Introduction
Pancreatic cancer is a leading cause of
cancer-related mortality and an aggressive malignancy with
mortality rates almost identical to incidence rates (1). The curative therapies of pancreatic
cancer have translated into <5% (2). The diagnosis of pancreatic cancer
represents a particularly bleak prognosis for patients and their
families. At present, due to the non-specific presenting symptoms
of pancreatic cancer, to diagnose pancreatic cancer at early stages
is impossible. Surgical resection is the only curative option for
pancreatic cancer patients, yet only 20% of patients present with
early-stage operable tumors (3).
Patients with unresectable disease consequently receive
chemotherapy and radiotherapy, which, however, also do not result
in longer survival (4). Therefore,
improving therapeutic options and diagnostic tools for pancreatic
cancer patients is anticipated. The genetic changes of pancreatic
cancer patients are important in identifying molecules for the
development of therapies. To improve the treatment of pancreatic
cancer, innovative approaches including new targeted molecules are
needed.
Cancer is a genetic disease caused by accumulation
of oncogenes and tumor suppressor gene changes (5). Bone morphogenetic protein (BMP)-family
members are divided into three subgroups based on their similar
amino acid sequence, including the BMP2/4 group, osteogenic
protein-1 (OP-1) group (BMP5, BMP6, BMP7 and BMP8) and BMP9/10
group (6). BMPs are well known to
play critical roles in diverse developmental phases, which are
frequently disrupted in cancer, and BMPs have, to date, gained
increasing attention in cancer research. However, the studies were
mainly carried out in the most studied tumor types, such as breast
and prostate cancer and hepatocellular carcinoma (7–10).
BMP2 and BMP9 are reported to play important roles in breast cancer
cell growth, and BMP6 and BMP7 are also detected in breast cancer
cell lines and patient samples (11–14).
However, there are only a few studies on the function of BMP8 in
the development of cancer, and only little information of BMP8 is
related to the progression of pancreatic cancer.
In the present study, as there is currently not
enough complete information on human BMP8A, we determined the roles
of BMP8B in pancreatic cancer. We found the expression of BMP8B was
downregulated in pancreatic cancer tissue compared with the normal
tissue adjacent to the tumors. Moreover, we demonstrated that BMP8B
serves as a key regulator in the apoptosis and survival of
pancreatic cancer cells by knocking down BMP8B or by overexpression
of BMP8B. Exploring the effects of BMP8B on cancer cell growth and
survival may provide a novel potential target for pancreatic cancer
therapy.
Materials and methods
Materials
The antibodies against BMP8B, Bcl-2, Bax, Bim and
β-actin were purchased from Santa Cruz Biotechnology Inc., Santa
Cruz, CA, USA. Kits of caspase-3 and -9 activities, and LDH assay
kit were from Beyotime Institute of Biotechnology (Haimen, China).
All other reagents were from common commercial sources.
Cell line preparation and culture
BxPC-3 and PANC-1, two human pancreatic cancer cell
lines, were obtained from the American Type Culture Collection
(Rockville, MD, USA). Both cell lines were routinely cultured at
37°C with RPMI-1640 medium supplemented with 10% fetal calf serum,
penicillin (100 U/ml) and streptomycin (100 μg/ml) in an incubator
with 95% air and 5% CO2.
Generation of BMP8B shRNA expression and
overexpression lentivirus
One shRNA targeting human BMP8B and one scrambled
shRNA (used as a negative control) were designed and synthesized by
Shanghai GenePharma Co., Ltd., Shanghai, China. The resultant
lentivirus containing BMP8B shRNA sequence and negative control
sequence were termed BMP8B shRNA and NC, respectively. The
lentivirus of BMP8B overexpression was also purchased from Shanghai
GenePharma Co., Ltd. We termed the BMP8B overexpression lentivirus
and blank vector BMP8B and vector, respectively.
Assessment of cell viability
The assessment of cell viability was determined by
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
assay. BxPC-3 and PANC-1 cells were split into a 96-well plate
(~5×103/well), and then subjected to growth arrest for
24 h before different treatments as the indicated groups. The cells
were divided into negative control and BMP8B shRNA, as well as
vector and BMP8B overexpression for 72 h. After 96 h of incubation
at 37°C, the cells were incubated in a medium containing 0.5% MTT
at 5 g/l and 20 μl/well for 4 h, and MTT was a yellow mitochondrial
dye and prepared in phosphate-buffered saline (PBS). After adding
DMSO to the medium followed by incubation for 10 min at 37°C, the
MTT reaction was terminated, and then the absorbance was read by a
spectrophotometer at 540 nm. All experiments were carried out in
triplicate and repeated thrice.
