Introduction
Gastric cancer (GC) is the fourth most common type
of cancer worldwide (1). Following
lung cancer, GC is also the second leading cause of cancer-related
mortality in Asia. Most GC patients who undergo surgical resection
and postoperative adjuvant therapy die due to tumor recurrence and
metastasis, with a 5-year overall survival of no more than 50% in
China (2). Chemoresistance and
ineffectiveness of radiotherapy are leading causes of therapy
failure in GC patients. Hence, elucidating the mechanism of
chemoresistance will further enable us to choose appropriate
chemotherapeutic drugs to treat GC, thereby improving the survival
of GC patients.
CD133 is a transmembrane glycoprotein and its
expression in cell surface downregulates quickly as cell
differentiated (3). CD133 has been
used widely as a marker to identify cancer stem cells (CSCs) in
colon, lung, brain and pancreas (4–7).
Furthermore, CD133 expression is correlated with chemoresistance
and early recurrence of GC (8).
Earlier studies on CD133+ cancer cells displayed
resistance to many chemotherapeutic agents such as paclitaxel
(9), etoposide, 5-fluorouracil
(5-FU) and cisplatin (5,10). Furthermore, our previous study
(11) showed that CD133+
GC cells were resistant to 5-FU. Although it has been reported that
CD133 plays a key role in chemoresistance, its cellular mechanisms
remain unclear. Thus, we performed further research to elucidate
these mechanisms.
A number of cellular mechanisms that contribute to
chemoresistance include upregulation of the multidrug-resistance
(MDR) gene product and B-cell lymphoma 2 (Bcl-2) protein and its
family members. Bcl-2 primarily mediates its pro-survival effects
by binding to the pro-apoptotic proteins Bcl-2-associated X protein
(Bax) and inhibiting its ability to release apoptogenic proteins
such as cytochrome c from the mitochondria (12). The overexpression of P-glycoprotein
(P-gp) has been most extensively studied in MDR. Preliminary
studies have attributed the high expression levels of specific
adenosine triphosphate-binding cassette drug transporters to the
increased resistance of CD133+ cancer cells to
chemotherapeutic agents (13,14).
In addition, Ma et al demonstrated that CD133+
CSCs appear to express higher levels of Bcl-2 than their
CD133− counterparts in the human hepatocellular cancer
cell line Huh7 (15).
Akt, a serine/threonine kinase, is a key molecule in
protecting cells from apoptosis, and the Akt-mediated survival
signaling pathway is an attractive target for cancer chemotherapy
(16,17). The expression of Akt is altered in
various types of human tumor and this aberrant expression may
contribute to chemoresistance (18–20).
Akt-mediated chemoresistance is likely to result from overall
anti-apoptotic activity of Akt and activation of the
phosphoinositide 3-kinase (PI3K) signaling cascade, which leads to
MDR.
Increasing evidence strongly suggests the functional
association of CD133+ CSC with Akt signaling.
CD133+ tumor cells derived from hepatoma, colon cancer
and neuroblastoma consistently displayed increased phospho-Akt
(p-Akt) levels compared with matched CD133− tumor cells
(15,21,22).
However, very few similar studies were reported for GC cells. Based
on the coincidental finding of CD133+ cancer cells being
resistant to chemotherapeutic induced apoptosis, the present study
investigated whether CD133 expression serves a functional role in
triggering MDR in GC cells. To investigate this potential
relationship, low-expressing CD133 cell lines (SGC7901 cells) were
employed (23). To overcome such
chemoresistance, it is necessary to define the CD133-dependent
molecular pathway and elucidate the optimal blocking strategy.
Therefore, identification of suitable biomarkers for predicting
patient prognosis and chemosensitivity is important for improving
the therapeutic effects for patients with advanced GC.
The present study investigated the role of CD133 in
the expression of P-gp and Bcl-2 and their family-mediated
PI3K/Akt/p70S6K pathway inducible chemoresistance and to overcome
this resistance by small interfering ribonucleic acid (siRNA)
and/or 5-FU/LY294002 combination treatment.
Materials and methods
Chemicals
LY294002, epidermal growth factor (EGF) and 5-FU
were purchased from Sigma (St. Louis, MO, USA).
Cell lines and cultures
Human GC cell lines SGC7901 and MKN45 were provided
by the Shanghai Institute of Cell Biology, CAS (Shanghai, China).
