Introduction
As a result of the increasing number of breast
carcinomas, breast cancer has become the most common malignancy and
is currently one of the leading causes of mortality in women
(1–2). Although systematic chemotherapy
remains a frequently used clinical strategy, due to the development
of multidrug resistance and severe side-effects (3,4), there
is still a need to identify novel agents to enhance the
effectiveness of breast cancer treatment.
In the past years, synthesis and characterization of
novel antitumor compounds with known biological activity have
represented a field of research that has created expectations for
more specific and less toxic therapies (5). Isatin (an alternative name for
1H-indole-2,3-dione; formula,
C8H5O2N), an endogenous indole in
mammalian tissues and fluids, possesses a wide range of biological
activities, such as anxiogenic, sedative and anticonvulsant
activities, and is a potent antagonist of atrial natriuretic
peptide receptors in vitro (6–8).
However, previous studies have focused on its potent anticancer
properties (9,10). In addition, many indole-based
compounds appear to act as inhibitors of various protein kinase
families, particularly receptor tyrosine kinases (RTKs) and
serine/threonine-specific protein kinases such as the
cyclin-dependent kinases (CDKs) (11,12).
Oxindole sunitinib malate, as a kinase inhibitor, was recently
approved by US FDA for the treatment of advanced renal carcinoma
and gastrointestinal stromal tumors, which underscores the
increasing interest in isatins as a new class of antineoplastic
agents. It was also shown that isatin and its analogs inhibited the
proliferation of some cancer cells, including colon HT29, breast
MCF-7, lung A549 and melanoma UACC903 cells and is a dual inhibitor
of tubulin polymerization and the Akt pathway (13). Therefore the aim of this study was
to further clarify the anticancer mechanism of isatin itself, which
may offer an opportunity to design effective safe drugs with
minimal toxicity for the treatment of breast carcinoma.
It is widely believed that the mitochondrial pathway
plays a critical role in the apoptotic process (14), particularly the mitochondrial Bcl-2
protein family. This family consists of a large group of
apoptosis-regulating proteins that modulate the apoptotic
mitochondrial pathway (15) and
includes both anti-apoptotic proteins, such as Bcl-2, Bcl-xl and
Mcl-1, and pro-apoptotic proteins, including Bax, Bad and Bak
(16). These proteins regulate
mitochondrial membrane permeability by either promoting or
suppressing the release of apoptogenic proteins from organelles.
Therefore, we sought to assess the in vitro effects of
isatin in MCF-7 cells and to further confirm the anticancer effect
of isatin through a mitochondrial pathway.
Materials and methods
Cell line and cell culture
The human MCF-7 cell line was obtained from Peking
Union Medical College. The cells were maintained in DMEM medium
supplemented with 15% heat-inactivated fetal calf serum at 37°C in
a tissue culture incubator with 5% CO2 and 98% relative
humidity. When 70% of the plate was covered by cells, isatin
(dissolved in 200 μl DMSO to obtain a 5 mmol/l solution) was added
to a final concentration of between 50 and 200 μmol/l. Following a
48 h incubation period, the cells were harvested and used for
apoptosis, mRNA and protein analysis.
Nuclear staining
The cells used for nuclear staining were seeded in
6-well plates (105 cells/well). After 48 h of treatment
with isatin, the cells were fixed with 4% paraformaldehyde for 1 h
at room temperature, washed with PBS, stained with 10 μg/ml Hoechst
33258 (Sigma) for 10 min at 37°C in the dark and then washed with
PBS. The apoptotic features of cell death were established by
staining cell nuclei with the DNA-binding fluorochrome Hoechst
33258 and assessing chromatin condensation by fluorescence
microscopy (BX-50, Olympus, Tokyo, Japan).
Flow cytometric analysis
The treated cells were harvested by centrifugation
and washed three times with PBS. The cells were fixed with ice-cold
75% ethanol for 18 h, stained with propidium iodide (PI) and then
analyzed by flow cytometry (CantoII; Becton-Dickinson, USA) to
detect apoptotic cells. A minimum of 10,000 events were analyzed in
each experiment.
Analysis of Bcl-2 and Bax mRNA
Total RNA was extracted from the cultured cells
(107) cultured in the presence or absence of isatin for
48 h using a TRIzol RNA isolation kit (Life Technologies,
Gaithersburg, MD, USA) and detected by reverse
transcription-polymerase chain reaction (RT-PCR), according to the
manufacturer’s protocol. Primers were: for GAPDH,
5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′ to give a
product of 452 bp; Bcl-2, 5′-GGAGGATTGTGGCCTTCTTTG-3′ and
5′-GGTGCCGGTTCAGGTACTCA-3′ to give a product of 120 bp; Bax,
5′-TCCACCAAGAAGCTGAGCGAG-3′ and 5′-GTCCAGCCCATGATGGTTCT-3′ to give
a product of 257 bp. Each RT-PCR assay was performed in triplicate.
Primers specific for human GAPDH cDNA were added to a parallel
reaction to standardize for variations in the PCR between samples.
PCR products were resolved on a 1.0% agarose gel, visualized under
UV light and quantified using a JS-380B Imager (Shanghai Peiqing,
China).
