Introduction
Rectal cancer (RC) is a major health issue and is
one of the leading causes of cancer-related death worldwide
(1). Neoadjuvant chemoradiotherapy
(NCRT) followed by surgical resection is the current standard
treatment for locally advanced rectal cancer (LARC). It offers
improved local control, reduced toxicity and higher rates of
sphincter preservation without compromising oncological outcome
compared with post-operative treatment (2,3). A
pathologic complete response (pCR) is one of the best predictive
markers of a favourable prognosis. However, approximately 15–30% of
patients experience a pCR, whereas the majority of patients have
some degree of residual disease after NCRT (4). Thus, if patients with tumours that are
responsive to NCRT could be identified at the time of diagnosis,
then NCRT could be administered in a more individualised
manner.
Recent studies have attempted to identify predictive
biomarkers such as Ki-67, p53, p21, p27, bax, BCL-2, vascular
endothelial growth factor (VEGF), epidermal growth factor receptor
(EGFR), survivin and thymidylate synthase (5,6).
However, clinical use of these biomarkers requires further
evaluation in prospective clinical trials (7).
Angiogenesis is necessary for tumour growth and
malignant progression, with VEGF being a key angiogenic factor.
High VEGF expression was found to be associated with poor survival
in colorectal cancers (8). In
particular, bevacizumab, a humanised monoclonal antibody inhibiting
VEGF-A, in combination with standard chemotherapy regimens was
beneficial both in terms of response rate and survival as first-and
second-line treatment of patients affected by metastatic colorectal
cancer. In patients affected by LARC who underwent radical surgery
and adjuvant chemoradiation, tumour VEGF overexpression was found
to be associated with a statistically higher risk of local
recurrence and metastasis (9). Not
only VEGF, but placental growth factor (PlGF) are potentially
useful predictive factors in rectal cancer (10).
Hypoxia is one of the key stimuli for the release of
angiogenic markers (AMs) necessary for angiogenesis and tumour
growth. Hypoxic tumours not only have a more aggressive nature
(11,12), but studies in cervical and head and
neck cancer demonstrated that tumour hypoxia decreases the response
to radiation treatment (13). The
effect of tumour hypoxia on the response to radiation therapy is
relevant to the management of rectal cancer.
Hypoxia-inducible factor 1α (HIF-1α) is a protein
involved in the cellular response to hypoxia centrally. It
activates a variety of downstream genes involved in anaerobic
metabolism and angiogenesis (14–16).
These downstream gene protein products, which include VEGF, stromal
cell-derived factor 1α (SDF-1α) and PlGF, promote cell survival
under hypoxic conditions. Among patients with colorectal cancer,
expression of these 4 AMs has been shown to correlate with rates of
lymph node or liver metastasis, disease-free survival, and overall
survival (17–20). However, the effects of pre-operative
treatment on expression of AMs in rectal cancer remain unclear. The
4 AMs chosen for this study include HIF-1α, VEGF, SDF-1α and
PlGF.
The aims of this exploratory study were to a)
characterise expression of AMs in LARC before NCRT, b) investigate
the change in AM expression after NCRT and c) evaluate the
relationship between AM expression and tumour response.
Patients and methods
Patients and neoadjuvant
chemoradiotherapy regimen
Between March 2005 and August 2009 at Soonchunhyang
University Hospital, a total of 55 patients with non-metastatic,
locally advanced (radiological T3–T4 or N+ and/or
clinically bulky) and biopsy-proven primary rectal cancer received
NCRT. The whole pelvic field received 25 fractions of 180 cGy/day
over 5 weeks, for a total of 4,500–5,040 cGy, using a four-field
box technique. Chemotherapy was administered intravenously and
consisted of 5-fluorouracil (5-FU; 425 mg/m2/day) and
leucovorin (20 mg/m2/day) during the first and fifth
weeks of radiotherapy. Surgical resection at 6–8 weeks was
performed following the completion of NCRT. All data were collected
and recorded prospectively, and the clinical and pathological
features were reviewed retrospectively. The patients were
classified according to the 6th edition of the American
Joint Committee on Cancer staging system (21). Surgical specimens were evaluated for
histopathologic staging as well as for pathologic response to NCRT.
