Introduction
Colorectal cancer is a common malignant tumor of the
digestive system, and the mortality associated with this disease is
ranked second highest among all malignant neoplasms in
industrialized countries (1). The
role of cyclooxygenase-2 (COX-2) in colorectal cancer development
and progression has been extensively studied. COX-2 is
overexpressed in large and high-grade dysplasia adenomas, and COX-2
over-expression is associated with carcinogenesis, progression,
invasion, metastasis and a poor prognosis (2–5).
Deregulation of COX-2 expression leads to an increased abundance of
prostaglandin E2 (PGE2), which can
potentially affect most of the events in cancer development,
including proliferation, resistance to apoptosis, angiogenesis,
immune suppression and invasion (6,7).
Because of its important role in tumor formation, progression,
invasion and metastasis, COX-2 is considered as a promising target
for cancer therapy (8,9).
There is increasing evidence demonstrating that
inhibition of expression of COX-2 has antitumor activity against
gastrointestinal carcinoma (10).
It has been confirmed that selective COX-2 inhibitors are effective
in preventing adenoma recurrence and reducing the incidence of
colorectal cancer to some extent (11). However, previous research has
revealed that unexpected cardiovascular side effects result when
selective COX-2 inhibitors are used in the long term (12). Consequently, the development of more
effective and low-toxicity selective COX-2 inhibitors is an
important area of study.
Paeonol (2-hydroxy-4-methoxyacetophenone), a major
active extract from the root bark of Paeonia suffruticosa
Andrews, possesses a number of biological activities, including
anti-inflammatory (13,14), anti-oxidant (15), anti-angiogenic (16), anti-allergic (17) and anti-oxidation (18). Paeonol has been shown to exhibit
anti-proliferative effects and apoptosis-inducing activities in
various types of tumor cell lines in vitro and in
vivo (19,20). Our previous study also revealed that
paeonol induced apoptosis in colorectal cancer cells (21). Here, in the present study, we
further investigated the underlying mechanisms of paeonol in
inducing apoptosis and whether its antitumor effect is associated
with reduction in COX-2 expression and a decrease in the levels of
PGE2 in colorectal cancer cells.
Materials and methods
Materials
Colorectal cancer cell lines HCT116, SW620 and LoVo
were obtained from the Cell Bank of the Shanghai Institute of
Biochemistry and Cell Biology, Chinese Academy of Sciences
(Shanghai, China). Paeonol was purchased from Natura Pharmaceutical
Co., Ltd. (purity >99.0%; Ningbo, China). DMEM/F-12 medium,
fetal bovine serum (FBS), penicillin-streptomycin, pancreatin and
glutamine and the bicinchoninic acid (BCA) protein assay kit were
purchased from Beyotime Institute of Biotechnology (Suzhou, China).
The Annexin V-FITC/propidium iodide (PI) apoptosis kit was
purchased from Roche Ltd. (Shanghai, China). Celecoxib,
3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT)
and Rhodamine 123 were purchased from Sigma-Aldrich (St. Louis, MO,
USA). The 2X Taq PCR Master Mix was obtained from Tiangen Biotech
Co., Ltd. (Beijing, China). Primers for human COX-2 and β-actin
were designed by Sangon Biotech Co., Ltd. (Shanghai, China), and
the sequences were as follows: forward, 5′-AAT GAG TAC CGA AAA
TTC-3′ and reverse, 5′-CAT CTA GTC CGG ACC GGG AAG-3′ for COX-2;
forward, 5′-ACC ACA GTC CAT GCC ATC AC-3′ and reverse, 5′-TCC ACC
ACC CTG TTG CTG TA-3′ for GAPDH. COX-2 siRNA was purchased from
Shanghai GenePharma Co., Ltd. (Shanghai, China). Lipofectamine 2000
reagent was purchased from Invitrogen (Carlsbad, CA, USA). The
primary antibodies against human COX-2, Bax, Bcl-2, caspase-3,
caspase-9, NF-κB, IKKα, IκBα and actin were obtained from Cell
Signaling Technology (Beverly, MA, USA). The secondary antibodies
were purchased from Wuhan Boster Biological Engineering Co., Ltd.
(Wuhan, China). All other chemicals were of reagent grade and were
obtained from commercial sources. Animals were maintained according
to animal care guidelines from the Institutional Animal Care and
Use Committee of Wuhan University. The study was approved by the
Institutional Review Board of Renmin Hospital of Wuhan
University.
Cell culture
All the cell lines were cultured in a 5%
CO2 and 95% humidified air atmosphere at 37°C in
DMEM/F-12 medium supplemented with heat-inactivated 10% FBS, 1%
antibiotics (100 IU penicillin and 100 μg/ml streptomycin).
Cell viability assay
MTT assay was used to analyze the viability of the
cell lines after test agent treatment. Briefly, all cancer cell
lines were seeded into 96-well plates (6.0×103
cells/well) and allowed to attach overnight. After cellular
adhesion, the medium was replaced with fresh medium supplemented
with various concentrations of test agents and further cultivated
for the indicated periods. Test agents were added to the culture
medium at various indicated concentrations. The control culture
received only the culture medium. Following further incubation, MTT
was added at a concentration of 5 mg/ml, and the cells were
incubated for another 4 h at 37°C. The medium was discarded, and
DMSO was added to dissolve the MTT formazan crystals. The
absorbance reading of each well was determined using a microplate
reader (Bio-Rad, Hercules, CA, USA) at a wavelength of 570 nm. The
wells without test agents and the free cells (culture medium alone)
were used as background. The cell growth inhibitory rates were
defined as the relative absorbance of the treated vs. the untreated
cells.
Cell apoptosis assay
To quantify apoptosis, cells were stained with
Annexin V and PI using the Annexin V-FITC/PI Apoptosis kit
according to the manufacturer’s instructions. Briefly, colorectal
cancer cells were cultured in 6-well plates with medium for 24 h.