Western blot analysis
MCF-7 cells (107) were cultured in the
presence or absence of isatin for 48 h. The cells were scraped and
lysed in buffer (20 mmol/l Tris-HCl, pH 7.4, 137 mmol/l NaCl, 10%
glycerol, 1% Nonidet P-40, 2 mmol/l sodium vanadate and 100 mmol/l
sodium fluoride) for 20 min on ice, before centrifugation at 12,000
× g for 2 min. The protein concentration of the supernatants was
determined using Bradford protein assay reagent (Bio-Rad),
separated in 10% SDS-PAGE and blotted onto a PVDF membrane. The
blots were blocked in non-fat milk in Tris-buffered saline (TBS)
containing 0.1% Tween-20 (TBS-T) for 2 h at room temperature,
before incubation with the monoclonal primary antibodies,
anti-Bcl-2, anti-Bax and anti-inhibitor of caspase-activated DNase
(ICAD) (1:1,000; New England Biolabs, Inc., Beverly, MA, USA)
overnight at 4°C. The blots were washed for 3×5 min in TBS-T and
then incubated in a 1:2,000 dilution of peroxidase-conjugated
donkey anti-rabbit secondary antibodies for 2 h at room
temperature. The blots were again washed for 3×5 min in TBS-T and
proteins were detected using an enhanced chemiluminescence plus kit
(Amersham Biosciences, Buckinghamshire, UK).
Measurement of the mitochondrial membrane
potential (ΔΨm)
Rhodamine 123 is widely used for mitochondrial
staining due to its rapid cellular uptake and equilibration. Viable
cells appear as a highly fluorescent population, whereas apoptotic
cells exhibit a lower fluorescence (17). Cells were treated with isatin (50,
100, 200 μmol/l) for 48 h, washed with PBS and then incubated in
PBS containing 5 μmol/l Rhodamine 123 at room temperature for 30
min. After two washes and a final resuspension in PBS, the
fluorescence of the cells was measured by flow cytometry (CantoII;
Becton-Dickinson, USA).
Detection of cytochrome c release from
the mitochondria to the cytosol
Cytochrome c determination in cytosolic and
mitochondrial fractions was performed using western blot analysis.
The cells were harvested after the respective treatments and washed
once with ice-cold PBS. For the isolation of mitochondria and
cytosol, cells were sonicated in a buffer containing 10 mM
Tris-HCl, pH 7.5, 10 mmol/l NaCl, 175 mmol/l sucrose and 12.5
mmol/l EDTA and the cell extracts were centrifuged at 1,000 × g for
10 min to pellet the nuclei. The supernatant obtained was
centrifuged at 18,000 × g for 30 min to pellet the mitochondria.
The resulting supernatant was termed the cytosolic fraction. The
pellet was lysed and the protein content was estimated in both
fractions using Bradford’s method. Equal amounts of protein were
separated on 15% SDS-PAGE and electrotransferred to a PVDF
membrane. The membrane was then incubated in 5% non-fat milk in TBS
for 2 h followed by overnight incubation with the primary antibody
(Sigma, 1:1,000). The incubated membranes were extensively washed
with TBST before incubation for 2 h with the secondary antibody.
After extensive washing with TBST, the immune complexes were
detected using an enhanced chemiluminescence detection kit.
Caspase-9 and -3 assay
Caspase-9 and -3 were analyzed by flow cytometry
using CaspGLOW™ Fluorescein Active caspase-9 staining kit
(eBioscience, USA) and PE-conjugated monoclonal active caspase-3
antibody apoptosis kit (BD Biosciences, USA). Cells were collected
and washed in cold PBS, resuspended at a concentration of
1×106 cells/0.5 ml. Samples were washed twice with wash
buffer, before FITC-LEHD-FMK (a specific inhibitor of caspase-9)
(eBioscience, 1:300) and PE-conjugated monoclonal anti-active
caspase-3 antibodies (BD Biosciences, 1:500) were added and the
cells were incubated for 30 min at room temperature. The marked
samples were analyzed by flow cytometry (CantoII;
Becton-Dickinson).
Statistical analysis
Each experiment was performed at least three times.
All data are expressed as the mean ± SD. Statistical analysis was
performed using one-way ANOVA. A level of P<0.05 was considered
to indicate a statistically significant difference.
