Classification of Colorectal Carcinoma
(13) (summarized in Table II). The median number of lymph
nodes found by the traditional palpation method was eight (range,
3–23) and four (range, 0–15) in the first and second lymph node
regions, respectively. Next, the median number of lymph nodes
searched by the re-palpation method was 0.5 (range, 0–3) in the
first lymph node region and 0 (range, 0–3) in the second lymph node
region. Finally, the median count examined by the fat-dissociation
method was two (range, 0–8) in the first lymph node region and one
(range, 0–4) in the second lymph node region (Table III). The use of the
fat-dissociation method resulted in a significant increase in the
total number of lymph nodes found compared to the traditional
method (Fig. 4). The Wilcoxon
singed-rank test showed the significant difference between the
fat-dissociation and re-palpation methods (P=0.0003).
 | Table IISummary of 19 patients. |
Table II
Summary of 19 patients.
| | | | | Positive/total lymph
nodes | | | |
|---|
| | | | |
| | | |
|---|
| Case | Gender | Age (years) | BMI | Operation | 1st Palpation | 1st Re-palpation | 1st
Fat-dissociation | 2nd Palpation | 2nd Re-palpation | 2nd
Fat-dissociation | T | N | Stage |
|---|
| 1 | M | 81 | 23.2 | S | 0/4 | 0/0 | 0/2 | 0/4 | 0/3 | 0/0 | T1b | N0 | I |
| 2 | F | 75 | 21.8 | L | 0/5 | 0/0 | 0/0 | 0/2 | 0/0 | 0/1 | T2 | N0 | I |
| 3 | F | 63 | 21.6 | LAR | 0/6 | 0/0 | 0/0 | 0/5 | 0/0 | 0/1 | T2 | N0 | I |
| 4 | M | 62 | 21.8 | LAR | 0/7 | 0/1 | 0/6 | 0/2 | 0/1 | 0/2 | T3 | N0 | II |
| 5 | M | 65 | 23.3 | LAR | 0/14 | 0/0 | 0/0 | 0/5 | 0/0 | 0/1 | T3 | N0 | II |
| 6 | F | 67 | 20.8 | LAR | 0/14 | 0/1 | 0/8 | 0/3 | 0/0 | 0/3 | T3 | N0 | II |
| 7 | M | 76 | 18.7 | T | 0/7 | 0/0 | 0/3 | 0/0 | 0/0 | 0/0 | T3 | N0 | II |
| 7 | M | 76 | 18.7 | APR | 0/10 | 0/1 | 0/2 | 0/4 | 0/1 | 0/0 | T4b | N0 | II |
| 8 | F | 67 | 22.5 | sLAR | 3/8 | 0/0 | 0/2 | 0/1 | 0/0 | 0/2 | T3 | N1 | IIIa |
| 9 | F | 70 | 25.1 | S | 3/3 | 0/0 | 0/2 | 0/0 | 0/0 | 0/2 | T2 | N1 | IIIa |
| 10 | M | 74 | 18.7 | S | 0/21 | 0/3 | 0/7 | 0/12 | 0/3 | 0/3 | T3 | N1 | IIIa |
| 11 | M | 82 | 18.5 | S | 1/23 | 0/1 | 0/2 | 0/4 | 0/0 | 0/1 | T3 | N1 | IIIa |
| 12 | M | 68 | 22.9 | AR | 1/5 | 0/1 | 0/1 | 0/3 | 0/0 | 0/3 | T4a | N1 | IIIa |
| 13 | F | 77 | 17.6 | sLAR | 3/19 | 0/0 | 0/3 | 0/5 | 0/0 | 0/1 | T3 | N1 | IIIa |
| 14 | F | 71 | 23.6 | L | 1/7 | 0/1 | 0/1 | 0/5 | 0/1 | 0/0 | T1b | N1 | IIIa |
| 15 | M | 57 | 22.7 | R | 3/8 | 0/1 | 0/2 | 0/2 | 0/1 | 0/4 | T4a | N1 | IIIa |
| 16 | F | 60 | 24.2 | LAR | 3/19 | 0/2 | 0/1 | 0/8 | 0/0 | 0/1 | T3 | N1 | IIIa |
| 17 | F | 57 | 29.8 | L | 4/16 | 0/0 | 0/0 | 0/6 | 0/0 | 0/1 | T2 | N2 | IIIb |
| 18 | M | 71 | 21.4 | AR | 5/6 | 1/1 | 2/3 | 1/2 | 0/0 | 1/1 | T4a | N2 | IV |
| 19 | F | 69 | 25.0 | LAR | 5/10 | 0/0 | 0/2 | 1/15 | 0/1 | 0/2 | T3 | N2 | IV |
 | Table IIIMedian number of lymph nodes by each
method. |
Table III
Median number of lymph nodes by each
method.