Cells were maintained in RPMI-1640 culture medium supplemented with
100 g/ml streptomycin, 100 U/ml penicillin and 10% fetal bovine
serum (both from HyClone, USA) at 37°C in a humidified atmosphere
containing 5% carbon dioxide.
Immunomagnetic cell sorting
The cells were subcultured every 2–3 days. The third
to fifth subcultures were harvested, and CD133+ GC cells
were isolated utilizing a CD133 immunomagnetic cell sorting kit
(Miltenyi Biotec, Bergisch Gladbach, Germany). The
CD133+ cells were cultured in serum-free 1640 medium at
37°C in a humidified atmosphere containing 5% carbon dioxide
(23,24).
Liposome-mediated siRNA silencing of
CD133
CD133-specific siRNA fragments were designed and
synthesized from the CD133 gene sequence (Shanghai GenePharma,
Shanghai, China); sense strand, 5′-GUCCUUCCUAUAGAACAAUTT-3′ and
antisense strand, 5′-AUUGUUCUAUAGGAAGGACTT-3′. A non-specific siRNA
sequence was synthesized as a negative control; sense strand,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense strand,
5′-ACGUGACACGUUCGGAGAATT-3′. Unsorted SGC7901 cell concentration
was adjusted to 1.5×105 cells/ml and spread on three
6-well plates (2 ml/well) (control, negative control group; and
CD133, siRNA group), and cultured overnight. Transfection solution
A was prepared as follows: the siRNAs were dissolved in deionized
water at 20 μmol/l and mixed with RPMI-1640 at a ratio of 10:250
μl/well. Transfection solution B was prepared by mixing
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) and RPMI-1640 at
a ratio of 5:250 μl/well. After 5 min, the two transfection
mixtures were mixed together and allowed to stand for 20 min. This
transfection solution of CD133 siRNA was added to the corresponding
wells. RPMI-1640 (500 μl/well) was added to the uninterfered group
as a control. After 24-h of transfection, the solution was
exchanged with serum-containing RPMI-1640.
Stable transfection of CD133
Cells were cultured up to a 60–80% confluence state.
The CD133 complementary deoxyribonucleic acid (cDNA) plasmid was
extracted with plasmid extraction pail (Qiagen, Düsseldorf,
Germany) and transfected using Lipofectamine® LTX
reagent (Invitrogen, Tokyo, Japan) in accordance with the
manufacturer’s protocol. Following transfection, cells were
cultured for 72 h and intermediate samples were collected at 24 and
48 h for further analysis.
Western blotting
Quantified protein lysates were resolved on sodium
dodecyl sulfate-polyacrylamide gel electrophoresis, transferred
onto polyvinylidene difluoride membrane (Millipore, Billerica, MA,
USA), and immunoblotted with mouse anti-human CD133/1 (1:100;
Miltenyi Biotec), P-gp (1:500; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), rabbit anti-human P-Akt (Ser473, 1:1,000),
Akt (1:1,000), Bcl-2 (1:1,000), Bax (1:1,000) and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:2,000) (Cell
Signaling Technology Inc., Boston, MA, USA). After immunoblotting,
lysates were incubated with horseradish peroxidase-labeled goat or
mouse anti-rabbit immunoglobulin G secondary antibody (1:2,000;
Jackson, Mukilteo, WA, USA) at room temperature. Blots were
visualized using enhanced chemiluminescence (Amersham Biosciences
Inc., Piscataway, NJ, USA).