Results
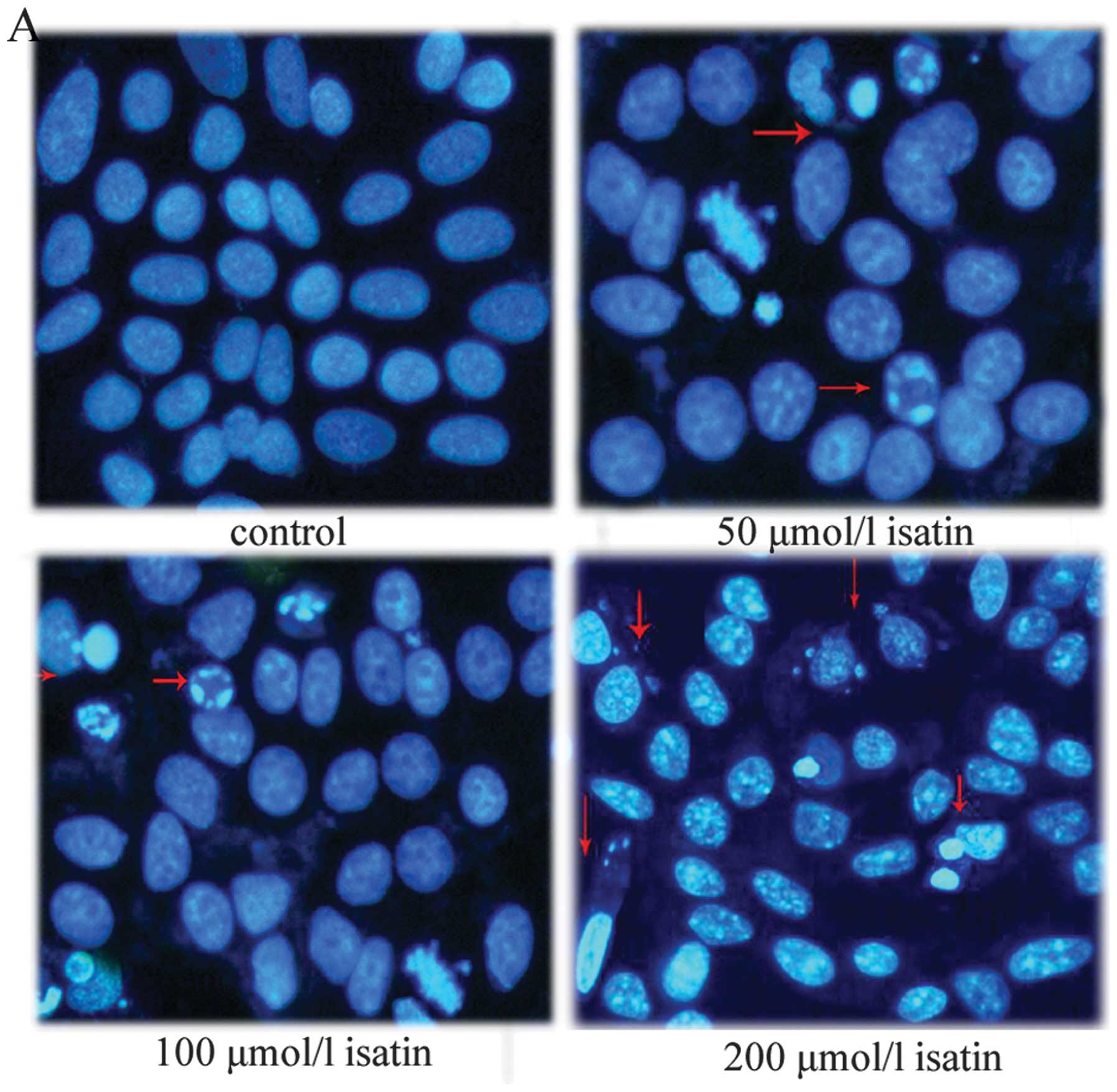
Effect of isatin on MCF-7 cell
apoptosis
Apoptotic cells have special morphologic features
such as cell shrinkage, chromatin condensation and margination as
well as forming apoptotic bodies. Hoechst 33258 staining was used
to determine whether the isatin-induced reduction in cell viability
was attributable to the induction of apoptosis. As shown in
Fig. 1A, the treatment of MCF-7
cells with isatin resulted in the induction of chromatin
condensation and fragmentation, which was visualized as intense
pycnotic bluish-white fluorescence within the cell nuclei. To
further confirm the isatin-induced apoptosis, we detected it by
flow cytometry. When cells were treated with isatin (50, 100 and
200 μmol/l) for 48 h, cell populations in the apoptotic phases
increased from 11.25±2.6 and 15.63±2.40 to 23.47±5.06%, compared