| Palpation
method | Re-palpation
method | Fat-dissociation
method |
|---|
| 1st LNs
(range) | 8 (3–23) | 0.5 (0–3) | 2 (0–8) |
| 2nd LNs
(range) | 4 (0–15) | 0 (0–3) | 1 (0–4) |
| Total LNs
(range) | 11.5 (3–33) | 1 (0–6) | 3 (1–11) |
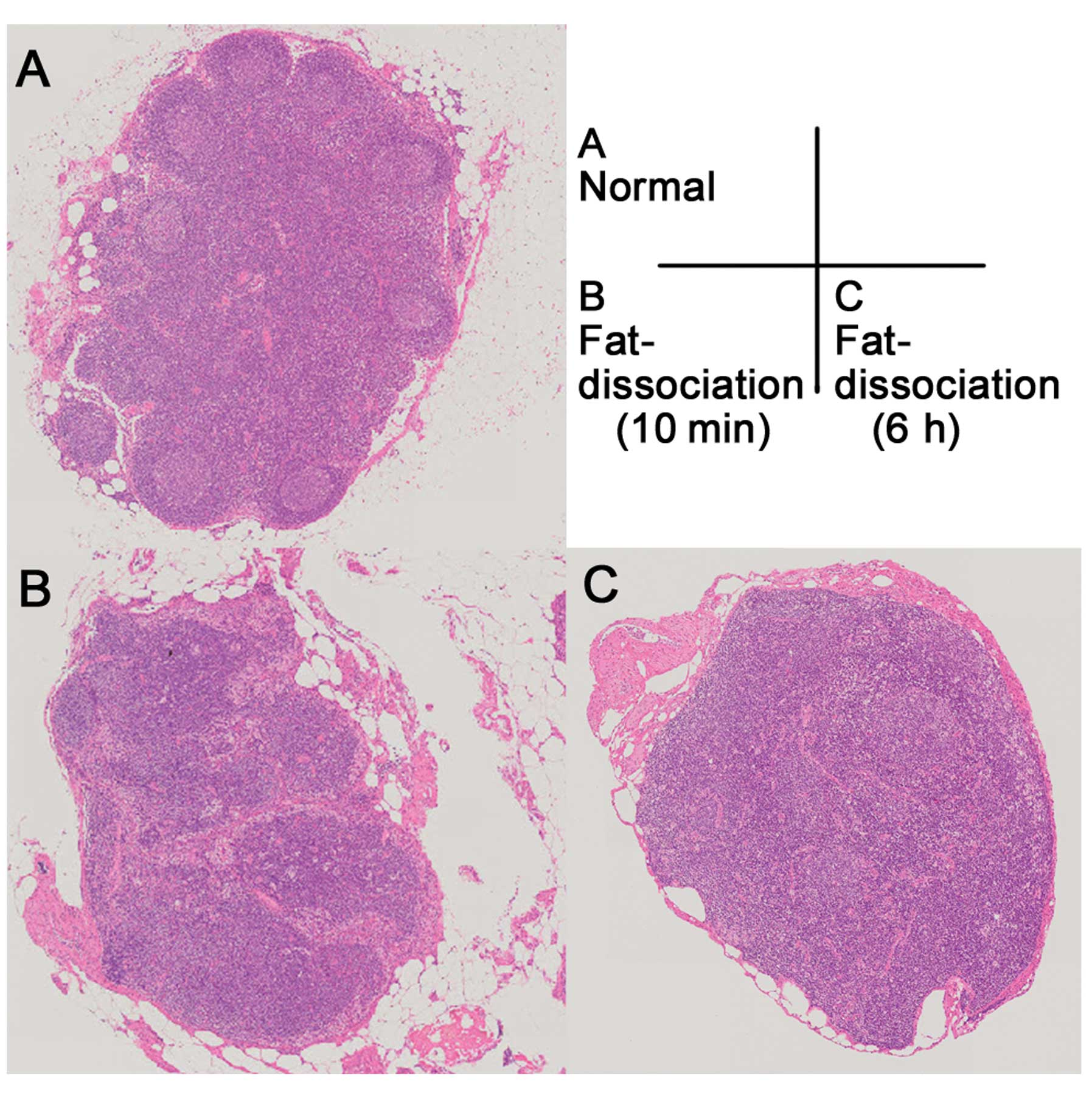
The lymph nodes were all pathologically examined,
and those explored by the fat-dissociation method were examined
without any difficulties (Fig.