The cells were then treated for a further 48 h with the test
agents. After treatment, the cells were washed twice with cold
phosphate-buffered saline (PBS) following treatment and resuspended
in 195 μl Annexin V-FITC binding buffer. Annexin V-FITC (5 μl) was
added and mixed gently, and the cells were incubated for 15 min at
room temperature in the dark. The cells were then centrifuged at
1,000 × g for 5 min and gently resuspended in 190 μl Annexin V-FITC
binding buffer. Following this, 10 μl PI staining solution was
added and gently mixed. The cells were kept on ice in the dark and
immediately subjected to flow cytometry (FACScan; BD Biosciences,
San Diego, CA, USA).
Determination of PGE2
production
To quantify PGE2 in the cell culture
supernatant, a competitive ELISA was performed according to the
manufacturer’s instructions (Thermo Scientific, Rockford, IL, USA).
Briefly, colorectal cancer cells were cultured in the 6-well plates
with medium for 24 h. The cells were then treated for a further 48
h with various concentrations of paeonol. After treatment, the LoVo
cell supernatant was diluted 1:4 for analysis. The samples were
tested in duplicate for each of three independent experiments.
Optical density at 405 nm was analyzed using a SpectraMax M2 plate
reader. The concentration of PGE2 was determined using
SoftMax Pro 5.4.2 plate reader software (Molecular Devices,
Sunnyvale, CA, USA). A standard concentration curve was generated
for each independent experiment.
COX-2 siRNA synthesis and
transfection
Colorectal cancer cells (2×105 in 2 ml of
DMEM/F-12 without antibiotics) were plated in 6-well plates. After
24 h, the human-specific COX-2 siRNA mix with Lipofectamine 2000
was overlaid on the cells according to the manufacturer’s protocol.
After 48 h of transfection, cells were harvested for the cell
viability assay, the cell apoptosis assay, reverse
transcription-polymerase chain reaction (RT-PCR) analysis and
western blot analysis.
Measurement of mitochondrial membrane
potential
Mitochondrial membrane potential was measured by
Rhodamine 123 staining. Colorectal cancer cells were cultured in
6-well plates and allowed to attach overnight. After cellular
adhesion, the cells were then treated for a further 48 h with
various indicated concentrations of the test agents. Cells were
harvested and washed twice with PBS and then incubated with 20 μl
Rhodamine 123 staining solution at 37°C in the dark for 30 min, and
then washed twice with PBS and centrifuged at 500 × g for 10 min.
Finally, the absorbance was determined using a spectrofluorometer
at an excitation wavelength of 505 nm and an emission wavelength of
534 nm.
RT-PCR analysis
Total RNA was isolated using TRIzol reagent and 1 μg
RNA was used as a template for the synthesis of cDNA using the
RevertAid First Strand cDNA synthesis kit (Fermentas, Waltham, MA,
USA) according to the manufacturer’s instructions. PCR analysis was
performed in a final volume of 25 μl using PCR Master Mix. PCR
products were separated in 1.5% agarose gel, stained with ethidium
bromide and images were captured.
Western blot assay
Protein expression levels were analyzed by western
blot analysis. Briefly, colorectal cancer cells were seeded in
6-well plates at a density of 2×105 cells and were then
incubated overnight at 37°C before treatment. After cells were
treated with various indicated concentrations of the test agents
for 48 h, the cells were washed with PBS and lysed with lysis
buffer, and incubated at 4°C for 1 h. The extracts were cleared by
centrifugation at 13,000 rpm for 20 min at 4°C. The concentration
of protein was determined using a BCA protein assay kit according
to the manufacturer’s instructions. Protein was loaded at a
concentration of 40 μg/lane, separated on a 12.5% sodium dodecyl
sulfate-polyacrylamide (SDS-PAGE) gel, and then transferred onto a
nitrocellulose membrane using a wet transfer system. Next, the
membrane was blocked with 10% non-fat dry milk in Tris-buffered
saline with Tween-20 (TBST, pH 8.0) and then incubated with the
primary antibodies: COX-2, Bax, Bcl-2, caspase-3, caspase-9, NF-κB,
IKKα, IκBα and actin overnight at 4°C. The appropriate horseradish
peroxidase (HRP) conjugated secondary antibodies were used at
1:1,000 for all antibodies. Positive antibody reactions were
detected with the enhanced chemoluminescence system and Hyperfilm
X-ray film.
Xenograft tumors in mice
Male BALB/c nude mice, 4–5 weeks of age, obtained
from the Center of Experimental Animals of Wuhan University, were
used in all of the experiments. LoVo cells, 5×106,
suspended in 100 μl PBS, were subcutaneously inoculated into the
lower right flank of the nude mice. After 7 days, the nude mice
were randomly divided into four groups (n=6 in each group): the
control group received 100 μl PBS (intragastric administration);
the three paeonol groups were intragastrically administered paeonol
(100, 200 and 400 mg/kg/day). There were no statistical differences
among the sizes of all the groups. Drugs were administrated daily
by gavage for 11 day. On day 12, all mice were sacrificed and the
tumor xenografts were removed and weighed. The tumor growth
inhibitory rate was calculated using the following formula:
Inhibition rate (%) = (1 - mean test tumor weight/mean control
tumor weight) × 100.