Crystal violet assay
Cells in different groups (~5×103/well)
were seeded into 6-well plates and cultured for 7 days and the
medium was switched every 2–3 days. Subsequently, the cells were
washed twice with pre-warmed PBS, and then cells remaining were
stained for 2 h with a crystal violet solution (0.5% crystal
violet, 20% methanol). After removal of the crystal violet
solution, the plates were washed three times by immersion in a
beaker filled with tap water. Plates were left to dry at 37°C, and
then the images were photographed by camera.
Measurement of caspase-3 activity
Caspase-3 activity was measured by its chromogenic
caspase substrate cleavage. The protein samples were prepared as
the indicated groups. Then, ~50 μg total proteins of every
indicated group were added to the reaction buffer containing
Ac-DEVD-pNA, incubated for 4 h at 37°C, and the absorbance of
yellow pNA cleavage from its corresponding precursors was measured
by using a spectrometer at 405 nm. The specific caspase-3 activity
was normalized to total proteins of cell lysates, and expressed as
fold of the baseline caspase activities of control cells.
Measurement of caspase-9 activity
Caspase-9 activity was measured as per the
manufacturer’s instructions. We measured the cleavage of
Ac-LEHD-pNA, the chromogenic caspase substrates of caspase-9, and
normalized the specific caspase-9 activity to total proteins of
cell lysates. Then, the specific caspase-9 activity was expressed
as fold of the baseline caspase activities of control cells.
LDH assay
The activity of lactate dehydrogenase (LDH) release
into the culture media was measured using a cytotoxicity detection
kit. Percentage of injured cells in cultures is represented by the
LDH activities of medium relative to total LDH activity after
complete cell lysis. Briefly, a portion of culture medium was
reacted with an equal volume of LDH substrate solution for 30 min.
The reaction was stopped by adding 5 volume of 0.1 M NaOH, and
absorbance was then measured at 440 nm in a spectrophotometer.
Sister cultures were treated with 1/100 volume of 10% Triton X-100
and incubated at 37°C for 30 min. Total LDH activities were
determined using medium containing Triton-lysed cellular
supernatant.
Western blotting
The methodology was described in previous reports
(15). Briefly, 5×105
cells were sonicated in RIPA buffer and homogenized. Debris was
removed by centrifugation at 12,000 × g for 10 min at 4°C. The
samples containing 50 μg protein were electrophoresed on
polyacrylamide SDS gels, and transferred to polyvinylidene
difluoride (PVDF) membranes. The membranes were blocked with 3%
BSA, incubated with primary antibodies, and subsequently with
alkaline phosphatase-conjugated secondary antibody. They were
developed with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue
tetrazolium (Tiangen Biotech Co. Ltd., Beijing, China). Blots were
also stained with anti-β-actin antibody as an internal control for
the amounts of target proteins.
Real-time PCR
Real-time PCR (qPCR) was performed with an Applied
Biosystems 7300 Fast Real-Time PCR System. Primers were
specifically designed using Applied Biosystems Primer Express 3.0.
The primer used for BMP8B was: forward, 5′-AGGTGGCTTCCTTATCTGCG-3′
and reverse, 5′-ATGTGCCAACTCTGCTTCGT-3′, with a product length of
165 bp The specificity of the primers was confirmed with a BLAST
program. Each 20 μl reaction contained 1X SYBR® Premix
Ex Taq™ II, 10 μM forward and reverse primers, 0.4 μl ROX reference
dye and 2 μl of cDNA. ABI 7300 Sequence Detector (Perkin-Elmer
Applied Biosystems) was programmed for the PCR conditions: 95°C for
30 sec, 40 cycles of 95°C for 5 sec and 60°C for 31 sec, followed
by routine melting curve analysis. Relative quantitation (RQ) of
target gene expression was calculated by the 2−ΔΔCT
method (16). The first step in the
RQ analysis is to normalize target gene expression level to β-actin
(ΔCt). The second step is to compare the difference between
normalized target gene expression between different samples (ΔΔCt).