5).
Discussion
The fat-dissociation method reported is effective
and applicable to searching for lymph nodes in the
surgically-resected mesentery. In the present study, upon
evaluation of the time required for the traditional palpation
(median, 50 min) and the fat-dissociation method (median, 18 min),
it was revealed that more time is required to search for lymph
nodes in the mesenteric fat by the palpation method. The
fat-dissociation method can save time on lymph node examination and
exploration. Generally, searching for lymph nodes around vessels is
difficult as surrounding vascular sheaths and nerves are fixed and
hard to analyze carefully. The fat-dissociation method can change
the mesenteric structure, making the mesenteric fat soft and
reducing the volume, which results in easier examination of the
lymph nodes around vessels. In our previous study, collagenase and
trypsin were used to generate primary culture from
surgically-resected specimens, generating in vitro culture
of the clinical samples (14,15).
The fat-dissociation method requires only 10 min to clear the
mesenteric fat, resulting in visualization of the lymph nodes. In
routine study, short incubation times may be risky, therefore we
also evaluated whether the incubation time can be extended. When we
used the solution at half its concentration and at several
temperatures, the mesenteric fat dissociated in 45 min, and even
after 12 h, the lymph nodes kept stable. This indicates that the
half-concentration solution would be feasible and applicable in
several institutions. We also evaluated the lymph node metastases.
In case 18, lymph node metastases were diagnosed after the
fat-dissociation method as well as the normal conventional
palpation method (Fig. 6). In the
Japanese classification of CRC, tumor nodule is defined as lymph
node metastasis (13). Tumor nodule
exists separately from intestine without lymph node structure. We
were able to evaluate the tumor nodule and there were no
differences of pathological findings between these two methods.
The patients of CRC stage III and high risk stage II
are recommended to receive adjuvant chemotherapy after surgery
(16–21); hence, adequate searching for lymph
nodes is necessary and important. The impact of the
fat-dissociation method is in enabling all clinicians to examine
the lymph nodes easily and effectively by modifying the condition
of the solution according to their individual work style.
In conclusion, we described a novel and effective
technique to dissociate the mesenteric fat, resulting in easier
examination of the lymph nodes. This new method, called the
fat-dissociation method, can visualize lymph nodes easily and
effectively in surgically-resected mesentery compared to the
conventional palpation method.
Acknowledgements
The authors thank Dr T. Fukata, Dr T. Umeda and Dr
T. Hara for searching for lymph nodes in the surgically-resected
mesentery.
References
|
1
|
Thun MJ, DeLancey JO, Center MM, Jemal A
and Ward EM: The global burden of cancer: priorities for
prevention. Carcinogenesis. 31:100–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Center for Cancer Control and Information
Services NCC. Japan recent cancer statistics. 2011, http://ganjoho.jp/public/statistics/pub/statistics01.html.