The detailed characteristics of the patients are listed in Table I. Our study was approved by the
Clinical Ethics Review Committee of the Soonchunhyang University
Hospital, Cheonan, Republic of Korea. Clinical consent was obtained
from all patients.
 | Table IAssociation between AMs and
clinicopathological parameters. |
Table I
Association between AMs and
clinicopathological parameters.
| Clinicopathological
parameters | HIF-1α
expression | VEGF expression | PlGF expression | SDF-1α
expression |
|---|
|
|
|
|
|---|
| Negative | Positive | P-value | Negative | Positive | P-value | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Total patients, n
(%) | 29 (52.7) | 26 (47.3) | | 24 (43.6) | 31 (56.4) | | 19 (34.5) | 36 (65.5) | | 16 (29.1) | 39 (70.9) | |
| Age (years) | | | 0.153 | | | 0.877 | | | 0.957 | | | 0.466 |
| <65 | 21 | 14 | | 15 | 20 | | 12 | 23 | | 9 | 26 | |
| ≥65 | 8 | 12 | | 9 | 11 | | 7 | 13 | | 7 | 13 | |
| Gender | | | 0.385 | | | 0.876 | | | 0.733 | | | 1.000 |
| Male | 24 | 19 | | 19 | 24 | | 14 | 29 | | 13 | 30 | |
| Female | 5 | 7 | | 5 | 7 | | 5 | 7 | | 3 | 9 | |
| Pre-treatment
tumour stage | | | 0.418 | | | 0.505 | | | 1.000 | | | 0.712 |
| 3 | 22 | 22 | | 18 | 26 | | 15 | 29 | | 12 | 32 | |
| 4 | 7 | 4 | | 6 | 5 | | 4 | 7 | | 4 | 7 | |
| Pre-treatment nodal
stage | | | 0.220 | | | 0.078 | | | 0.570 | | | 0.388 |
| 0 | 8 | 3 | | 8 | 3 | | 5 | 6 | | 5 | 6 | |
| 1 | 9 | 13 | | 7 | 15 | | 6 | 16 | | 5 | 17 | |
| 2 | 12 | 10 | | 9 | 13 | | 8 | 14 | | 6 | 16 | |
| Post-treatment
tumour stage | | | 0.938 | | | 0.926 | | | 0.262 | | | 0.648 |
| 0 | 4 | 2 | | 3 | 3 | | 3 | 3 | | 2 | 4 | |
| 1 | 1 | 0 | | 0 | 1 | | 0 | 1 | | 0 | 1 | |
| 2 | 2 | 6 | | 3 | 5 | | 5 | 3 | | 1 | 7 | |
| 3 | 20 | 18 | | 17 | 21 | | 10 | 28 | | 12 | 26 | |
| 4 | 2 | 0 | | 1 | 1 | | 1 | 1 | | 1 | 1 | |
| Post-treatment
nodal stage | | | 0.138 | | | 0.281 | | | 0.489 | | | 0.277 |
| 0 | 23 | 15 | | 18 | 20 | | 15 | 23 | | 14 | 24 | |
| 1 | 4 | 8 | | 5 | 7 | | 2 | 10 | | 0 | 12 | |
| 2 | 2 | 3 | | 1 | 4 | | 2 | 3 | | 2 | 3 | |
Tissue microarray (TMA) construction
Areas representative of cancer were marked on
haematoxylin and eosin-stained slides and TMAs were constructed.
TMAs were created from formalin-fixed by 10% neutral buffered
formalin, paraffin-embedded tissues using a 2-mm-diameter punch
(Unitma; Unitech Science, Seoul, Korea). TMA blocks were assembled
by obtaining duplicate cores from one patient block and
re-embedding the two cores in an arrayed recipient block (Unitma;
Unitech Science). A TMA block contains 60 cores from 30
samples.
Tumour response
Clinical stage was performed by an independent
review conducted by a radiologist, and pathologic stage was
reviewed by two independent pathologists. Downstaging was defined
as staging reduction from pre-treatment stage (cStage) to
pathologic stage (ypStage) (i.e. cIII to ypII, ypI or yp0; cII to
ypI or yp0). Pathologic response (tumour regression) to NCRT was
semiquantitatively determined by the amount of viable tumour versus
the amount of fibrosis, ranging from no evidence of any NCRT effect
to a complete response with no viable tumour identified, as
described by Dworak et al (22). The following were characteristics of
each grade: grade 0, no regression; grade 1, minor regression
(dominant tumour mass with obvious fibrosis in 25% or less of the
tumour mass); grade 2, moderate regression (dominant tumour mass
with obvious fibrosis in 26–50% of the tumour mass); grade 3, good
regression (dominant fibrosis outgrowing the tumour mass; i.e.
>50% tumour regression); and grade 4, total regression (no
viable tumour cells, only fibrotic mass). Patients with tumour
regression grade (TRG) of 3 or 4 were considered as the responder
group in our study.
Immunohistochemical (IHC) staining
The TMAs were sectioned at 4-μm intervals,
deparaffinised three times in xylene for 30 min and rehydrated with
graded alcohols (100% ethyl alcohol for 5 min, 95% ethyl alcohol
for 3 min and 75% ethyl alcohol for 3 min) and then heated in
antigen retrieval solution (sodium citrate, pH 6.0) in a microwave
for 20 min. Sections were incubated in H2O2
for 10 min at room temperature. Furthermore, the sections were
incubated with 150 ml of the primary antibodies, VEGF (1:200;
Millipore, USA), PlGF (1:200; R&D system, USA), HIF-1α (1:50;
Proteintech, USA) and SDF-1α (1:100; Novus Biologicals, USA) at 4°C
overnight. Subsequently, the sections were washed in PBS buffer
three times for 3 min, treated with 150 ml secondary antibody for 1
h at room temperature and stained with DAB solution (Dako, USA).