Statistical analysis
All continuing values are expressed as the mean ±
SD. Student’s t-test was used for comparison of the values between
two groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
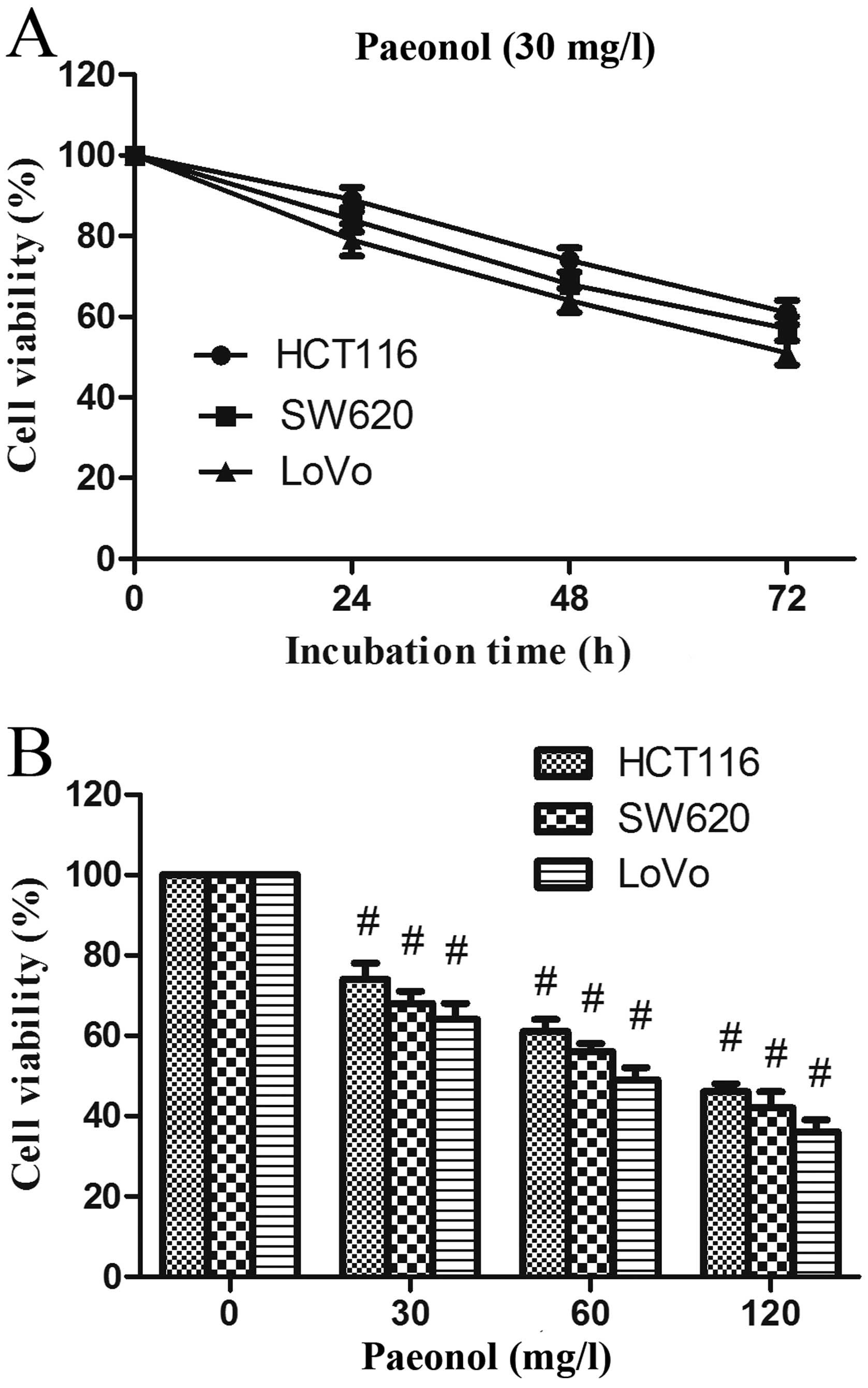
Paeonol inhibits human colorectal cancer
cell proliferation
The three human colorectal cancer cell lines were
treated with 30 mg/l paeonol, and the viability of cells was
assessed by MTT assay from 24 to 72 h. After treatment, the
proliferation of these cell lines was significantly inhibited,
especially in the LoVo cells (Fig.
1A). Furthermore, the growth rate of LoVo cells was greatly
decreased by incubation with 60 and 120 mg/l paeonol (Fig. 1B). The viability of LoVo cells
treated with paeonol was decreased in a dose- and time-dependent
manner.
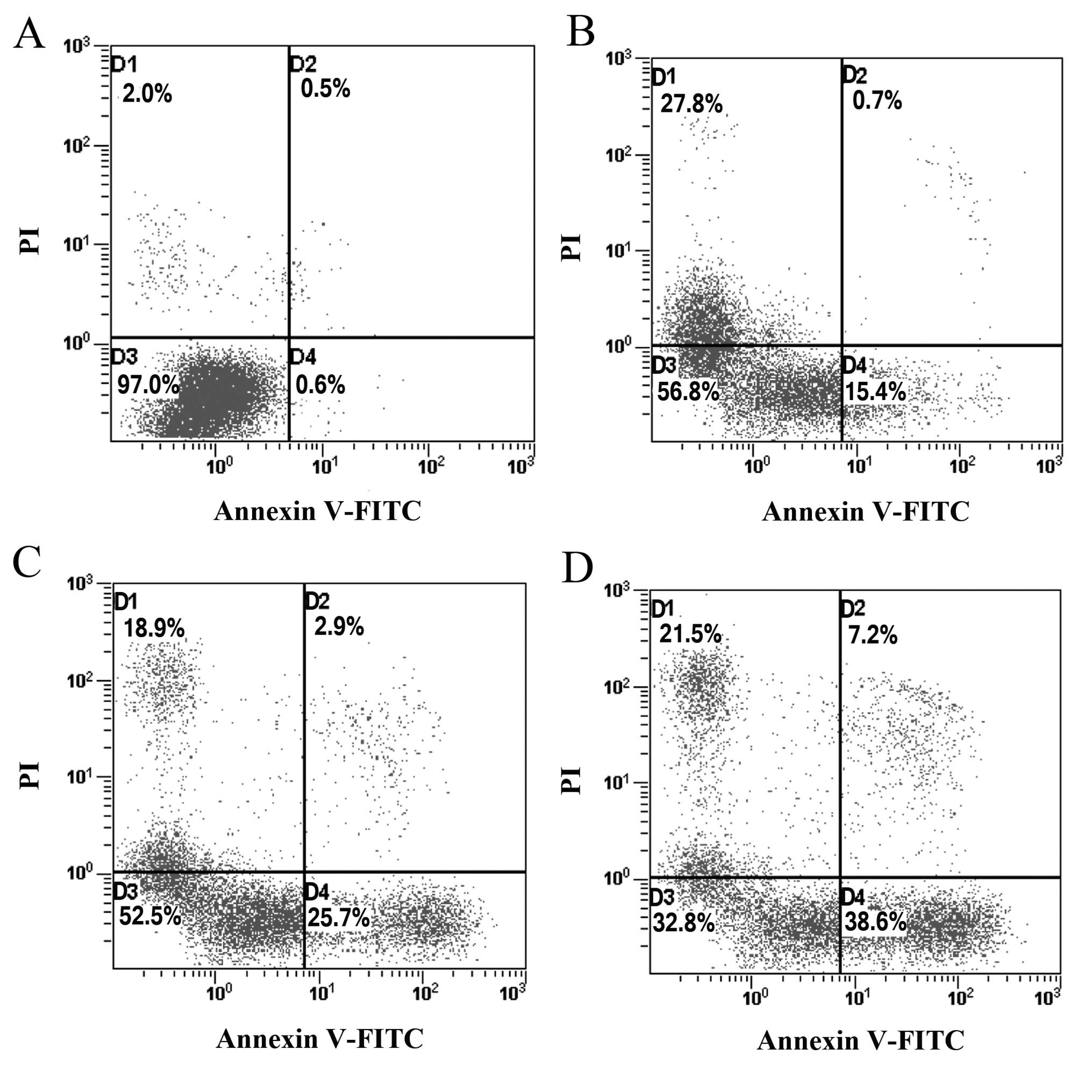
Paeonol induces cell apoptosis in
colorectal cancer cells
To determine whether the growth-inhibitory effect of
paeonol is related to the induction of apoptosis, colorectal cancer
cells treated with paeonol for 48 h were analyzed using flow
cytometric analysis. The proportion of apoptotic cells increased
from 15.4 to 38.6% in a dose-dependent manner (Fig. 2). The percentage of apoptotic cells
treated with paeonol was significantly higher compared with that in
the control group (P<0.01), indicating that paeonol may inhibit
the growth of colorectal cancer cells by inducing apoptosis.
Paeonol downregulates the expression of
COX-2 in colorectal cancer cells
COX-2, overexpressed in various types of cancers,
plays an important role in tumor formation, progression, invasion
and metastasis. To elucidate the interaction between COX-2 and