Semi-quantitative reverse
transcriptase-polymerase chain reaction (sqRT-PCR)
Total ribonucleic acid (RNA) was extracted using
TRIzol reagent (Invitrogen). The extracted RNA (500 ng) was
reverse-transcribed into cDNA using a commercial kit (Takara Bio
Inc., Otsu, Shiga, Japan) under the following conditions: 42°C for
30 min, 99°C for 5 min and 5°C for 5 min. The cDNA was used as a
template for PCR amplification with forward and reverse primers
(Shanghai Sangon, Shanghai, China), as shown in Table I. The primer annealing temperatures
were: CD133 at 57°C, multidrug resistance protein 1 at 72°C, Bcl-2
at 72°C and Bax at 72°C. GAPDH was used as an internal control for
the PCR reaction (Shanghai Sangon) and the annealing temperature
was 55°C. The final products of RT-PCR amplification were checked
by agarose gel electrophoresis (Bio-Rad, Hercules, CA, USA). The
results were photographed on a gel imaging system (Bio-Rad) and the
relative gray value of each DNA band was estimated. Each
measurement was repeated thrice.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Primer sequence
(5′-3′) | Length (bp) |
|---|
| CD133 | F:
TTACGGCACTCTCACCT
R: TATTCCACAAGCAGCAAA | 172 |
| MDR1 | F:
GCTTATGCGAAAGCTGGAGCAGTT
R: TGGCCGTGATGGCTTTCTTTATGC | 151 |
| Bcl-2 | F:
TTGGATCAGGGAGTT
R: TGTCCCTACCAACCAGAAGG | 295 |
| Bax | F:
GTTGCCCTCTTCTACTTTG
R: AGCCACCCTGGTCTTG | 142 |
| GAPDH | F:
ACGGATTTGGTCGTATTGGGCG
R: CTCCTGGAAGATGGTGATGG | 197 |
Cell proliferation and cytotoxicity
assay
The Cell Counting Kit-8 (CCK-8) assay was used to
assess the cell viability. Cells were seeded onto 96-well plates at
a concentration of 8×103/well and incubated overnight
under the usual culture conditions. Cells were exposed to each of
5-FU at various concentrations (0, 0.1, 1, 10, 100 and 1,000 μM;
dissolved in dimethyl sulfoxide). After 10 μl of CCK-8 solution was
added in each well, the plates were incubated for 1 h at 37°C. The
absorbance of individual wells was read at 490 nm (test wavelength)
and 530 nm (reference wavelength) using a microplate reader
(Bio-Rad Laboratories Inc.). The sensitivity of tumor cells to 5-FU
(5-FU treatment alone or in combination with LY294002) was
determined by estimating the IC50 values (doses that
induce 50% growth inhibition) for 5-FU from the dose-response
curves. Cell growth was examined using a CCK-8 kit (Cayman, Ann
Arbor, Michigan, USA). Cell growth inhibition (%) was calculated as
follows: [1-optical density (OD) values of 5-FU+/OD values of
5-FU-] × 100.
Hoechst 33258 staining
A staining solution of Hoechst 33258 was prepared
immediately before use. After drug treatment, cells were incubated
with Hoechst 33258 on 6-well plates (1 ml/well) for 20–30 min and
washed thrice with phosphate-buffered saline. Cells were then
assessed for Hoechst fluorescence using Nikon Intensilight C-HGF1
fluorescent microscope (Nikon, Tokyo, Japan) (magnification,
×200).
Statistical analysis
Statistical analyses were performed using SPSS
version 13.0 software (Chicago, IL, USA). The results are expressed
as means and standard deviations (± SD). Comparisons between groups
were performed using one-way analysis of variance (ANOVA). Values
of P<0.05 were considered to indicate a statistically
significant difference.
Results
CD133+ GC cells confer
chemoresistance to 5-FU
Investigation of the correlation between CD133 and
chemoresistance to 5-FU in GC cells was performed. First,
CD133+ and CD133− GC cell lines (SGC7901 and
MKN45 GC cell lines) were treated with 5-FU (0–1,000 μM).
CD133+ GC cells were found to be significantly resistant
to 5-FU compared to autologous unsorted and CD133− GC
cells (Fig. 1A). The
IC50 values of CD133+, unsorted and
CD133− cells were: 37.74±1.32 to 26.39±2.04, 15.80±0.14
to 11.25±1.37 and 5.46±0.98 to 3.05±0.32 μM, respectively. Then,
the presence of apoptosis was confirmed by Hoechst 33258 staining
(Fig. 1B and C), which showed less
frequent peripheral chromatin condensation and nuclear
fragmentation in CD133+ GC cells than unsorted and
CD133− GC cells. Western blotting (Fig. 1D) and RT-PCR (Fig. 1E) were performed to detect the
expression of P-gp, Bcl-2 and Bax in GC cells. The results showed
that CD133+ GC cells had a tendency to express much
higher amounts of P-gp and Bcl-2 than unsorted and
CD133− cells, but lower expression of Bax. These data
suggest that CD133 may contribute to the observed resistance to
apoptosis of CD133+ GC cells. Notably, overexpression of
P-gp and Bcl-2 may protect CD133+ GC cells from
apoptosis induced by 5-FU.