The sections were then washed in PBS buffer for 10 min. Finally,
the sections were counterstained with hematoxylin for 3 min at room
temperature, washed in distilled water 3 times for 3 min and
mounted with coverslips.
IHC analysis
The VEGF, PlGF, HIF-1α and SDF-1α stained tissue
cores were examined by 2 independent pathologists and a consensus
score was determined for each specimen. A positive reaction for
both antibodies was scored into 4 grades, according to the
intensity of the staining: 0, 1+, 2+ and 3+. The percentages of
positive cells were also scored into 4 categories: 0 (0%), 1
(1–33%), 2 (34–66%), and 3 (67–100%). The final score, calculated
as the product of the intensity score multiplied by the percentage
score, was classified as follows: 0 for negative; 1–3 for weak; 4–6
for moderate; and 7–9 for strong.
Statistical analysis
The correlations between expression levels of
hypoxia-related proteins and pathologic response to NCRT were
evaluated by the χ2 or Fisher’s exact test. The
univariate and multivariate analyses between response to NCRT and
clinical or histopathologic parameters were performed by binary
logistic regression model. All P-values quoted were two-sided, and
P<0.05 was considered to indicate a statistically significant
difference. All the analyses were performed using SPSS v. 17.0
(SPSS, Inc., Chicago, IL, USA).
Results
Association between AM expression and
clinicopathological variables
The mean age of the 55 patients with LARC was 56
years (range, 18–82 years). In regards to gender, 43 (78.2%) were
male, and 12 (21.8%) were female. Regarding the stage of disease,
11 (20.0%) were at stage II, and 44 (80.0%) were at stage III.
Concerning the T stage, 44 (80.0%) were T3 and 11 (20.0%) were T4.
The number of negative lymph node metastases was 11 (20.0%); N1 was
22 (40.0%), and N2 was 22 (40.0%). A pCR was obtained in 9.1% cases
(5 patients). Patient characteristics are summarised in Table I. As shown in Table I, expression levels of AMs were not
statistically correlated to the clincopathological variables.