paeonol, LoVo cells were exposed to 0, 30, 60 and 120 mg/l paeonol
for 48 h, and the expression of COX-2 was assessed using western
blot analysis. Paeonol treatment was associated with reduced
expression of COX-2 (Fig. 3A). The
expression of COX-2 in cells treated with 30 mg/l paeonol was
observed to be lower than that in the controls. Treatment with 120
mg/l paeonol led to a further decrease, indicative of a
dose-dependent decrease in COX-2 expression.
Paeonol reduces PGE2 synthesis
in colorectal cancer cells
As the COX-2 metabolite, PGE2 has been
implicated in COX-2-mediated effects including cancer cell
proliferation and apoptosis, we further assessed the levels of
PGE2 in the paeonol-treated cells. Treatment with
paeonol for 48 h resulted in a significant reduction in the
production or synthesis of PGE2 in the LoVo cells in a
concentration-dependent manner, suggesting that paeonol-induced
reduction in PGE2 production is associated with the
inhibitory effect of paeonol on COX-2 expression, inhibition of
cell proliferation, and induction of cell apoptosis (Fig. 3B).
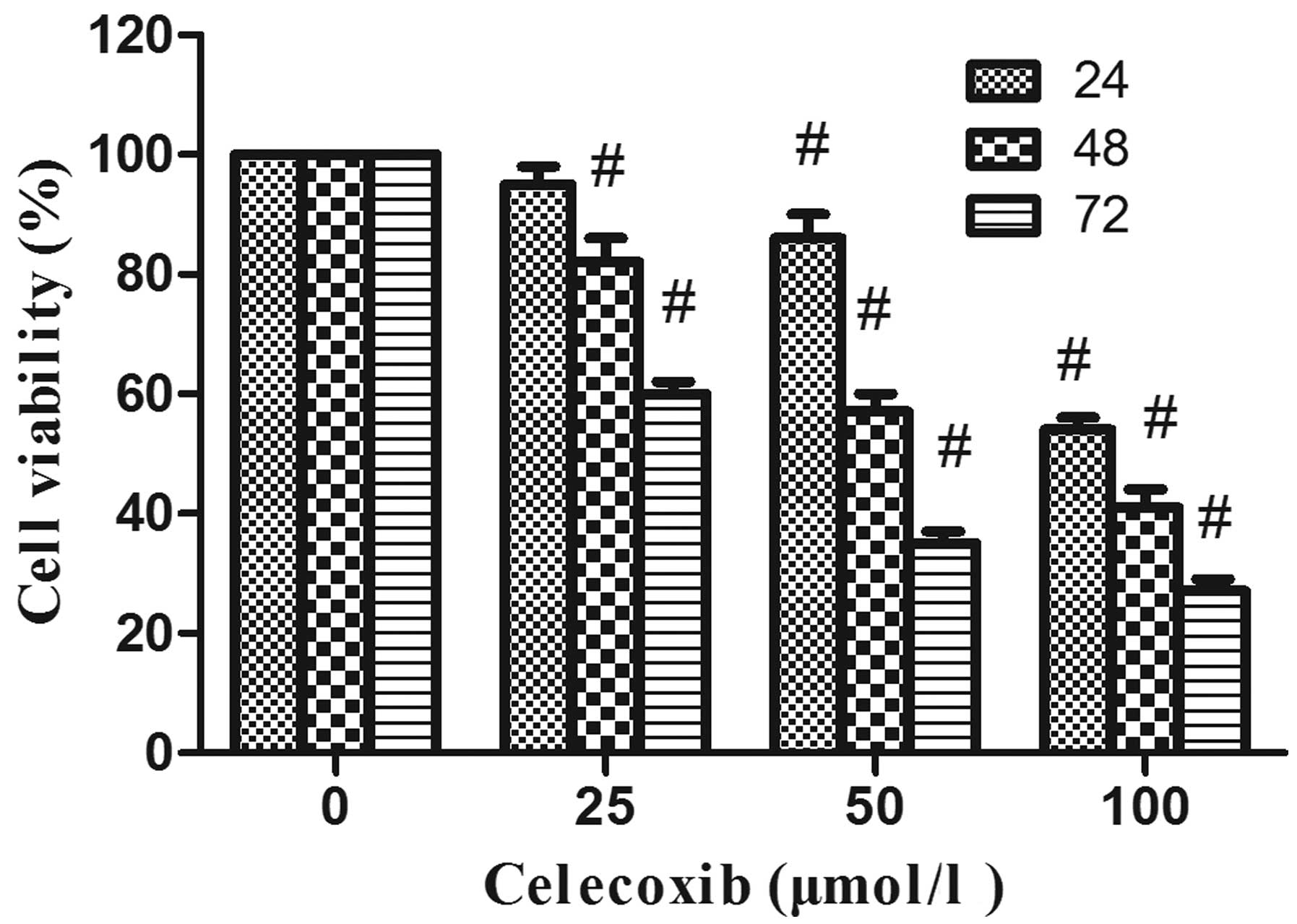
Celecoxib, a selective COX-2 inhibitor,
inhibits colorectal cancer cell proliferation and induces cell
apoptosis
This experiment was performed to explore whether the
antitumor effect of paeonol on colorectal cancer cells is mediated
through its inhibitory effect on COX-2 expression. For this
purpose, the viability of LoVo cells was assessed by MTT assay
after treatment with various concentrations of celecoxib (0, 25, 50
and 100 μmol/l), a well-known inhibitor of COX-2, from 24 to 72 h.
Treatment of the cells with celecoxib resulted in a dose- and