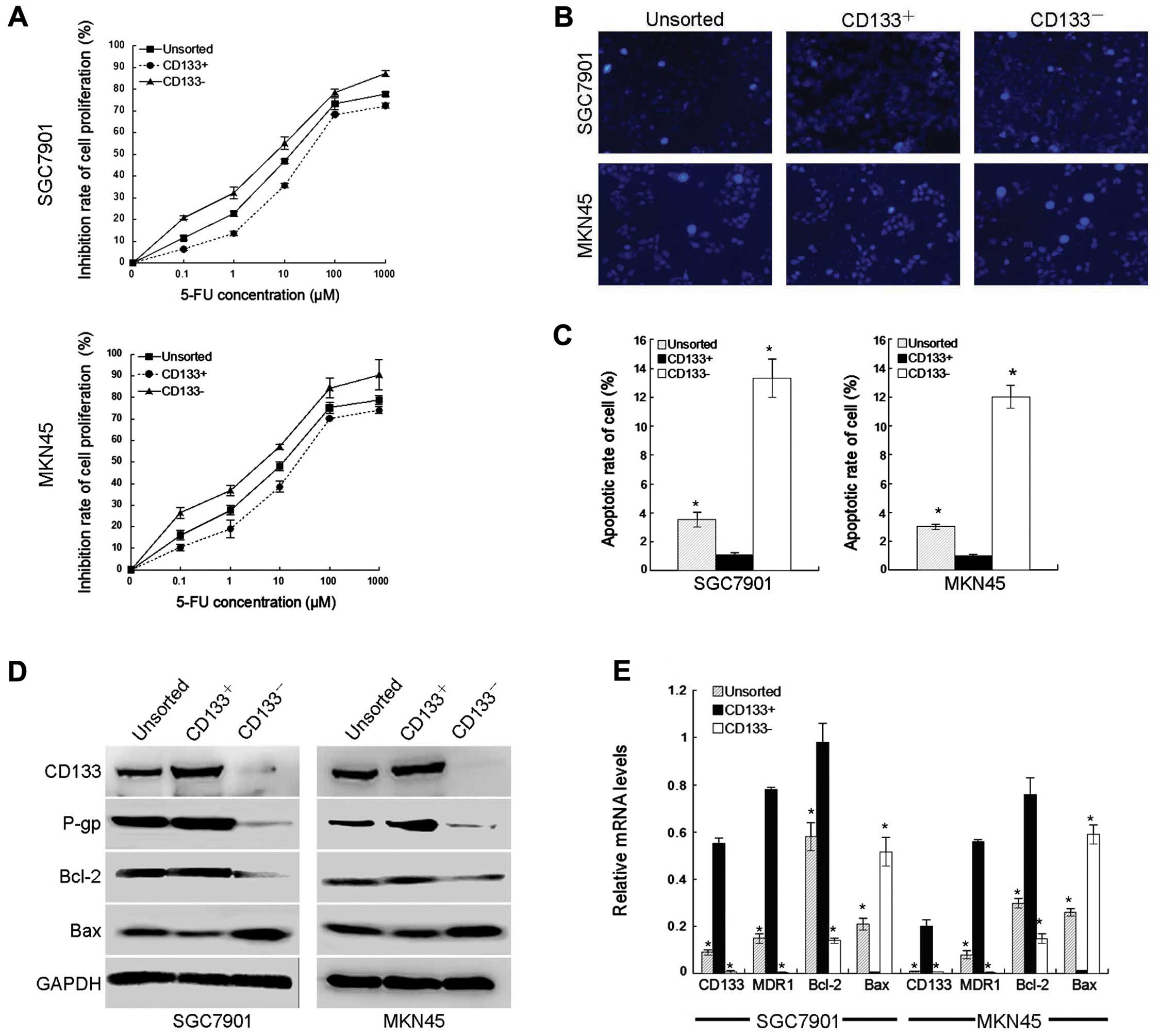 | Figure 1CD133+ cells confer
resistance to 5-fluorouracil (5-FU) in gastric cancer (GC) cells.