Change in AM expression in LARC before
NCRT and after surgery
The positive expression rate of HIF-1α, VEGF, PlGF
and SDF-1α was 47.3% (26/55), 56.4% (31/55), 65.5% (36/55) and
70.9% (39/55) before NCRT, respectively. Weak, moderate and strong
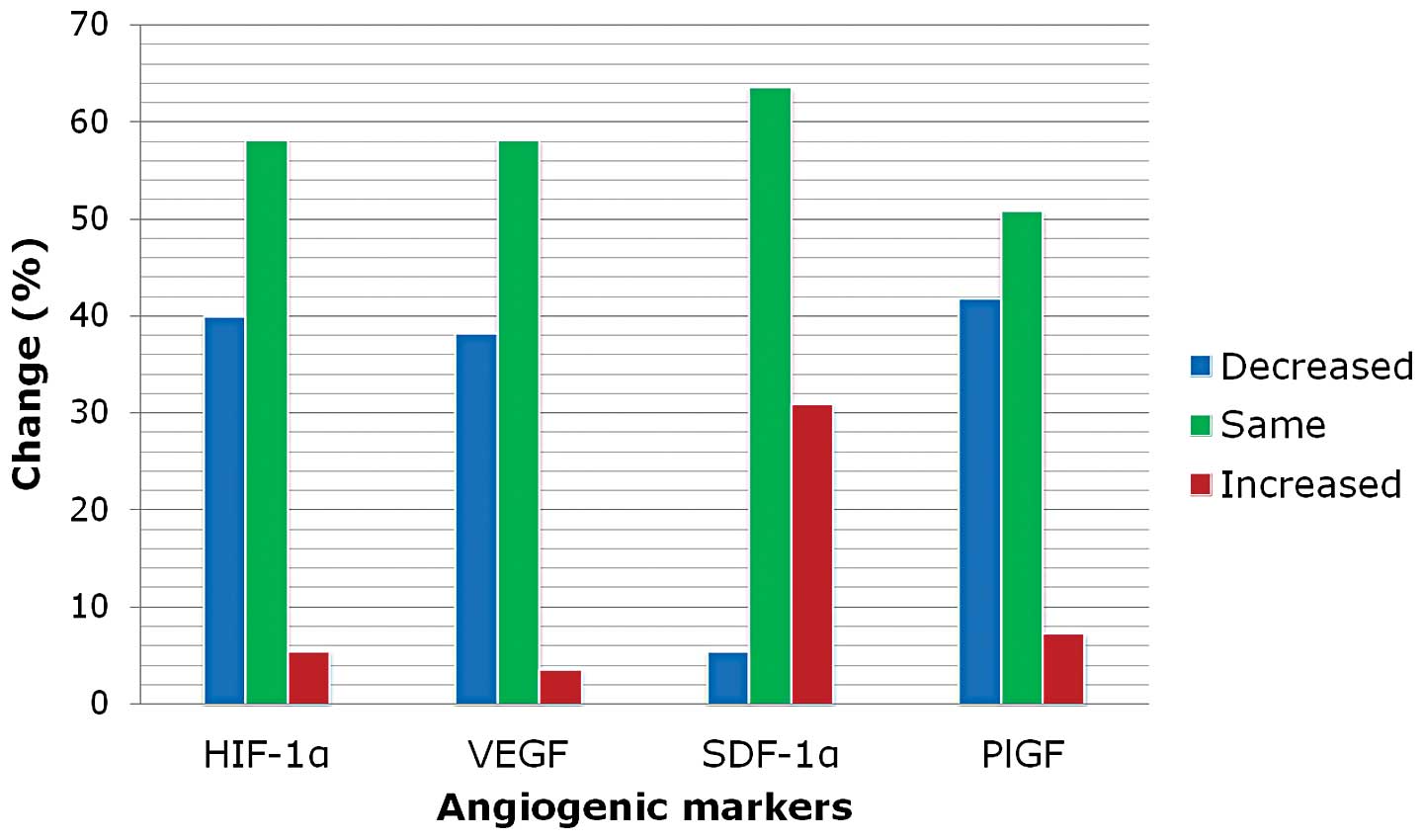
staining intensity of AMs is illustrated in Fig. 1. The expression rate of HIF-1α,
VEGF, SDF-1α and PlGF was increased by 1.8% (1/55), 3.6% (2/55),
30.9% (17/55) and 7.3% (4/55) after NCRT, respectively. Expression
of VEGF, PlGF and HIF-1α protein was downregulated after NCRT in
the rectal cancer tissues (P<0.001, P=0.001 and P=0.044,
respectively). However, SDF-1α was upregulated after NCRT
(P<0.001; Table II, Fig. 2).
 | Table IIResults of AM immunoreactivity before
NCRT and after surgery. |
Table II
Results of AM immunoreactivity before
NCRT and after surgery.
| HIF-1α | VEGF | SDF-1α | PlGF |
|---|
|
|
|
|
|
|---|
| Staining score | Before NCRT | After surgery | Before NCRT | After surgery | Before NCRT | After surgery | Before NCRT | After surgery |
|---|
| Negative | 29 | 48 | 24 | 39 | 16 | 10 | 19 | 30 |
| Weak | 17 | 7 | 16 | 12 | 17 | 12 | 19 | 19 |
| Moderate | 8 | 0 | 11 | 2 | 15 | 19 | 8 | 4 |
| Strong | 1 | 0 | 4 | 2 | 7 | 14 | 9 | 2 |
Relationship between tumour response to
NCRT and clinicopathological variables
Upregulated expression of SDF-1α (P<0.016) and
positive PlGF staining (P=0.001) after NCRT were significantly
associated with resistance to NCRT. However, other
clinicopathologic variables showed no correlation with tumour
response (Table III). In
multivariate analyses, positive PlGF staining after NCRT was found
to be associated with resistance to NCRT [P=0.013; OR=0.197, 95%
confidence interval (CI), 0.055–0.705]. Only low pre-treatment
tumour lymph node staging was associated with pCR (P=0.002;
Table IV).
 | Table IIIAssociation between tumour response
and clinicopathological parameters. |
Table III
Association between tumour response
and clinicopathological parameters.