time-dependent reduction in the cell viability of LoVo cells as
compared with non-celecoxib-treated controls (P<0.05) (Fig. 4). Celecoxib also could induce cell
apoptosis. After treatment with celecoxib for 48 h, the proportion
of apoptotic cells increased from 11.5 to 34.2% in a dose-dependent
manner (Fig. 5). These data suggest
that the inhibition of COX-2 expression is linked to the inhibition
of cell proliferation and induction of cell apoptosis.
siRNA knockdown of COX-2 leads to
inhibition of cell proliferation and induction of cell
apoptosis
We further verified the role of COX-2 in cell
proliferation and apoptosis through siRNA knockdown of COX-2 in the
colorectal cells and examined whether it would lead to the
inhibition of cell proliferation and the induction of cell
apoptosis. After 48 h of transfection, the expression of COX-2 was
analyzed by RT-PCR and western blot analysis. The mRNA expression
and protein expression levels of COX-2 were significantly decreased
in the COX-2 siRNA group compared with the control siRNA group. We
assessed the potential effects of downregulation of COX-2 on the
proliferation in LoVo cells (Fig.
6A). The transfection of LoVo cells with COX-2 siRNA resulted
in a significant reduction in the cell proliferation of LoVo cells
after 48 h as compared to that of the control siRNA-transfected
LoVo cells (Fig. 6B). We also
analyzed the effect of COX-2 siRNA on cell apoptosis in LoVo cells
using Annexin V-FITC/PI staining. After 48 h of transfection, the
proportion of apoptotic cells was significantly increased in the
COX-2 siRNA group compared with that of the control siRNA group
(Fig. 6C).