Accessed December 30, 2013
|
|
3
|
Van Cutsem E, Nordlinger B and Cervantes
A; ESMO Guidelines Working Group. Advanced colorectal cancer: ESMO
Clinical Practice Guidelines for treatment. Ann Oncol. 21(Suppl 5):
v93–v97. 2010.
|
|
4
|
Labianca R, Nordlinger B, Beretta GD, et
al: Early colon cancer: ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 24(Suppl 6):
vi64–vi72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Network NCC NCCN guidlines for treatment
of cancer by site: Colon/Rectal Cancer. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
Accessed December 30, 2013
|
|
6
|
Hida J, Mori N, Kubo R, et al: Metastases
from carcinoma of the colon and rectum detected in small lymph
nodes by the clearing method. J Am Coll Surg. 178:223–228.
1994.PubMed/NCBI
|
|
7
|
Morikawa E, Yasutomi M, Shindou K, et al:
Distribution of metastatic lymph nodes in colorectal cancer by the
modified clearing method. Dis Colon Rectum. 37:219–223. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chapman B, Paquette C, Tooke C, et al:
Impact of Schwartz enhanced visualization solution on staging
colorectal cancer and clinicopathological features associated with
lymph node count. Dis Colon Rectum. 56:1028–1035. 2013. View Article : Google Scholar
|
|
9
|
Gregurek SF and Wu HH: Can GEWF solution
improve the retrieval of lymph nodes from colorectal cancer
resections? Arch Pathol Lab Med. 133:83–86. 2009.PubMed/NCBI
|
|
10
|
Newell KJ, Sawka BW, Rudrick BF and Driman
DK: GEWF solution. Arch Pathol Lab Med. 125:642–645.
2001.PubMed/NCBI
|
|
11
|
ESMO Oncology Clinical Practice Guidlines.
http://www.esmo.org/Guidelines-Practice/Clinical-Practice-Guidelines.
Accessed December 30, 2013
|
|
12
|
Japanese Society for Cancer of the Colon
and Rectum. JSCCR Guidelines 2010 for the Treatment of Colorectal
Cancer; Kanehara, Tokyo. pp. 13–15. 2010
|
|
13
|
Japanese Society for Cancer of the Colon
and Rectum. Japanese Classification of Colorectal Carcinoma. 8th
edition. Kanehara, Tokyo: pp. 11–14. 2013
|
|
14
|
Miyoshi N, Ishii H, Nagai K, et al:
Defined factors induce reprogramming of gastrointestinal cancer
cells. Proc Natl Acad Sci USA. 107:40–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyoshi N, Ishii H, Nagano H, et al:
Reprogramming of mouse and human cells to pluripotency using mature
microRNAs. Cell Stem Cell. 8:633–638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Figueredo A, Charette ML, Maroun J,
Brouwers MC and Zuraw L: Adjuvant therapy for stage II colon
cancer: a systematic review from the Cancer Care Ontario Program in
evidence-based care’s gastrointestinal cancer disease site group. J
Clin Oncol. 22:3395–3407. 2004.
|
|
17
|
Gill S, Loprinzi CL, Sargent DJ, et al:
Pooled analysis of fluorouracil-based adjuvant therapy for stage II
and III colon cancer: who benefits and by how much? J Clin Oncol.
22:1797–1806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benson AB III, Schrag D, Somerfield MR, et
al: American Society of Clinical Oncology recommendations on
adjuvant chemotherapy for stage II colon cancer. J Clin Oncol.
22:3408–3419. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
André T, Boni C, Mounedji-Boudiaf L, et
al: Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment
for colon cancer. New Engl J Med. 350:2343–2351. 2004.
|
|
20
|
André T, Boni C, Navarro M, et al:
Improved overall survival with oxaliplatin, fluorouracil, and
leucovorin as adjuvant treatment in stage II or III colon cancer in
the MOSAIC trial. J Clin Oncol. 27:3109–3116. 2009.PubMed/NCBI
|
|
21
|
Kuebler JP, Wieand HS, O’Connell MJ, et
al: Oxaliplatin combined with weekly bolus fluorouracil and
leucovorin as surgical adjuvant chemotherapy for stage II and III
colon cancer: results from NSABP C-07. J Clin Oncol. 25:2198–2204.
2007. View Article : Google Scholar
|