| Clinicopathological
parameters | Tumour
response | P-value |
|---|
|
|---|
| R | NR |
|---|
| Age (years) | | | 0.877 |
| <65 | 20 | 15 | |
| ≥65 | 11 | 9 | |
| Gender | | | 0.416 |
| Male | 23 | 20 | |
| Female | 8 | 4 | |
| Pre-treatment
tumour stage | | | 0.180 |
| 3 | 27 | 17 | |
| 4 | 4 | 7 | |
| Pre-treatment nodal
stage | | | 0.517 |
| 0 | 4 | 7 | |
| 1 | 15 | 7 | |
| 2 | 12 | 10 | |
| Pre-treatment VEGF
staining | | | 0.773 |
| Negative | 13 | 11 | |
| Positive | 18 | 13 | |
| Pre-treatment PlGF
staining | | | 0.190 |
| Negative | 13 | 6 | |
| Positive | 18 | 18 | |
| Pre-treatment
SDF-1α staining | | | 0.235 |
| Negative | 11 | 5 | |
| Positive | 20 | 19 | |
| Pre-treatment
HIF-1α staining | | | 0.368 |
| Negative | 18 | 11 | |
| Positive | 13 | 13 | |
| Post-treatment VEGF
staining | | | 0.074 |
| Negative | 19 | 20 | |
| Positive | 12 | 4 | |
| Post-treatment PlGF
staining | | | 0.001 |
| Negative | 23 | 7 | |
| Positive | 8 | 17 | |
| Post-treatment
SDF-1α staining | | | 0.159 |
| Negative | 8 | 2 | |
| Positive | 23 | 22 | |
| Post-treatment
HIF-1α staining | | | 0.686 |
| Negative | 28 | 20 | |
| Positive | 3 | 4 | |
| Change of staining
status |
| VEGF | | | 0.400 |
| Decreased | 10 | 11 | |
| Same | 20 | 12 | |
| Increased | 1 | 1 | |
| PlGF | | | 0.568 |
| Decreased | 12 | 11 | |
| Same | 19 | 9 | |
| Increased | 0 | 4 | |
| SDF-1α | | | 0.016 |
| Decreased | 3 | 0 | |
| Same | 22 | 13 | |
| Increased | 6 | 11 | |
| HIF-1α | | | 0.343 |
| Decreased | 11 | 11 | |
| Same | 19 | 13 | |
| Increased | 1 | 0 | |
 | Table IVAssociation between pCR and
clinicopathological parameters. |
Table IV
Association between pCR and
clinicopathological parameters.
| Clinicopathological
parameters | pCR | P-value |
|---|
|
|---|
| (−) | (+) |
|---|
| Age (years) | | | 1.000 |
| <65 | 32 | 3 | |
| ≥65 | 18 | 2 | |
| Gender | | | 0.298 |
| Male | 40 | 3 | |
| Female | 10 | 2 | |
| Pre-treatment
tumour stage | | | 0.571 |
| 3 | 39 | 5 | |
| 4 | 11 | 0 | |
| Pre-treatment nodal
stage | | | 0.002 |
| 0 | 7 | 4 | |
| 1 | 21 | 1 | |
| 2 | 22 | 0 | |
| Pre-treatment VEGF
staining | | | 0.643 |
| Negative | 21 | 3 | |
| Positive | 29 | 2 | |
| Pre-treatment PlGF
staining | | | 0.327 |
| Negative | 16 | 3 | |
| Positive | 34 | 2 | |
| Pre-treatment
SDF-1α staining | | | 0.622 |
| Negative | 14 | 2 | |
| Positive | 36 | 3 | |
| Pre-treatment
HIF-1α staining | | | 0.355 |
| Negative | 25 | 4 | |
| Positive | 25 | 1 | |
| Post-treatment VEGF
staining | | | 1.000 |
| Negative | 35 | 4 | |
| Positive | 15 | 1 | |
| Post-treatment PlGF
staining | | | 0.056 |
| Negative | 25 | 5 | |
| Positive | 25 | 0 | |
| Post-treatment
SDF-1α staining | | | 0.220 |
| Negative | 8 | 2 | |
| Positive | 42 | 3 | |
| Post-treatment
HIF-1α staining | | | 1.000 |
| Negative | 43 | 5 | |
| Positive | 7 | 0 | |
| Change of staining
status |
| VEGF | | | 0.817 |
| Decreased | 19 | 2 | |
| Same | 29 | 3 | |
| Increased | 2 | 0 | |
| PlGF | | | 0.835 |
| Decreased | 21 | 2 | |
| Same | 25 | 3 | |
| Increased | 4 | 0 | |
| SDF-1α | | | 0.053 |
| Decreased | 2 | 1 | |
| Same | 31 | 4 | |
| Increased | 17 | 0 | |
| HIF-1α | | | 0.418 |
| Decreased | 21 | 1 | |
| Same | 28 | 4 | |
| Increased | 1 | 0 | |
Relationship with AM expression
Before NCRT, an association was identified between
HIF-1α expression and SDF-1α (P=0.034). HIF-1α was not correlated
with VEGF and PlGF. However, SDF-1α had an association with PlGF
(P=0.005). After surgery, HIF-1α expression was not correlated with
SDF-1α (P=0.621), and SDF-1α tended to be associated with PlGF
(P=0.052).
Discussion
Recently, studies have attempted to identify
predictive biomarkers, yet various studies only compared
pre-treatment and post-treatment changes in biomarker expression
(5). In this study, we investigated
the predictive relevance of AM expression both in pre-treatment
biopsies and in corresponding surgical specimens of 55 patients
with LARC treated with standadised 5-FU-based NCRT. Comparing
pre-treatment biopsies and surgical specimens, we observed a
downregulation of VEGF, PlGF and HIF-1α. However, SDF-1α was
upregulated after NCRT. In addition, upregulated SDF-1α after NCRT
was significantly associated with resistance to NCRT. Our findings
suggest that SDF-1α is one of the important targets for resistance
to NCRT and this finding is significant.