(A) SGC7901 and MKN45 cells cultured in 6-cm plates were treated
with various concentrations of 5-FU (0, 1, 10, 100 or 1,000 μM) for
48 h. Cell Counting Kit-8 was used to analyze the inhibition of
cell proliferation. Values represent means ± standard deviation. (B
and C) Hoechst 33258 staining and fluorescence microscopy showed
morphological change in GC cells (magnification, ×200). (D and E)
Protein and messenger ribonucleic acid (mRNA) expression of CD133,
P-glycoprotein, B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X
proteins were determined by western blotting (D), and
semi-quantitative reverse transcription-polymerase chain reaction
(E), respectively. Glyceraldehyde 3-phosphate dehydrogenase protein
and mRNA were used as internal controls. Control, untreated
control. *P<0.05 vs. unsorted or CD133−
cells. |
Downregulation of CD133 gene in SGC-7901
GC cells protects cells from apoptosis resulting from 5-FU
To further investigate the importance of CD133, gene
silencing by RNA interference was performed. As shown in Fig. 2A, the expression of CD133 in GC
cells was successfully knocked down by transfecting with CD133
siRNA. In addition, P-gp and Bcl-2 were decreased in CD133
siRNA-expressing cells compared to control siRNA-expressing cells,
while the expression of Bax was increased (Fig. 2A and B). Furthermore, it was found
that CD133 silencing increased the cytotoxicity of 5-FU in GC cells
compared to cells without CD133 silencing (Fig. 2C). The IC50 values of
control, negative control and siRNA CD133 were: 16.65±4.54,
16.94±4.55 and 9.66±2.01 μM, respectively. The presence of
apoptosis was confirmed by Hoechst 33258 staining (Fig. 2D and E), which showed more frequent
peripheral chromatin condensation and nuclear fragmentation in
CD133 siRNA-expressing cells than in control siRNA cells. Taken
together, these results indicate that downregulation of CD133 may
enhance the cytotoxicity of 5-FU in GC cells by regulating the
expression of P-gp and Bcl-2 family.
Activation of CD133 promotes resistance
to apoptosis resulting from 5-FU
To confirm the role of CD133 in 5-FU resistance in
GC cells, CD133 was activated by transfecting the CD133 gene into
SGC7901 cancer cells (Fig. 3A).
Western blotting (Fig. 3B and C)
showed that CD133 expression was stably increased in
CD133-expressing cells compared with vector control cells.
Moreover, P-gp and Bcl-2 were increased in CD133-expressing cells
compared to vector control cells, while the expression of Bax was
decreased (Fig. 3D). In addition,
the IC50 values of control, vector control and
Flag-CD133 were: 8.69±1.03, 8.87±1.20 and 26.03±3.18 μM,
respectively after treatment with 5-FU for 48 h (Fig. 3E), which indicated that CD133
overexpression increased resistance to 5-FU. The Hoechst 33258
staining (Fig. 3F and G) showed
less frequent peripheral chromatin condensation and nuclear
fragmentation in CD133-expressing cells than vector control cells.
These data demonstrate that CD133 protects GC cells from
5-FU-induced cell death.
CD133 activation enhances PI3K/Akt/p70S6K
activity
Recently, it was reported that Akt overexpression
decreases the chemosensitivity of GC cells to 5-FU in vitro
(26). Although CD133 is a
downstream substrate of Akt, CD133 was shown to enhance Akt
phosphorylation in several types of cancer cells (16,22,23,27).
Thus, the present study investigated whether CD133-induced 5-FU
resistance is attributed to Akt/p70S6K activation in GC cells. As a
result, it was found that although levels of total Akt proteins
were similar between CD133+ and CD133− tumor
cells, the phosphorylation of Akt on S473 and p70S6K was markedly
upregulated in CD133+ GC cells compared with matched
CD133− GC cells (Fig. 4A and
B). Thus, these findings suggest that elevated Akt activity may
be a distinctive feature of CD133+ GC cells.
Furthermore, western blotting (Fig. 4C
and D) showed that CD133 activation and silencing directly
increased and decreased the expressions of p-Akt and p-p70S6K, the
active form of Akt and p70S6K, respectively.
CD133 enhances 5-FU resistance through
PI3K/Akt/p70S6K activity
To confirm whether the positive correlation between
CD133 and 5-FU resistance is mediated by Akt, the present study
assessed the effects of LY294002 and EGF treatment on 5-FU-induced
cytotoxicity. Western blotting (Fig.