with 6.33±1.40% of apoptotic cells in the control (Fig. 1B).
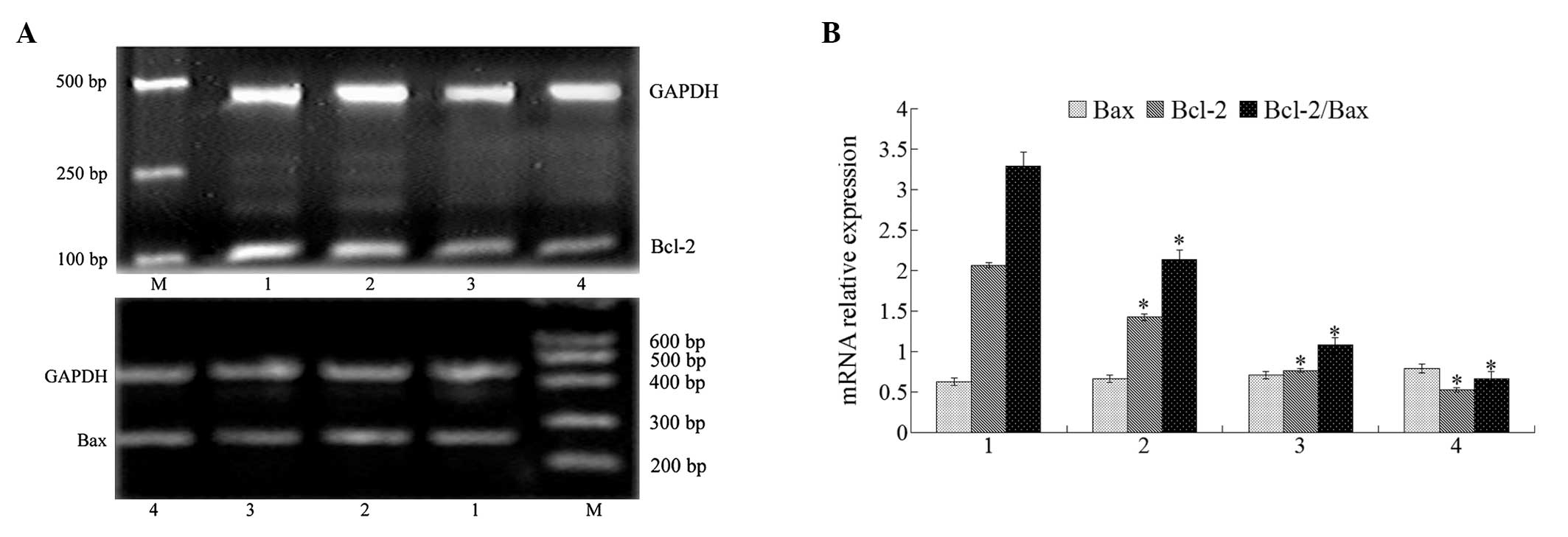
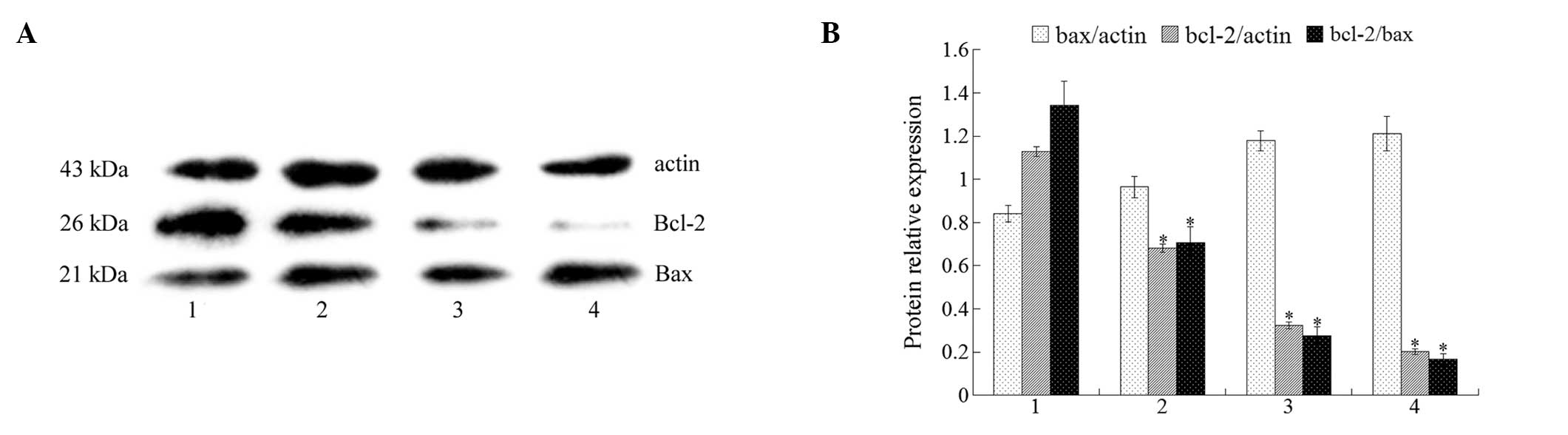
Effect of isatin on Bcl-2 and Bax
expression
Bcl-2 family members actively participate in the
apoptotic process either via pro-apoptotic proteins such as Bax, or
via anti-apoptotic ones including Bcl-2. The ratio of pro- and
anti-apoptotic molecules is essential to apoptosis. Therefore, we
studied the effect of isatin on Bax and Bcl-2 mRNA expression. The
RT-PCR results showed that isatin decreased the expression of Bcl-2
mRNA and while it did not modulate the expression of Bax mRNA
(Fig. 2), the Bcl-2/Bax ratio was
decreased. The Bcl-2 and Bax protein expression results from the
western blot analysis were consistent with those from mRNA analysis
(Fig. 3). These results demonstrate
that isatin possesses a pro-apoptotic effect on MCF-7 cells.