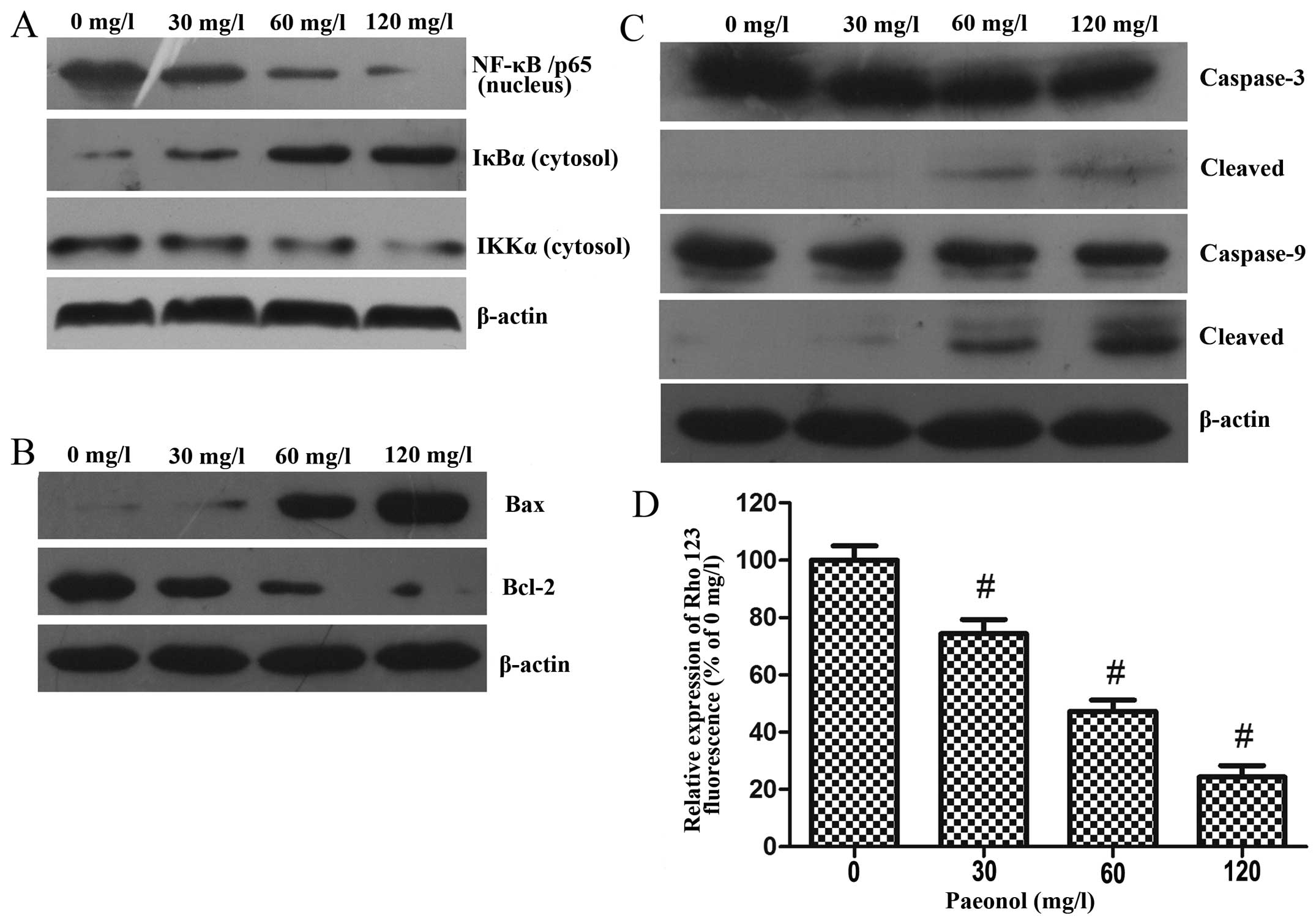
Paeonol inhibits the activation of NF-κB
in colorectal cancer cells
NF-κB is an upstream regulator of COX-2; therefore,
we further examined whether paeonol could also decrease the
activation of NF-κB in colorectal cancer cells.
For this purpose, after treatment with paeonol (0,
30, 60 and 120 mg/ml) for 48 h, LoVo cells were harvested and whole
cell lysates and nuclear lysates were prepared.
The results of the western blot analysis indicated
that paeonol reduced the nuclear translocation of NF-κB/p65 in a
dose-dependent manner. In addition, treatment with paeonol resulted
in the downregulation of IKKα and degradation of IκBα, which was
responsible for the inactivation of NF-κB and its translocation to
the nucleus (Fig. 7A).
Paeonol induces cell apoptosis through
activation of the mitochondrial pathway in colorectal cancer
cells
To examine the mechanism of apoptosis induced by
paeonol in the colorectal cancer cells, we analyzed mitochondrial
features of the intrinsic apoptotic pathway. After treatment with
paeonol (0, 30, 60 and 120 mg/l) for 48 h, western blot analysis
was performed to assess the protein expression levels of Bax,
Bcl-2, caspase-3 and caspase-9 (Fig. 7B
and C). Treatment with increasing doses of paeonol led to
increased expression of Bax and decreased expressions of
anti-apoptotic Bcl-2, and the stimulation of caspase-3 and
caspase-9 activity, which is considered as a hallmark of the
apoptotic process.
Paeonol decreases mitochondrial membrane
potential in colorectal cancer cells
Disruption of mitochondrial integrity is a critical
step occurring in cells undergoing apoptosis, and a decreasing
mitochondrial membrane potential is related to mitochondrial
dysfunction. Moreover, loss of mitochondrial membrane potential
plays a vital role in mitochondrial-mediated apoptosis. After
treatment with various concentrations of paeonol for 48 h, the
fluorescence intensity of Rhodamine 123 was significantly decreased
in the LoVo cells, suggesting that paeonol treatment of colorectal
cancer cells induced apoptosis through the mitochondrial apoptosis
pathway (Fig. 7D).
Antitumor effect of paeonol on colorectal
cancer cells in vivo
After the investigation of apoptosis induction in
LoVo cells in vitro, we further evaluated the antitumor
effect of paeonol. LoVo cells were subcutaneously inoculated into
the lower right flank of nude mice. After 7 days, the nude mice
were treated with different concentrations of paeonol via oral
gavage every day. Tumor weight was obtained at the end of the
experiment. Tumor weight in the paeonol groups was significantly
decreased (P<0.05 vs. control). The tumor growth inhibitory
rates in the paeonol groups were 22.35, 31.32 and 36.54%,
respectively (Table I).
 | Table IInhibitory effect of paeonol on LoVo
cell xenotrans-planted tumors in BALB/c mice. |
Table I
Inhibitory effect of paeonol on LoVo
cell xenotrans-planted tumors in BALB/c mice.
| Group | n | Tumor weight
(g) | Inhibitory rate
(%) |
|---|
| Control | 6 | 0.613±0.062 | - |
| 100 mg/kg
paeonol | 6 | 0.476±0.044a | 22.35 |
| 200 mg/kg
paeonol | 6 | 0.421±0.071a | 31.32 |
| 400 mg/kg
paeonol | 6 | 0.389±0.054a | 36.54 |
Discussion
Colorectal cancer is the second most common cause of
cancer-related mortality in the world. On a world-wide basis, there
were more than 1,200,000 new cases in 2012, with more than 600,000
deaths (22). Although much
progress in treatment and diagnosis has been achieved in recent
years, the survival rate and survival period of colorectal cancer
patients have not significantly improved. Of patients with newly
diagnosed colorectal cancer, 15–25% have metastatic disease, which
is usually lethal (23). Therefore,
additional research is urgently required to investigate new
treatment options and innovative therapeutic strategies.