5A–C) showed that LY294002 treatment for 48 h effectively
blocked p-Akt and p-p70S6K expression in CD133-expressing cells,
while EGF-treatment for 48 h reversed the effect of CD133 siRNA on
these two phosphorylated proteins. Moreover, the changes of P-gp
and Bcl-2 were in accordance with the phosphorylated levels of Akt
and p70S6K. In contrast, the expression of Bax changed in the
opposite direction (Fig. 5A). In
addition, CD133-expressing cells treated with LY294002 showed
higher 5-FU cytotoxicity, while EGF reversed the effect of CD133
siRNA on 5-FU cytotoxicity (Fig.
5D). In addition, the IC50 values of groups 1–4
were: 27.3±3.18, 18.01±0.18, 8.63±1.22 and 24.30±8.08 μM,
respectively. Notably, Hoechst 33258 staining (Fig. 5E and F) illustrated that treatment
with LY294002 or transfection with CD133-siRNA caused more
apoptosis in 5-FU-treated CD133-expressing cells, while EGF partly
reversed this change. Collectively, the above results indicate that
CD133 enhances 5-FU resistance through regulation of P-gp and Bcl-2
family mediated by the PI3K/Akt/p70S6K pathway.
 | Figure 5Effects of phosphoinositide 3-kinase
(PI3K) inhibitor (LY294002) and PI3K activator [epidermal growth
factor (EGF)] on phospho-Akt (p-Akt) and phospho-p70S6 kinase
(p-p70S6K) expression and 5-fluorouracil resistance. (A–C) CD133
cells treated with either 10 μM LY294002 for 48 h
(CD133+) or 0.1 ng/ml EGF for 48 h (CD133−).
Western blotting was performed with p-Akt, Akt, p70S6K, p-p70S6K,
P-glycoprotein, B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X
protein antibodies. (D) Cells were cultured in the presence or
absence of 20 μM LY294002 or 0.1 ng/ml EGF for 48 h, and then
treated with various concentrations of 5-FU (0, 1, 10, 100 or 1,000
μM) for 48 h. Cell Counting Kit-8 was used to analyze the
inhibition of cell proliferation. Values represent means ± standard
deviation. (E and F) Hoechst 33258 staining and fluorescence
microscopy show morphological changes after the above treatments in
gastric cancer cells (magnification, ×200). Group 1, Flag-CD133;
group 2, Flag-CD133+ LY294002; group 3,
Flag-CD133+ CD133 small interfering ribonucleic acid
(siRNA); and group 4, Flag-CD133+ CD133 siRNA+EGF.
*P<0.05 group 2 vs. group 1; group 4 vs. group 3. |
Discussion
Increasing evidence has shown that CD133 is not only
a biomarker, but it also functions in cell growth, development and
tumor biology. CD133 has been reported to be related to
chemoresistance in various cancer cells (27–29).
Although occurrence of resistance to chemotherapy of GC is rather
frequent, involvement of CD133 in the chemoresistance of GC is
rarely reported. The present study demonstrated the correlation
between CD133 and chemoresistance in GC cells. Furthermore, this
resistance may be related to CD133 with higher expression of P-gp
as well as Bcl-2 and lower expression of Bax mediated by
PI3K/Akt/p70S6K signaling.
In the present study, it was found that
CD133+ GC cells were significantly resistant to 5-FU
compared to autologous unsorted and CD133− GC cells,
which was in accordance with Ma et al (15). There was also a significant
difference in expression of P-gp, Bcl-2 and Bax between
CD133+ and CD133− GC cells, which may explain
the above phenomenon.
Gene modulation is a powerful method for analyzing
gene function. In the present study, the function of CD133 in 5-FU
resistance was confirmed in two GC cells by modulation of CD133
activation using two different approaches (expression of CD133 gene
and CD133-siRNA). It was found that knockdown of the expression of
CD133 caused corresponding changes in expression of P-gp, Bcl-2 and
Bax. As a result, CD133 silencing increased cytotoxicity of 5-FU in
GC cells compared to cells without CD133 silencing. In addition,
activation of CD133 increased the 5-FU resistance in GC cells along
with higher expression of P-gp and Bcl-2, which was consistent with
Angelastro and Lamé (29).