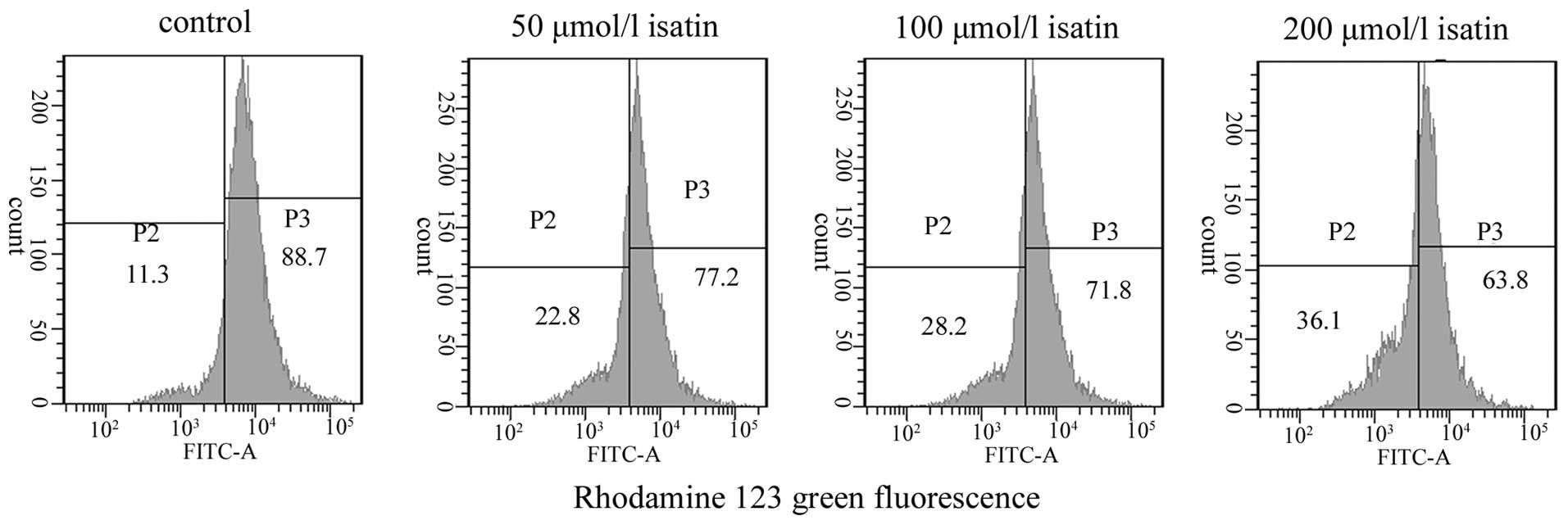
ΔΨm determination
Disruption of mitochondrial integrity is one of the
early events leading to apoptosis. Thus, when cells are stained
with a potential-sensitive mitochondrial specific probe, the
staining and the fluorescence intensity are directly correlated
with the mitochondrial polarization status. To assess whether
isatin affects mitochondrial function, changes in the ΔΨm were
analyzed through the use of a fluorescent mitochondrial dye,
Rhodamine 123. As shown in Fig. 4,
exposure to isatin for 48 h resulted in a decrease in the
fluorescence intensity by ~77.00±2.27, 71.47±2.12 and 63.67±4.40%
at 50, 100 and 200 μmol/l, respectively, which was lower than that
in control MCF-7 cells (88.40±6.15%). This suggests that treatment
with isatin for 48 h results in decreases in the ΔΨm.
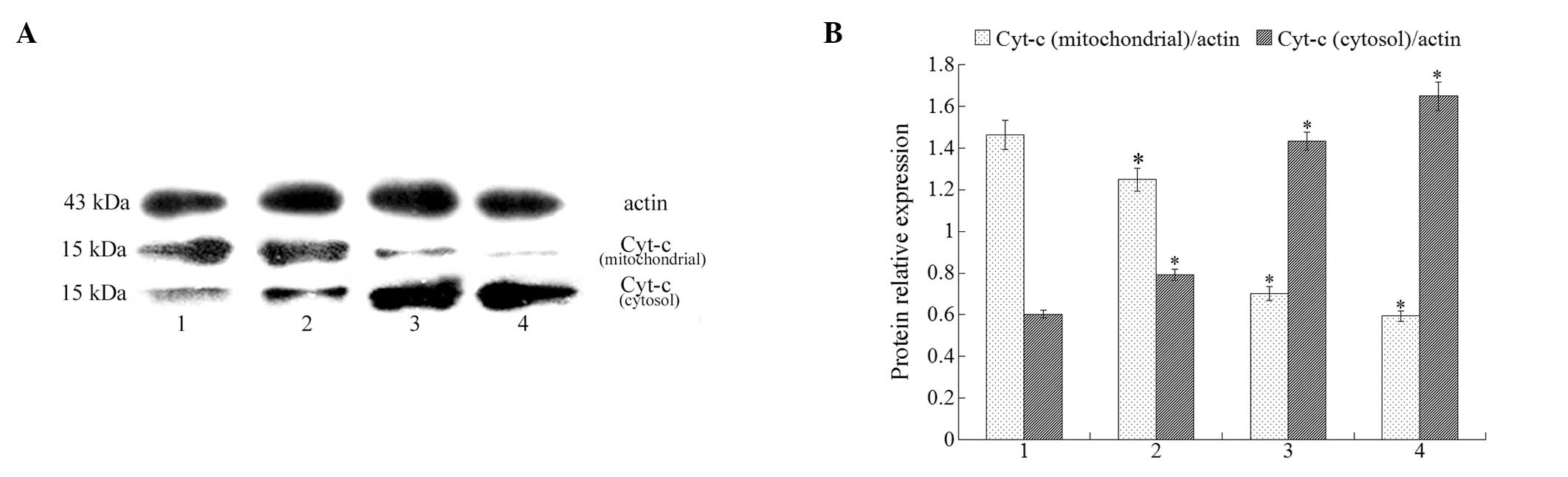
Cytochrome c release from mitochondria to
cytosol
Cytochrome c release from mitochondria is a
critical step in the apoptotic cascade since this activates
downstream caspases. To investigate cytochrome c results in
isatin-treated cells, we conducted western blot analysis in both
cytosolic and mitochondrial fractions. The results demonstrate a
clear increase in cytosolic cytochrome c after treatment
with isatin (at 50, 100, and 200 μmol/l). At the same time, a
decrease in cytochrome c was detected in the mitochondrial
fraction (Fig. 5).