Previous studies have revealed that paeonol exhibits
anti-proliferative effects in various tumor cell lines in
vitro and in vivo (19–21).
However, the underlying mechanisms remain unknown. In the present
study, our results showed that paeonol effectively inhibited
HCT116, SW620 and LoVo cell proliferation, particularly in LoVo
cells, in a dose- and time-dependent manner in vitro. In the
xenograft tumor-bearing nude mouse model, paeonol was revealed to
have significant anticancer effect. It also revealed that paeonol
resulted in apoptosis of treated cells in a dose-dependent manner.
These results indicated that paeonol may inhibit the proliferation
of colorectal cancer cells by activating the apoptotic signaling
pathway.
Apoptosis, a tightly regulated signaling process
that involves the coordination of both anti-apoptotic and
pro-apoptotic proteins, is vital for anti-carcinogenesis (24). The pro-apoptotic Bcl-2 family
members, such as Bax and Bcl-2, are essential for the initiation of
mitochondrial dysfunction during apoptosis. The results showed that
the expression of pro-apoptotic factor Bax was markedly upregulated
in the paeonol treatment group. However, anti-apoptotic factor
Bcl-2 was significantly reduced. Moreover, the paeonol-induced
apoptotic response involved caspase-3 and caspase-9 activation in
the colorectal cancer cells. Paeonol also induced loss of
mitochondrial membrane potential in the LoVo cells. This suggests
that Bcl-2 inhibited Bax activity, which reduced mitochondrial
membrane potential, leading to caspase-3 upregulation and cell
apoptosis (25). Together these
results indicate that paeonol induced apoptosis in colorectal
cancer cells by activating the mitochondrial-mediated apoptosis
pathway.
COX-2, the inducible enzyme that regulates
PGE2 synthesis, is frequently overexpressed in various
types of cancers (26,27). COX-2 and PGE2 play a
central role in orchestrating multiple events of cancer invasion,
metastasis and tumor development (28–30).
Thus, COX-2 is considered as a promising target for cancer therapy
(8,9). Three controlled randomized trials
(PreSAP, APC and Approve) were launched to evaluate the efficacy
and safety of cyclooxygenase-2 inhibitors (COXIBs) in preventing
the recurrence of sporadic colorectal adenomas. The data showed
that the use of COXIBs was associated with a significant reduction
in the risk of adenoma recurrence, particularly of advanced
adenomas in all of these studies (31–33).
However, the cardiovascular toxicity associated with the use of
COXIBs limits their use in healthy individuals. Therefore, the
search for novel and low-toxic inhibitors of COX-2 may provide a
better option for the treatment of colorectal cancer and this may
prove to be a better strategy for its prevention or treatment. In
the present study, paeonol inhibited the expression of COX-2 and
the production of PGE2. These results indicated that the
effects of paeonol on cell grow inhibition and apoptosis were
associated with the inhibion of COX-2 expression and
PGE2 synthesis. This was supported by evidence that
treatment of LoVo cells with celecoxib, a selected COX-2 inhibitor,
resulted in a reduction in the cell viability and an increase in
cell apoptosis. Similar effects were noted when the LoVo cells were
transfected with COX-2 siRNA. Protein encoded by COX-2 genes is a
type of oncogenic protein, which could catalyze the conversion of
arachidonic acid to PGE2. Research has confirmed that
overexpression of COX-2 promotes cell proliferation by weakening
the anti-proliferative effect of transforming growth factor-β
(TGF-β) (34). A previous study
demonstrated that COX-2 is a regulatory factor in the Bcl-2
upstream sequences through the phosphatidylinositol 3-kinase (PI3K)
signaling pathway, and eventually inhibits the apoptosis of cancer
cells (35). It has also been
confirmed that COX-2 inhibits the apoptosis of cancer cells by
inducing the mutation of P53 and weakening the apoptotic signal
mediated by Fas protein (36).
These findings indicate that paeonol induces apoptosis by
suppressing the expression of COX-2, which leads to downregulation
of Bcl-2 and upregulation of Bax.
NF-κB is a family of dimeric transcription factors
that regulate a wide spectrum of biological processes, including
inflammation, immune responses, cell proliferation and apoptosis
(37–39). Moreover, NF-κB downstream effectors
including COX-2, Bax and Bcl-2 are key mediators of apoptosis and
cell cycle arrest. In the present study, treatment of paeonol
resulted in the downregulation of IKKα and degradation of IκBα,
eventually leading to the inactivation of NF-κB and its
translocation to the nucleus. As previously mentioned, paeonol also
affected the NF-κB-regulated apoptosis-related proteins including
Bax and Bcl-2.
In conclusion, the results from this study suggest
that paeonol exhibits a marked antitumor effect. One of the
antitumor mechanisms of paeonol may be that its inhibition of NF-κB
and COX-2 leads to reduced proliferation and induction of
apoptosis, connected with caspase-dependent mitochondrial
dysfunction. These results provide a rationale to continue research
on paeonol, and further mechanism-based studies are required. We
expect that paeonol may replace selective COX-2 inhibitors due to
their toxic effects, and may become a new strategy for the therapy
of colorectal cancer.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of Hubei Province (no. 2013CFA076). We
sincerely thank Mr. Hong Xia from the Key laboratory of Hubei
Province for Digestive System Disease for his outstanding
administrative support in this study.