Collectively, these observations clearly demonstrate that CD133 is
protective of 5-FU-induced cytotoxicity in GC cells and blockage of
CD133 could be an effective approach to improve anticancer efficacy
of 5-FU in GC patients.
It is generally accepted that Akt is a critical
survival signal involved in cancer development and progression as
well as chemoresistance (30).
Although cancer cells acquire resistance to anticancer agents
through Akt, either constitutive or induced by anticancer drugs,
the molecular mechanisms underlying anticancer drug-induced Akt
activation have yet to be fully elucidated. p-Akt is overexpressed
in GC specimens (31). A previous
study showed that Akt overexpression decreased the chemosensitivity
of GC cells to 5-FU in vitro (25). Hence, the present study investigated
the involvement of Akt in the CD133 regulation of 5-FU cytotoxicity
in GC cells. In the present study, the phosphorylations of Akt on
S473 and p70S6K was markedly upregulated in CD133+ GC
cells compared with matched CD133− GC cells.
Constitutive activation and silencing of CD133 increased and
decreased the p-Akt and p-p70S6K expression in GC cells,
respectively, suggesting that CD133 activates PI3K/Akt/p70S6K
signaling. In addition, it was found that treatment of CD133
gene-expressing GC cells with the PI3K/Akt inhibitor LY294002
restored the 5-FU cytotoxicity suppressed by CD133 overexpression.
Notably, PI3K/Akt activator EGF inhibited the 5-FU cytotoxicity
enhanced by CD133 downregulation. Hence, CD133 and Akt appear to
have similar effects on 5-FU chemoresistance in GC cells. The
present study results agree with those of Wang et al
(22), who showed CD133-induced
PI3K/Akt activation.
In conclusion, the results of the present study
suggest that concurrent blocking of CD133 and PI3K/Akt/p70S6K
pathways is an effective strategy for improving the anticancer
efficacy of 5-FU. Our results provide important insight into the
efficient 5-FU-design for future studies on GC treatment. Studies
on the association between CD133 and chemosensitivity in GC cells
using human GC specimens with animal models are warranted to verify
the usefulness of this strategy.
Acknowledgements
The present study was supported by funds from the
National Natural Science Foundation of China (grant no. 81101850)
and the Shanghai City Board of Education (grant no. 12YZ047).
References
|
1
|
Bertuccio P, Chatenoud L, Levi F, Praud D,
Ferlay J, Negri E, Malvezzi M and La Vecchia C: Recent patterns in
gastric cancer: a global overview. Int J Cancer. 125:666–673. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ji JF: Several surgical problems in the
treatment of gastric cancer. J Pract Oncol. 18:341–346. 2003.
|
|
3
|
Peichev M, Naiyer AJ, Pereira D, Zhu Z,
Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA and Rafii
S: Expression of VEGFR-2 and AC133 by circulating human
CD34+ cells identifies a population of functional
endothelial precursors. Blood. 95:952–958. 2000.PubMed/NCBI
|
|
4
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
5
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
7
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee HH, Seo KJ, An CH, Kim JS and Jeon HM:
CD133 expression is correlated with chemoresistance and early
recurrence of gastric cancer. J Surg Oncol. 106:999–1004. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu G, Yuan X, Zeng Z, Tunici P, Ng H,
Abdulkadir IR, Lu L, Irvin D, Black KL and Yu JS: Analysis of gene
expression and chemoresistance of CD133+ cancer stem
cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baba T, Convery PA, Matsumura N, Whitaker
RS, Kondoh E, Perry T, Huang Z, Bentley RC, Mori S, Fujii S, Marks
JR, Berchuck A and Murphy SK: Epigenetic regulation of CD133
and tumorigenicity of CD133+ ovarian cancer cells.
Oncogene. 28:209–218. 2009.
|
|
11
|
Zhu Y1, Jiang B, Cai C, Wang S, Wu J and
Yu J: Relationship between CD133 and chemoresistance in human
gastric cancer and its associated mechanism. Zhonghua Wei Chang Wai
Ke Za Zhi. 17:168–174. 2014.(In Chinese).
|
|
12
|
Kim R, Emi M and Tanabe K: Role of
mitochondria as the gardens of cell death. Cancer Chemother
Pharmacol. 57:545–553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dean M, Fojo T and Bates S: Tumor stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
14
|
Haraguchi N, Utsunomiya T, Inoue H, Tanaka
F, Mimori K, Barnard GF and Mori M: Characterization of a side
population of cancer cells from human gastrointestinal system. Stem
Cells. 24:506–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma S, Lee TK, Zheng BJ, Chan KW and Guan
XY: CD133+ HCC cancer stem cells confer chemoresistance
by preferential expression of the Akt/PKB survival pathway.