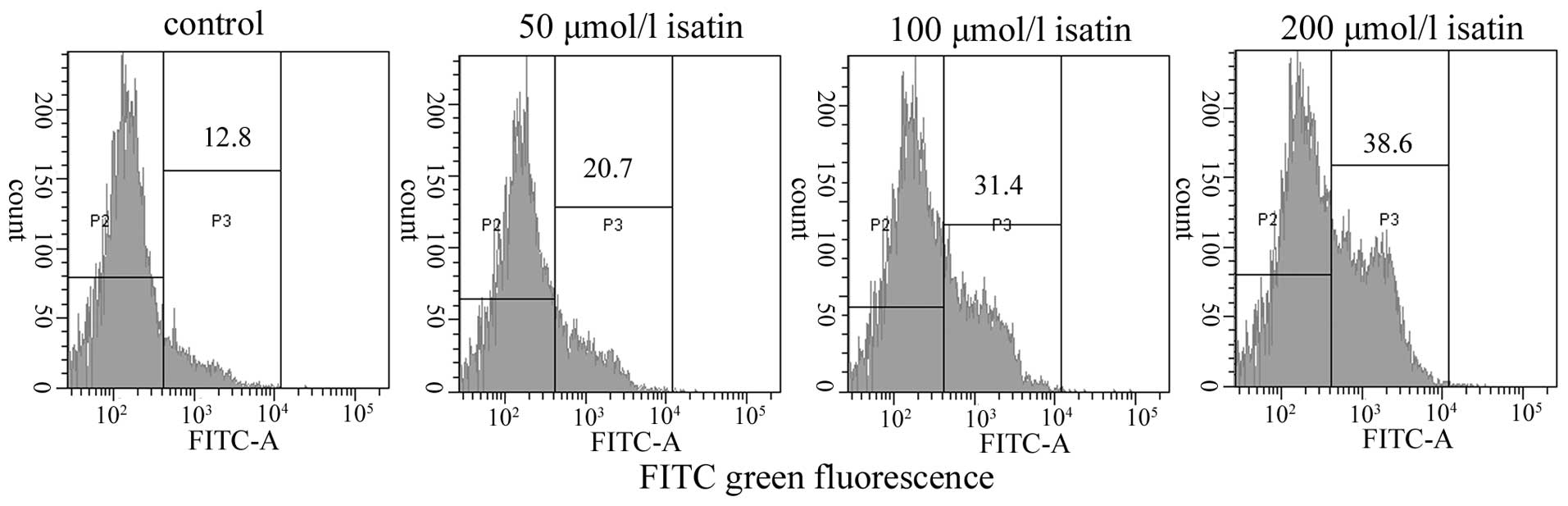
Analysis of caspase-9 and -3 protein
expression
We analyzed active caspase-9 and -3 in MCF-7 cells
treated for 48 h with isatin at 0, 50, 100, 200 μmol/l by flow
cytometry with specific inhibitors of caspase-9 and -3. As shown in
Fig. 6, exposure to isatin for 48 h
resulted in an increase in the expression of active caspase-9 by
~21.17±2.63, 31.77±3.56 and 38.73±4.30% at 50, 100 and 200 μmol/l,
respectively, which was higher than that in control MCF-7 cells
(13.07±2.81%). In addition, the expression of active caspase-3 was
increased in isatin-treated cells, but not in control cells
(Table I).
 | Table IEffect of isatin on caspase-3 activity
in MCF-7 cells measured by flow cytometry. |
Table I
Effect of isatin on caspase-3 activity
in MCF-7 cells measured by flow cytometry.
| Positive rate
(%) |
|---|
|
|
|---|
| Group | Unactivated
caspase-3 | Activated
caspase-3 |
|---|
| Control | 90.43±2.96 | 9.40±2.76 |
| Isatin |
| 50 μmol/l | 83.00±1.67a | 16.57±1.36a |
| 100 μmol/l | 72.47±4.22a | 26.40±4.21a |
| 200 μmol/l | 63.87±4.56a | 35.97±4.52a |
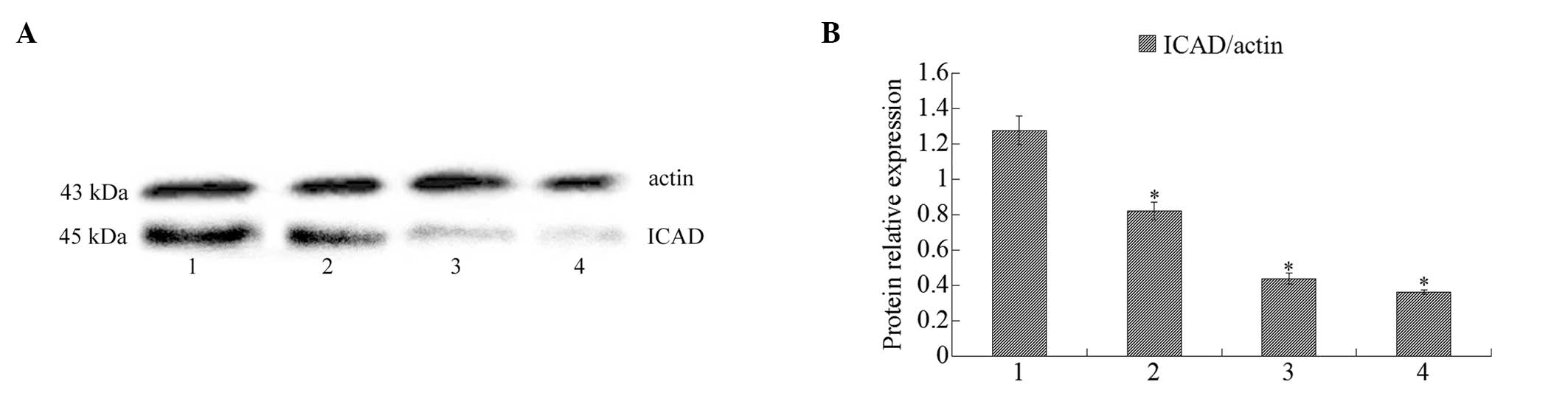
Western blot analysis of ICAD
Western blot analysis indicated that ICAD expression
was downregulated when MCF-7 cells were treated with isatin (50,
100, 200 μmol/l) for 48 h (Fig.