SDF-1α, also known as chemokine ligand 12 (CXCL12),
and its receptor CXCR4, play important roles in the onset and
progression of primary or metastatic cancer from various organs
(23–26). In colorectal cancer (CRC), elevated
SDF-1α expression is associated with metastasis and poor prognosis
(27,28). In our investigation, upregulation of
SDF-1α in surgical specimens was related to resistance to NCRT.
Thus, SDF-1α appears to be a predictive marker to chemoradiation
treatment. In an in vitro study using a CRC cell line, the
results indicate that CXCR4 antagonistic therapy might prevent
tumour cell dissemination and metastasis in CRC patients,
consequently improving survival (29). Therefore, the targeting of SDF-1α
represents an attractive adjuvant treatment to eradicate cancer
cells and induce anti-angiogenic effects in highly hypoxic tumours.
Further study evaluating the distinctive value of SDF-1α expression
in LARC patients receiving NCRT is warranted. However, we did not
observe a relationship between expression of AMs before NCRT and
tumour reponse. Therefore, it is not possible to choose the ‘right’
patients who may require additional therapeutics (such as
anti-angiogenesis), except NCRT, by analhysis of the specimen
before treatment. These findings are difficult for clinical
application.
We also found that positive expression of PlGF after
NCRT was correlated with resistance to NCRT in multivariate
analyses. PlGF is a cytokine in the VEGF family of growth factors,
with 53% homology to VEGF (30). It
primarily regulates the angiogenic switch under pathologic states
(31). PlGF recruits smooth muscle
precursors that envelop developing vessels in tumours and together
with VEGF produces more stable and mature vessels. PlGF may also
facilitate metastasis by increasing the motility and invasion of
malignant cells (32). Tumour
overexpression of PlGF and VEGF together is associated with
increased tumour angiogenesis and cancer growth (33,34).
However, in general, there was no correlation between elevated VEGF
expression and survival (35,36).
Our results suggest that PlGF, than VEGF, is also an important
target for resistance to NCRT. It would be worthwhile to determine
whether or not PlGF is a predictive biomarker for patients with
LARC receiving NCRT.
As shown in this study, an association was
identified between HIF-1α and SDF-1α (P=0.034). HIF-1α was not
correlated with VEGF and PlGF. However, SDF-1α had an association
with PlGF (P=0.005). Although HIF-1α expression is known to drive
expression of downstream proteins, differences in individual
protein half-lives may not allow for a direct relationship between
HIF-1α and other proteins (37).
Downstream proteins may have been influenced by other signaling
pathways independent of HIF-1α, making their expression levels
somewhat variable in relation to HIF-1α. The limited sample size
and the heterogeneity of intratumoural oxygenation may also be
responsible for these findings.
In summary, SDF-1α and PlGF are relevant for
resistance to NCRT. By comparison of pre-therapeutic and
post-therapeutic intratumoural SDF-1α and PlGF, our results suggest
that therapeutic strategies to downregulate expression of SDF-1α
and PlGF during pre-operative treatment or to inhibit SDF-1α/PlGF
mediated signaling pathways may further increase the individual
tumour response and, as a consequence, improve patient prognosis.
Based on our results, patients with increased expression of SDF-1α
or positive expression of PlGF after NCRT might benefit from
additional anti-SDF-1α/PlGF therapeutics.
Acknowledgements
The authors would like to thank Kim Hyung-Joo and
Park So-Young for providing excellent technical assistance.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Sauer R, Becker H, Hohenberger W, Rodel C,
Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF,
Karstens JH, Liersch T, Schmidberger H and Raab R; German Rectal
Cancer Study Group. Preoperative versus postoperative
chemoradiotherapy for rectal cancer. N Engl J Med. 351:1731–1740.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roh MS, Colangelo LH, O’Connell MJ,
Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L,
Ursiny CS, Petrelli NJ and Wolmark N: Preoperative multimodality
therapy improves disease-free survival in patients with carcinoma
of the rectum: NSABP R-03. J Clin Oncol. 27:5124–5130. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maas M, Nelemans PJ, Valentini V, Das P,
Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R,
Haustermans K, Mohiuddin M, Pucciarelli S, Small W Jr, Suárez J,
Theodoropoulos G, Biondo S, Beets-Tan RG and Beets GL: Long-term
outcome in patients with a pathological complete response after
chemoradiation for rectal cancer: a pooled analysis of individual
patient data. Lancet Oncol. 11:835–844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sprenger T, Rödel F, Beissbarth T, Conradi
LC, Rothe H, Homayounfar K, Wolff HA, Ghadimi BM, Yildirim M,
Becker H, Rödel C and Liersch T: Failure of downregulation of
survivin following neoadjuvant radiochemotherapy in rectal cancer
is associated with distant metastases and shortened survival. Clin
Cancer Res. 17:1623–1631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Edden Y, Wexner SD and Berho M: The use of
molecular markers as a method to predict the response to
neoadjuvant therapy for advanced stage rectal adenocarcinoma.