Each experiment was repeated 2–3 times in 3–4 samples.
Statistical analysis
The results are expressed as the mean values ±
standard deviation, and a Student’s t-test was used to evaluate
statistical significance. A value of <0.05 (p<0.05) was
considered to indicate statistically significant differences.
Results
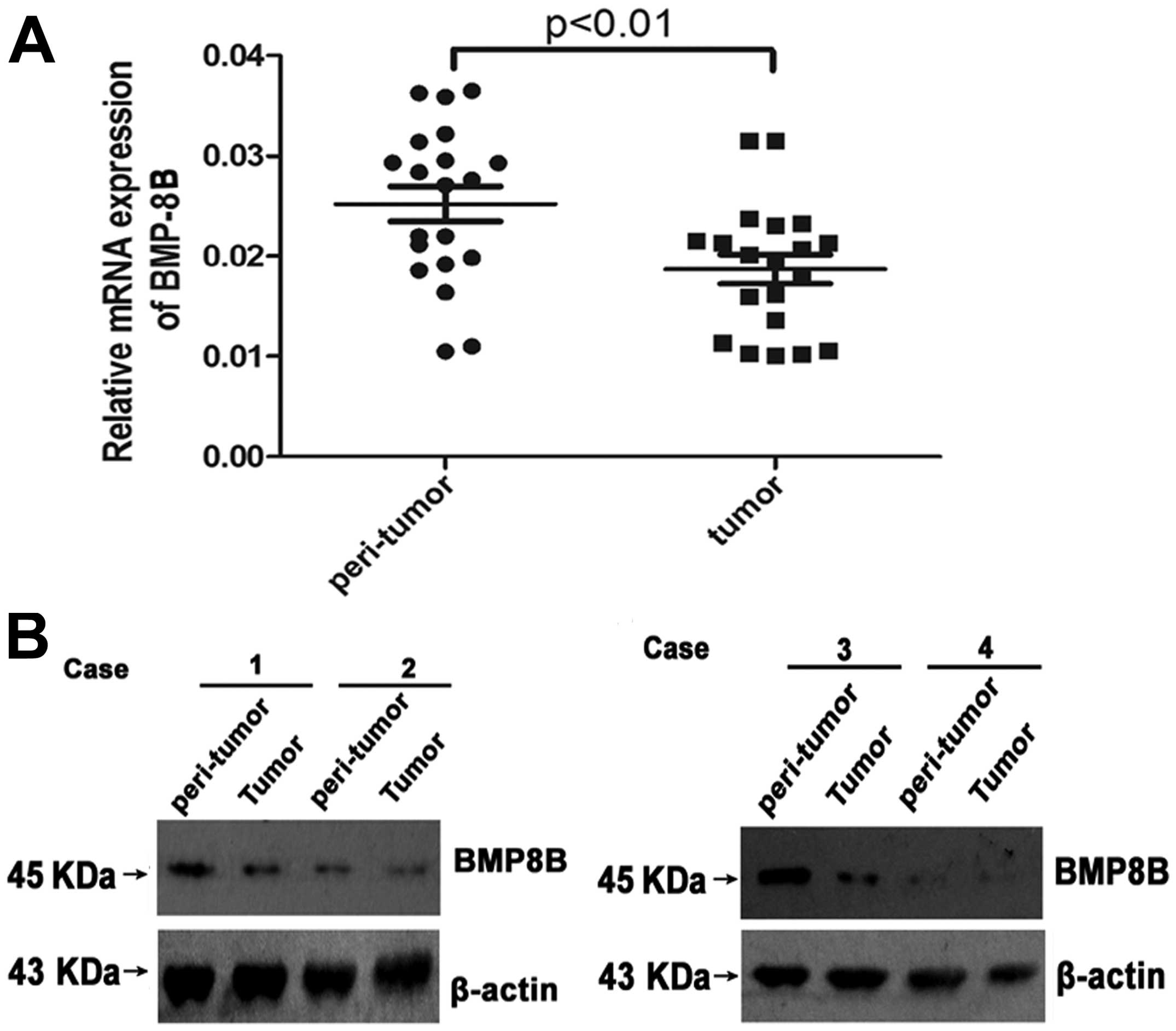
Expression of BMP8B is downregulated in
the tissue of pancreatic cancer
To determine whether BMP8B is involved in the
progression of pancreatic cancer, we first examined the expression
of BMP8B in the patients of pancreatic cancer with western blotting
and qPCR. We found that the protein and mRNA expression of BMP8B
was significantly lower in pancreatic cancer tissue compared with
the normal tissue adjacent to the tumors (Fig. 1A and B, P<0.05). The results
showed that BMP8B may play important roles in regulating the
development of pancreatic cancer.