7).
Discussion
The lack of selectivity of many anticancer agents
and the occurrence of intrinsic or acquired resistance of tumors to
chemotherapy are major obstacles in the treatment of cancer.
Studies have shown that isatin and its analogs display diverse
biological activities including anticancer activities as an
endogenous molecule in humans and other mammals (18). Therefore, isatin has gained
considerable attention due to its anticancer activities, and it is
expected to be a novel candidate for low toxic tumor therapy
(19,20). In this study, we used a commercially
available isatin and its inhibitory activities on the growth of
MCF-7 cells were assayed. The results demonstrated that isatin
strongly induced human breast cancer cell line apoptosis in
vitro.
In this context, we thoroughly investigated the
molecular pathways induced by isatin in breast cancer cells. Based
on morphologic observations, DNA fragmentation suggested that MCF-7
cell death induced by isatin involved an apoptotic mechanism. In
addition, the apoptotic rate, analyzed by flow cytometry, further
confirmed that isatin induced MCF-7 cell apoptosis in a
concentration-dependent manner. Apoptosis is a regulated biological
mode of cell death and includes two major pathways, the death
receptor-mediated extrinsic pathway and the mitochondria-dependent
intrinsic pathway (21). The Bcl-2
family of proteins, including both pro- and anti-apoptotic members,
play important roles in controlling the mitochondria-dependent
intrinsic pathway at critical checkpoints. However, studies have
demonstrated that the anticancer effects of many currently
available chemotherapeutic agents may be inhibited by upregulation
of Bcl-2 expression which blocks the apoptotic pathway (22). A promising antisense strategy to
downregulate Bcl-2 is under clinical evaluation for the treatment
of melanoma (23). Therefore, we
determined Bcl-2 and Bax proteins in MCF-7 cells following
treatment with various concentrations of isatin. The results showed
that, concomitant with an increase in isatin, Bcl-2 expression
decreased and the ratio of Bcl-2 to Bax significantly decreased
(P<0.05). These results indicated that the
mitochondria-dependent intrinsic pathway is an important pathway in
isatin-induced apoptosis. Previous studies have also demonstrated
that the ratio of Bax/Bcl-2 sets the threshold of susceptibility to
apoptosis in the mitochondrial pathway (24). Cytochrome c is one of a host
of pro-death molecules located within mitochondria and is a
universal feature of apoptosis. Studies have shown that the Bcl-2
family is closely involved in the regulation of cytochrome c
release into the cytosol (25). The
anti-apoptotic proteins such as Bcl-2 preserve the integrity of
mitochondria and block the release of cytochrome c. Bax is
located in the cytosol and can interact with the anti-apoptotic
protein Bcl-2. In response to apoptotic signals, Bax translocates
to the mitochondria and inserts into the outer mitochondrial
membrane, heterodimerizing with Bcl-2 to abrogate the inhibition of
apoptosis caused by Bcl-2 by promoting the release of cytochrome
c into the cytosol. Our results showed that cytochrome
c levels in the mitochondria were downregulated, suggesting
that it was released into the cytosol. The release of cytochrome
c from the mitochondria to the cytosol is an important step
in the apoptotic pathway.
Caspases are a family of cysteases, which cleave
protein substrates after their Asp residues and appear to be
involved in regulating the activation of apoptotic signal
transmission (26). It is well
known that mitochondrial damage caused by apoptotic stimuli
triggers the release of apoptogenic proteins including cytochrome
c and Smac. Cytochrome c triggers the activation of
caspases. Based on our current findings on the regulatory effects
of isatin on decreased mitochondrial membrane potential and the
release of cytochrome c, we hypothesized that caspase-9 and
-3 may play important roles in isatin-induced apoptosis. The
results of this study confirmed that caspase-9 and -3 activities
were increased following isatin treatment. To further confirm the
participation of caspase-3 in cell death, we examined the protein
expression of ICAD. ICAD is digested by caspase-3, resulting in
activation of caspase-activated DNase (CAD) and nuclear
internucleosomal DNA fragmentation (27). Our results showed that the
expression of ICAD was downregulated. Taken together, these results
suggest that the caspase cascade plays a critical role in
isatin-induced MCF-7 cell apoptosis.
In summary, isatin significantly inhibits the growth
of MCF-7 cells in vitro. This anticancer mechanism may
involve the deregulation of Bcl-2, a decrease in the ΔΨm and
activation of caspase-9 and -3. These results strongly support the
hypothesis that the mitochondrial pathway is involved in apoptosis.
However, as relatively little research has focused on its in
vitro effects, further study is required to confirm the
anticancer effect of isatin.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics, 2011. CA Cancer J Clin. 61:409–418. 2011.