Colorectal Dis. 14:555–561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trakarnsanga A, Ithimakin S and Weiser MR:
Treatment of locally advanced rectal cancer: controversies and
questions. World J Gastroenterol. 18:5521–5532. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chin KF, Greenman J, Gardiner E, Kumar H,
Topping K and Monson J: Pre-operative serum vascular endothelial
growth factor can select patients for adjuvant treatment after
curative resection in colorectal cancer. Br J Cancer. 83:1425–1431.
2000. View Article : Google Scholar
|
|
9
|
Cascinu S, Graziano F, Catalano V,
Staccioli MP, Rossi MC, Baldelli AM, Barni S, Brenna A, Secondino
S, Muretto P and Catalano G: An analysis of p53, BAX and vascular
endothelial growth factor expression in node-positive rectal
cancer. Relationships with tumour recurrence and event-free
survival of patients treated with adjuvant chemoradiation. Br J
Cancer. 86:744–749. 2002. View Article : Google Scholar
|
|
10
|
Willett CG, Duda DG, di Tomaso E, Boucher
Y, Ancukiewicz M, Sahani DV, Lahdenranta J, Chung DC, Fischman AJ,
Lauwers GY, Shellito P, Czito BG, Wong TZ, Paulson E, Poleski M,
Vujaskovic Z, Bentley R, Chen HX, Clark JW and Jain RK: Efficacy,
safety, and biomarkers of neoadjuvant bevacizumab, radiation
therapy, and fluorouracil in rectal cancer: a multidisciplinary
phase II study. J Clin Oncol. 27:3020–3026. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brizel DM, Scully SP, Harrelson JM,
Layfield LJ, Bean JM, Prosnitz LR and Dewhirst MW: Tumor
oxygenation predicts for the likelihood of distant metastases in
human soft tissue sarcoma. Cancer Res. 56:941–943. 1996.PubMed/NCBI
|
|
12
|
Nordsmark M, Alsner J, Keller J, Nielsen
OS, Jensen OM, Horsman MR and Overgaard J: Hypoxia in human soft
tissue sarcomas: adverse impact on survival and no association with
p53 mutations. Br J Cancer. 84:1070–1075. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koukourakis MI, Bentzen SM, Giatromanolaki
A, Wilson GD, Daley FM, Saunders MI, Dische S, Sivridis E and
Harris AL: Endogenous markers of two separate hypoxia response
pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase
9) are associated with radiotherapy failure in head and neck cancer
patients recruited in the CHART randomized trial. J Clin Oncol.
24:727–735. 2006. View Article : Google Scholar
|
|
14
|
Sowter HM, Ratcliffe PJ, Watson P,
Greenberg AH and Harris AL: HIF-1-dependent regulation of hypoxic
induction of the cell death factors BNIP3 and NIX in human tumors.
Cancer Res. 61:6669–6673. 2001.PubMed/NCBI
|
|
15
|
Lal A, Peters H, St Croix B, Haroon ZA,
Dewhirst MW, Strausberg RL, Kaanders JH, van der Kogel AJ and
Riggins GJ: Transcriptional response to hypoxia in human tumors. J
Natl Cancer Inst. 93:1337–1343. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1 alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
17
|
Ishigami SI, Arii S, Furutani M, Niwano M,
Harada T, Mizumoto M, Mori A, Onodera H and Imamura M: Predictive
value of vascular endothelial growth factor (VEGF) in metastasis
and prognosis of human colorectal cancer. Br J Cancer.
78:1379–1384. 1998. View Article : Google Scholar
|
|
18
|
Rajaganeshan R, Prasad R, Guillou PJ,
Poston G, Scott N and Jayne DG: The role of hypoxia in recurrence
following resection of Dukes’ B colorectal cancer. Int J Colorectal
Dis. 23:1049–1055. 2008.PubMed/NCBI
|
|
19
|
Wei SC, Liang JT, Tsao PN, Hsieh FJ, Yu SC
and Wong JM: Preoperative serum placenta growth factor level is a
prognostic biomarker in colorectal cancer. Dis Colon Rectum.
52:1630–1636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saigusa S, Toiyama Y, Tanaka K, Yokoe T,
Okugawa Y, Fujikawa H, Matsusita K, Kawamura M, Inoue Y, Miki C and
Kusunoki M: Cancer-associated fibroblasts correlate with poor
prognosis in rectal cancer after chemoradiotherapy. Int J Oncol.