BMP8B is overexpressed in PANC-1 and
BxPC-3 is efficiently infected with BMP8B-shRNA
We then constructed the stably transfected cell
lines of BMP8B overexpression in PANC-1 in order to further
demonstrate that BMP-8 is associated with pancreatic cancer cell
growth and survival. To confirm the transfection efficiency and to
achieve the overexpressed cells of BMP8B, we examined the
expression of BMP8B. As shown in Fig.
2A and B, the protein and mRNA expression of BMP8B were
considerably increased in PANC-1.
BxPC-3 was infected with BMP8B-shRNA to
knock down BMP8B expression
We found the protein and mRNA expression of BMP8B
was significantly decreased after applying BMP8B-shRNA in BxPC-3
compared to the negative control (NC) group (Fig. 2C and D, n=3, p<0.05), indicating
the adequate knocking down of the BMP-8 gene by BMP8B-shRNA.
Overexpression of BMP8B leads to cell
growth arrest and death, and weakens the colony formation in
pancreatic cancer cells
MTT was applied to determine the effects of BMP8B on
cell growth. The results showed that the cell viability of PANC-1
was inhibited after overexpression of BMP8B (Fig. 3A, n=3, p<0.05). Moreover, the
release of LDH, which occurs with cell death, was increased in
BMP8B-overexpressed cells (Fig. 3B,
n=3, p<0.05). The colony formation of tumor cells reflects the
tumorigenicity of tumor cells. We found that overexpression of
BMP8B resulted in the decreased colony formation activities in
PANC-1 (Fig. 3C, n=3, p<0.05).
These results demonstrated that overexpression of BMP8B blocked the
growth of pancreatic cancer cells.
Knockdown of BMP8B gene expression
promotes cell growth and colony formation
We examined the cell growth and colony formation in
BxPC-3 after silencing the BMP8B gene expression with BMP8B shRNA.
As shown in Fig. 4, knocking down
BMP8B expression increased cell growth and promoted colony
formation, and inhibited the release of LDH in BxPC-3. The results
were in accordance with the findings of Fig. 3.
BMP8B overexpression induces the
mitochondrial-dependent apoptosis through regulating Bim/Bcl-2/Bax
expression
Bim, Bcl-2 and Bax are important molecules localized
on mitochondrial outer membrane and control the stability of
mitochondria. Bim and Bax are pro-apoptotic proteins and Bcl-2 is
an anti-apoptotic protein. In our experiments, we found the
expression of Bim and Bax was upregulated by BMP8B overexpression,
and the overexpression of BMP8B blocked the expression of Bcl-2 in
PANC-1 (Fig. 5A and B, n=3,
p<0.05). Furthermore, caspase-3 and -9 are the key performers
during the mitochondrial pathway apoptosis. We then examined
whether BMP8B participated in regulating the activation of
caspase-3 and -9. The results showed that the activation of
caspase-3 and -9 was increased by BMP8B overexpression in PANC-1
(Fig. 5C, n=3, p<0.05). These
results demonstrate that the overexpression of BMP8B triggers the
mitochondrial-dependent apoptosis through regulating Bim/Bcl-2/Bax
expression.
The mitochondrial pathway of apoptosis is
inhibited by decreasing the BMP8B expression
We also examined the activation of caspase-3 and -9
and the expression of mitochondrial membrane proteins after
knocking down BMP8B gene expression in BxPC-3 to further support
our conclusion. Our results showed that silencing BMP8B gene
induced Bcl-2 expression and decreased the expression of Bim and
Bax (Fig. 6A and B, n=3,
p<0.05). Moreover, the activities of caspase-3 and -9 were
suppressed by BMP8B shRNA in BxPC-3 (Fig. 6C, n=3, p<0.05), indicating that
the apoptosis is inhibited by knocking down BMP8B expression.