Oncogene. 27:1749–1758. 2008.
|
|
16
|
Cardone MH, Roy N, Stennicke HR, Salvesen
GS, Franke TF, Stanbridge E, Frisch S and Reed JC: Regulation of
cell death protease caspase-9 by phosphorylation. Science.
282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo
P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME: AKT
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Curnock AP, Logan MK and Ward SG:
Chemokine signalling: pivoting around multiple phosphoinositide
3-kinases. Immunology. 105:125–136. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-κB. J Clin
Inves. 107:241–246. 2001.
|
|
20
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR,
Pfeffer LM and Donner DB: NF-κB activation by tumour necrosis
factor requires the AKT serine-threonine kinase. Nature. 401:82–85.
1999.
|
|
21
|
Sartelet H, Imbriglio T, Nyalendo C,
Haddad E, Annabi B, Duval M, Fetni R, Victor K, Alexendrov L,
Sinnett D, Fabre M and Vassal G: CD133 expression is associated
with poor outcome in neuroblastoma via chemoresistance mediated by
the AKT pathway. Histopathology. 60:1144–1155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang YK, Zhu YL, Qiu FM, Zhang T, Chen ZG,
Zheng S and Huang J: Activation of Akt and MAPK pathways enhances
the tumorigenicity of CD133+ primary colon cancer cells.
Carcinogenesis. 31:1376–1380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu RQ, Wu JG, Zhou GC, Jiang HG, Yu JW and
Jiang BJ: Sorting of CD133(+) subset cells in human gastric cancer
and the identification of their tumor initiating cell-like
properties. Zhonghua Wei Chang Wai Ke Za Zhi. 15:174–179. 2012.(In
Chinese).
|
|
24
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shin JY, Kim JO, Lee SK, Chae HS and Kang
JH: LY294002 may overcome 5-FU resistance via down-regulation of
activated p-AKT in Epstein-Barr virus-positive gastric cancer
cells. BMC Cancer. 10:4252010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei Y, Jiang Y, Zou F, Liu Y, Wang S, Xu
N, Xu W, Cui C, Xing Y, Liu Y, Cao B, Liu C, Wu G, Ao H, Zhang X
and Jiang J: Activation of PI3K/Akt pathway by CD133-p85
interaction promotes tumorigenic capacity of glioma stem cells.
Proc Natl Acad Sci USA. 110:6829–6834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Damdinsuren B, Nagano H, Kondo M, Natsag
J, Hanada H, Nakamura M, Wada H, Kato H, Marubashi S, Miyamoto A,
Takeda Y, Umeshita K, Dono K and Monden M: TGF-β1-induced cell
growth arrest and partial differentiation is related to the
suppression of Id1 in human hepatoma cells. Oncol Rep. 15:401–408.
2006.
|
|
28
|
Zhang Q, Shi S, Yen Y, Brown J, Ta JQ and
Le AD: A subpopulation of CD133+ cancer stem-like cells
characterized in human oral squamous cell carcinoma confer
resistance to chemotherapy. Cancer Lett. 289:151–160. 2010.
|
|
29
|
Angelastro JM and Lamé MW: Overexpression
of CD133 promotes drug resistance in C6 glioma cells. Mol Cancer
Res. 8:1105–1115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimamura H, Terada Y, Okado T, Tanaka H,
Inoshita S and Sasaki S: The PI3-kinase-Akt pathway promotes
mesangial cell survival and inhibits apoptosis in vitro via NF-κB
and Bad. J Am Soc Nephrol. 14:1427–1434. 2003.PubMed/NCBI
|
|
31
|
Bellacosa A, Kumar CC, Di Cristofano A and
Testa JR: Activation of AKT kinases in cancer: implications for
therapeutic targeting. Adv Cancer Res. 11:29–86. 2005. View Article : Google Scholar : PubMed/NCBI
|



