38:655–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM and Haller DG: AJCC Cancer Staging Handbook - TNM
Classification of Malignant Tumors. 6th edition. Springer-Verlag;
New York: 2002
|
|
22
|
Dworak O, Keilholz L and Hoffmann A:
Pathological features of rectal cancer after preoperative
radiochemotherapy. Int J Colorectal Dis. 12:19–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL,
Mohar A, Verastegui E and Zlotnik A: Involvement of chemokine
receptors in breast cancer metastasis. Nature. 410:50–56.
2001.PubMed/NCBI
|
|
24
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar
|
|
25
|
Schrader AJ, Lechner O, Templin M, Dittmar
KE, Machtens S, Mengel M, Probst-Kepper M, Franzke A, Wollensak T,
Gatzlaff P, Atzpodien J, Buer J and Lauber J: CXCR4/CXCL12
expression and signalling in kidney cancer. Br J Cancer.
86:1250–1256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taichman RS, Cooper C, Keller ET, Pienta
KJ, Taichman NS and McCauley LK: Use of the stromal cell-derived
factor-1/CXCR4 pathway in prostate cancer metastasis to bone.
Cancer Res. 62:1832–1837. 2002.PubMed/NCBI
|
|
27
|
Matsusue R, Kubo H, Hisamori S, Okoshi K,
Takagi H, Hida K, Nakano K, Itami A, Kawada K, Nagayama S and Sakai
Y: Hepatic stellate cells promote liver metastasis of colon cancer
cells by the action of SDF-1/CXCR4 axis. Ann Surg Oncol.
16:2645–2653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoshitake N, Fukui H, Yamagishi H,
Sekikawa A, Fujii S, Tomita S, Ichikawa K, Imura J, Hiraishi H and
Fujimori T: Expression of SDF-1 alpha and nuclear CXCR4 predicts
lymph node metastasis in colorectal cancer. Br J Cancer.
98:1682–1689. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heckmann D, Laufs S, Maier P, Zucknick M,
Giordano FA, Veldwijk MR, Eckstein V, Wenz F, Zeller WJ, Fruehauf S
and Allgayer H: A Lentiviral CXCR4 overexpression and knockdown
model in colorectal cancer cell lines reveals plerixafor-dependent
suppression of SDF-1α-induced migration and invasion. Onkologie.
34:502–508. 2011.PubMed/NCBI
|
|
30
|
Wei SC, Tsao PN, Yu SC, Shun CT, Tsai-Wu
JJ, Wu CH, Su YN, Hsieh FJ and Wong JM: Placenta growth factor
expression is correlated with survival of patients with colorectal
cancer. Gut. 54:666–672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carmeliet P, Moons L, Luttun A, Vincenti
V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D,
Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A,
Blacher S, VandenDriessche T, Ponten A, Eriksson U, Plate KH,
Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM,
Collen D and Persico MG: Synergism between vascular endothelial
growth factor and placental growth factor contributes to
angiogenesis and plasma extravasation in pathological conditions.
Nat Med. 7:575–583. 2001. View
Article : Google Scholar
|
|
32
|
Fischer C, Jonckx B, Mazzone M, Zacchigna
S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M,
De Mol M, Autiero M, Wyns S, Plaisance S, Moons L, van Rooijen N,
Giacca M, Stassen JM, Dewerchin M, Collen D and Carmeliet P:
Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors
without affecting healthy vessels. Cell. 131:463–475. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Landriscina M, Cassano A, Ratto C, Longo
R, Ippoliti M, Palazzotti B, Crucitti F and Barone C: Quantitative
analysis of basic fibroblast growth factor and vascular endothelial
growth factor in human colorectal cancer. Br J Cancer. 78:765–770.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Adini A, Kornaga T, Firoozbakht F and
Benjamin LE: Placental growth factor is a survival factor for tumor
endothelial cells and macrophages. Cancer Res. 62:2749–2752.
2002.PubMed/NCBI
|
|
35
|
Lee JC, Chow NH, Wang ST and Huang SM:
Prognostic value of vascular endothelial growth factor expression
in colorectal cancer patients. Eur J Cancer. 36:748–753. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Khorana AA, Ryan CK, Cox C, Eberly S and
Sahasrabudhe DM: Vascular endothelial growth factor, CD68, and
epidermal growth factor receptor expression and survival in
patients with stage II and stage III colon carcinoma: a role for
the host response in prognosis. Cancer. 97:960–968. 2003.
View Article : Google Scholar
|
|
37
|
Lee-Kong SA, Ruby JA, Chessin DB,
Pucciarelli S, Shia J, Riedel ER, Nitti D and Guillem JG:
Hypoxia-related proteins in patients with rectal cancer undergoing
neoadjuvant combined modality therapy. Dis Colon Rectum.
55:990–995. 2012. View Article : Google Scholar : PubMed/NCBI
|