Discussion
It is well known that promoting tumor cell
proliferation and inhibiting cell apoptosis both result in the
overgrowth of tumor cells, which accelerates the progression and
development of tumors. In the present study, we demonstrated that
BMP8B is downregulated in pancreatic cancer tissue compared with
the normal tissue adjacent to the tumors. Moreover, overexpression
of BMP8B induced apoptosis and inhibited the growth in pancreatic
cancer cells, and silencing BMP8B gene expression promoted
pancreatic cancer cell survival and decreased apoptosis. We provide
new evidence that BMP8B likely serves as a tumor suppressor gene to
inhibit the growth of pancreatic cancer cells, and silencing BMP8B
gene expression advances the progression of pancreatic cancer.
Mounting evidence indicates that an important
feature of tumor cells is infinitely excessive growth and
proliferation. Thus, examining the oncogene and tumor suppressor
gene is helpful to relieve the progression of cancer and to provide
potential therapeutic targets for treatment (17). Previous studies reported that BMP8B
plays a positive role in promoting cell proliferation and tumor
development (18). However, direct
evidence that BMP8B is associated with advancing the progression of
pancreatic cancer and BMP8B overexpression inhibiting the growth of
pancreatic cancer cells is lacking. In the present study, we found
that the protein and mRNA expression of BMP8B is decreased in
pancreatic cancer tissue compared with the normal tissue adjacent
to the tumors. Moreover, overexpression of BMP8B inhibited the
growth and colony formation of PANC-1, and silencing the BMP8B gene
expression promoted the colony formation and cell growth in BxPC-3.
We believe that the inhibition of the BMP8B pathway is required for
the survival of pancreatic cancer cells.
An important physiological change of the early
events of the mitochondrial apoptosis pathway is mitochondrial
dysfunction (19). In general, the
mitochondrial dysfunction results from the expression change of the
proteins, which are localized on the mitochondrial membrane and
control the opening of mitochondrial membrane pores. Then, the
release of cytochrome c from the mitochondria into the
cytoplasm induced by mitochondrial dysfunction activates caspase-9
and -3, and causes cell apoptosis (20). In the present study, we observed
that the overexpression of BMP8B induced the expression of Bim and
decreased the ratio of Bcl-2/Bax. On the contrary, knocking down
BMP8B expression resulted in the increased ratio of Bcl-2 to Bax
and downregulated the expression of Bim. Moreover, caspase-3 and -9
were activated in BMP8B-overexpressed PANC-1, and silencing BMP8B
expression inhibited the activation of caspase-3 and -9 in BxPC-3.
In brief, we demonstrated that BMP8B protects against
mitochondria-dependent apoptosis in pancreatic cancer cells.
Although we confirmed the important roles of BMP8B
in regulating pancreatic cancer cell survival, how the BMP8B
pathway promotes pancreatic cancer development remains to be
examined in further studies. In addition, it still needs to be
determined whether the BMP8B pathway is also involved in regulating
the death receptor pathway (the other important apoptosis pathway)
in pancreatic cancer.
In conclusion, it has been demonstrated that BMP8B,
which is downregulated in the tissue of pancreatic cancer, exerts
inhibitive effects on the apoptosis of pancreatic cancer cells.
These findings elucidate one possible mechanism of pancreatic
cancer progression influenced by BMP8B, providing a novel potential
therapeutic target for the treatment in the future.
Acknowledgements
This study was supported in part by grants from the
Science and Technology Research Project of the Education Department
of Heilongjiang Province (no. 12541814 to Z.X. Cheng), the Natural
Science Foundation of Heilongjiang Province (no. H201373 to Z.X.
Cheng), Jiamusi University Youth Foundation (q2013-024 to Z.X.
Cheng), Heilongjiang Provincial Health Department Fund (2013-238 to
Z.X. Cheng).
References
|
1
|
Danovi SA1, Wong HH and Lemoine NR:
Targeted therapies for pancreatic cancer. Br Med Bull. 87:97–130.