View Article : Google Scholar
|
|
3
|
Smith NZ: Treating metastatic breast
cancer with systemic chemotherapies: current trends and future
perspectives. Clin J Oncol Nurs. 16:E33–E43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gonzalez-Angulo AM, Morales-Vasquez F and
Hortobagyi GN: Overview of resistance to systemic therapy in
patients with breast cancer. Adv Exp Med Biol. 608:1–22. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ramachandran S: Synthesis and
antimicrobial evaluation of some novel schiff and mannich bases of
isatin derivatives. Int J Res Pharm Chem. 1:289–294. 2011.
|
|
6
|
Medvedev A, Igosheva N, Crumeyrolle-Arias
M and Glover V: Isatin: role in stress and anxiety. Stress.
8:175–183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pandeya SN, Smitha S, Jyoti M and Sridhar
SK: Biological activities of isatin and its derivatives. Acta
Pharm. 55:27–46. 2005.PubMed/NCBI
|
|
8
|
Medvedev A, Buneeva O and Glover V:
Biological targets for isatin and its analogues: implications for
therapy. Biologics. 1:151–162. 2007.PubMed/NCBI
|
|
9
|
Vine KL, Matesic L, Locke JM, Ranson M and
Skropeta D: Cytotoxic and anticancer activities of isatin and its
derivatives: a comprehensive review from 2000–2008. Anticancer
Agents Med Chem. 9:397–414. 2009.PubMed/NCBI
|
|
10
|
Cane A, Tournaire MC, Barritault D and
Crumeyrolle-Arias M: The endogenous oxindoles 5-hydroxyoxindole and
isatin are antiproliferative and proapoptotic. Biochem Biophys Res
Commun. 276:379–384. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun L, Liang C, Shirazian S, Zhou Y,
Miller T, Cui J, Fukuda JY, et al: Discovery of
5-[5-fluoro-2-oxo-1,2-dihydroindol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic
acid (2-diethylaminoethyl) amide, a novel tyrosine kinase inhibitor
targeting vascular endothelial and platelet-derived growth factor
receptor tyrosine kinase. J Med Chem. 46:1116–1119. 2003.
|
|
12
|
Polychronopoulos P, Magiatis P,
Skaltsounis AL, Myrianthopoulos V, Mikros E, Tarricone A, Musacchio
A, et al: Structural basis for the synthesis of indirubins as
potent and selective inhibitors of glycogen synthase kinase-3 and
cyclin-dependent kinases. J Med Chem. 47:935–946. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krishnegowda G, Prakasha Gowda AS, Tagaram
HR, Carroll KF, Irby RB, Sharma AK and Amin S: Synthesis and
biological evaluation of a novel class of isatin analogs as dual
inhibitors of tubulin polymerization and Akt pathway. Bioorg Med
Chem. 19:6006–6014. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang C and Youle RJ: The role of
mitochondria in apoptosis. Annu Rev Genet. 43:95–118. 2009.
View Article : Google Scholar
|
|
15
|
Volkmann N, Marassi FM, Newmeyer DD and
Hanein D: The rheostat in the membrane: BCL-2 family proteins and
apoptosis. Cell Death Differ. 21:206–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: roles in cell survival and oncogenesis. Oncogene.
24:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cottet-Rousselle C, Ronot X, Leverve X and
Mayol JF: Cytometric assessment of mitochondria using fluorescent
probes. Cytometry A. 79:405–425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pakravan P, Kashanian S, Khodaei MM and
Harding FJ: Biochemical and pharmacological characterization of
isatin and its derivatives: from structure to activity. Pharmacol
Rep. 65:313–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Premanathan M, Radhakrishnan S,
Kulangiappar K, Singaravelu G, Thirumalaiarasu V, Sivakumar T and
Kathiresan K: Antioxidant & anticancer activities of isatin
(1H-indole-2,3-dione), isolated from the flowers of Couroupita
guianensis Aubl. Indian J Med Res. 136:822–826. 2012.
|
|
20
|
de Cândido-Bacani PM, Mori MP, Calvo TR,
Vilegas W, Varanda EA and Cólus IM: In vitro assessment of the
cytotoxic, apoptotic, and mutagenic potentials of isatin. J Toxicol
Environ Health A. 76:354–362. 2013.PubMed/NCBI
|
|
21
|
Ashkenazi A and Dixit VM: Death receptors:
signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reed JC: Double identity for proteins of
the Bcl-2 family. Nature. 387:773–776. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bedikian AY, Millward M, Pehamberger H,
Conry R, Gore M, Trefzer U, Pavlick AC, et al: Bcl-2 antisense
(oblimersen sodium) plus dacarbazine in patients with advanced
melanoma: the oblimersen melanoma study group. J Clin Oncol.
24:4738–4745. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Indran IR, Tufo G, Pervaiz S and Brenner
C: Recent advances in apoptosis, mitochondria and drug resistance
in cancer cells. Biochim Biophys Acta. 1807:735–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuranaga E: Caspase signaling in animal
development. Dev Growth Differ. 53:137–148. 2011. View Article : Google Scholar
|
|
27
|
Yoshida A, Pommier Y and Ueda T:
Endonuclease activation and chromosomal DNA fragmentation during
apoptosis in leukemia cells. Int J Hematol. 84:31–37. 2006.
View Article : Google Scholar : PubMed/NCBI
|