2008. View Article : Google Scholar
|
|
2
|
Warshaw AL and Fernández-del Castillo C:
Pancreatic carcinoma. N Engl J Med. 326:455–465. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar
|
|
4
|
Bardeesy N and DePinho RA: Pancreatic
cancer biology and genetics. Nat Rev Cancer. 2:897–909. 2002.
View Article : Google Scholar
|
|
5
|
Alarmo EL and Kallioniemi A: Bone
morphogenetic proteins in breast cancer: dual role in
tumourigenesis? Endocr Relat Cancer. 17:R123–R139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyazono K, Kamiya Y and Morikawa M: Bone
morphogenetic protein receptors and signal transduction. J Biochem.
147:35–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim IY and Kim SJ: Role of bone
morphogenetic proteins in transitional cell carcinoma cells. Cancer
Lett. 241:118–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Langenfeld EM, Kong Y and Langenfeld J:
Bone morphogenetic protein 2 stimulation of tumor growth involves
the activation of Smad-1/5. Oncogene. 25:685–692. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Piccirillo SG, Reynolds BA, Zanetti N,
Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F and Vescovi
AL: Bone morphogenetic proteins inhibit the tumorigenic potential
of human brain tumour-initiating cells. Nature. 444:761–765. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bleuming SA, He XC, Kodach LL, Hardwick
JC, Koopman FA, Ten Kate FJ, van Deventer SJ, Hommes DW,
Peppelenbosch MP, Offerhaus GJ, Li L and van den Brink GR: Bone
morphogenetic protein signaling suppresses tumorigenesis at gastric
epithelial transition zones in mice. Cancer Res. 67:8149–8155.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arnold SF, Tims E and Mcgrath BE:
Identification of bone morphogenetic proteins and their receptors
in human breast cancer cell lines: importance of BMP2. Cytokine.
11:1031–1037. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ren W, Sun X, Wang K, Feng H, Liu Y, Fei
C, Wan S, Wang W, Luo J, Shi Q, Tang M, Zuo G, Weng Y, He T and
Zhang Y: BMP9 inhibits the bone metastasis of breast cancer cells
by downregulating CCN2 (connective tissue growth factor, CTGF)
expression. Mol Biol Rep. 41:1373–1383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Autzen P, Robson CN, Bjartell A, Malcolm
AJ, Johnson MI, Neal DE and Hamdy FC: Bone morphogenetic protein 6
in skeletal metastases from prostate cancer and other common human
malignancies. Br J Cancer. 78:1219–1223. 1998. View Article : Google Scholar
|
|
14
|
Alarmo EL, Pärssinen J, Ketolainen JM,
Savinainen K, Karhu R and Kallioniemi A: BMP7 influences
proliferation, migration, and invasion of breast cancer cells.
Cancer Lett. 275:35–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang SJ, Gao Y, Chen H, Kong R, Jiang HC,
Pan SH, Xue DB, Bai XW and Sun B: Dihydroartemisinin inactivates
NF-κB and potentiates the anti-tumor effect of gemcitabine on
pancreatic cancer both in vitro and in vivo. Cancer Lett.
293:99–108. 2010.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spender LC and Inman GJ: Developments in
Burkitt’s lymphoma: novel cooperations in oncogenic MYC signaling.
Cancer Manag Res. 6:27–38. 2014.
|
|
18
|
Mima K, Fukagawa T, Kurashige J, Takano Y,
Uchi R, Ueo H, Matsumura T, Ishibashi M, Sawada G, Takahashi Y,
Akiyoshi S, Eguchi H, Sudo T, Sugimachi K, Watanabe M, Ishii H,
Mori M, Baba H, Sasako M and Mimori K: Gene expression of bone
morphogenic protein 8B in the primary site, peripheral blood and
bone marrow of patients with gastric cancer. Oncol Lett. 6:387–392.
2013.PubMed/NCBI
|
|
19
|
Jung SH, Kang KD, Ji D, Fawcett RJ, Safa
R, Kamalden TA and Osborne NN: The flavonoid baicalin counteracts
ischemic and oxidative insults to retinal cells and lipid
peroxidation to brain membranes. Neurochem Int. 53:325–337. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Correa F, Soto V and Zazueta C:
Mitochondrial permeability transition relevance for apoptotic
triggering in the post-ischemic heart. Int J Biochem Cell Biol.
39:787–798. 2007. View Article : Google Scholar : PubMed/NCBI
|