References
|
1
|
Strimpakos AS, Cunningham D, Mikropoulos
C, Petkar I, Barbachano Y and Chau I: The impact of
carcinoembryonic antigen flare in patients with advanced colorectal
cancer receiving first-line chemotherapy. Ann Oncol. 21:1013–1019.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benamouzig R, Uzzan B, Martin A, et al:
Cyclooxygenase-2 expression and recurrence of colorectal adenomas:
effect of aspirin chemoprevention. Gut. 59:622–629. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang R, Guo L, Wang P, Yang W, Lu Y, Huang
Z and Tang C: Chemoprevention of cancers in gastrointestinal tract
with cyclooxygenase 2 inhibitors. Curr Pharm Des. 19:115–125.
2013.PubMed/NCBI
|
|
4
|
Cheng J and Fan XM: Role of
cyclooxygenase-2 in colorectal cancer development and progression.
World J Gastroenterol. 19:7361–7318. 2013. View Article : Google Scholar
|
|
5
|
Bocca C, Ievolella M, Autelli R, et al:
Expression of Cox-2 in human breast cancer cells as a critical
determinant of epithelial-to-mesenchymal transition and
invasiveness. Expert Opin Ther Targets. 18:121–135. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh B and Lucci A: Role of
cyclooxygenase-2 in breast cancer. J Surg Res. 108:173–179. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harris RE: Cyclooxygenase-2 (cox-2)
blockade in the chemoprevention of cancers of the colon, breast,
prostate, and lung. Inflammopharmacology. 17:55–67. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Méric JB, Rottey S, Olaussen K, Soria JC,
Khayat D, Rixe O and Spano JP: Cyclooxygenase-2 as a target for
anticancer drug development. Crit Rev Oncol Hematol. 59:51–64.
2006.PubMed/NCBI
|
|
9
|
Dannenberg AJ and Subbaramaiah K:
Targeting cyclooxygenase-2 in human neoplasia: rationale and
promise. Cancer Cell. 4:431–436. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elder DJ, Halton DE, Crew TE and Paraskeva
C: Apoptosis induction and cyclooxygenase-2 regulation in human
colorectal adenoma and carcinoma cell lines by the
cyclooxygenase-2-selective non-steroidal anti-inflammatory drug
NS-398. Int J Cancer. 86:553–560. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rostom A, Dubé C, Lewin G, et al:
Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2
inhibitors for primary prevention of colorectal cancer: a
systematic review prepared for the U.S. Preventive Services Task
Force. Ann Intern Med. 146:376–389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Solomon SD, Wittes J, Finn PV, et al:
Cardiovascular risk of celecoxib in 6 randomized placebo-controlled
trials: the cross trial safety analysis. Circulation.
117:2104–2113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chou TC: Anti-inflammatory and analgesic
effects of paeonol in carrageenan-evoked thermal hyperalgesia. Br J
Pharmacol. 139:1146–1152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang H, Chang EJ, Lee Y, Kim JS, Kang SS
and Kim HH: A genome-wide microarray analysis reveals
anti-inflammatory target genes of paeonol in macrophages. Inflamm
Res. 57:189–198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsieh CL, Cheng CY, Tsai TH, et al:
Paeonol reduced cerebral infarction involving the superoxide anion
and microglia activation in ischemia-reperfusion injured rats. J
Ethnopharmacol. 106:208–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SA, Lee HJ, Ahn KS, et al: Paeonol
exerts anti-angiogenic and anti-metastatic activities through
downmodulation of Akt activation and inactivation of matrix
metalloproteinases. Biol Pharm Bull. 32:1142–1147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SH, Kim SA, Park MK, et al: Paeonol
inhibits anaphylactic reaction by regulating histamine and
TNF-alpha. Int Immunopharmacol. 4:279–287. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun YC, Shen YX and Sun GP: Advances in
the studies of major pharmacological activity of paeonol. Zhong
Cheng Yao Zazhi. 26:579–582. 2004.(In Chinese).
|
|
19
|
Cai J, Chen S, Zhang W, Hu S, Lu J, Xing J
and Dong Y: Paeonol reverses paclitaxel resistance in human breast
cancer cells by regulating the expression of transgelin 2.
Phytomedicine. 21:984–991. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan L, Song B, Sun G, Ma T, Zhong F and
Wei W: Endoplasmic reticulum stress-induced resistance to
doxorubicin is reversed by paeonol treatment in human
hepatocellular carcinoma cells. PLoS One. 8:e626272013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li M, Tan SY, Zhang J and You HX: Effects
of paeonol on intracellular calcium concentration and expression of
RUNX3 in LoVo human colon cancer cells. Mol Med Rep. 7:1425–1430.
2013.PubMed/NCBI
|
|
22
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
23
|
Kindler HL and Shulman KL: Metastatic
colorectal cancer. Curr Treat Options Oncol. 2:459–471. 2001.
View Article : Google Scholar
|
|
24
|
Tang D, Lotze MT, Kang R and Zeh HJ:
Apoptosis promotes early tumorigenesis. Oncogene. 30:1851–1854.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Masferrer JL, Leahy KM, Koki AT, et al:
Antiangiogenic and antitumor activities of cyclooxygenase-2
inhibitors. Cancer Res. 60:1306–1311. 2000.PubMed/NCBI
|
|
27
|
Sheng H, Shao J, Kirkland SC, et al:
Inhibition of human colon cancer cell growth by selective
inhibition of cyclooxygenase-2. J Clin Invest. 99:2254–2259. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh T, Vaid M, Katiyar N, Sharma S and
Katiyar SK: Berberine, an isoquinoline alkaloid, inhibits melanoma
cancer cell migration by reducing the expressions of
cyclooxygenase-2, prostaglandin E2 and prostaglandin
E2 receptors. Carcinogenesis. 32:86–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reich R and Martin GR: Identification of
arachidonic acid pathways required for the invasive and metastatic
activity of malignant tumor cells. Prostaglandins. 51:1–17. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dubois RN, Abramson SB, Crofford L, et al:
Cyclooxygenase in biology and disease. FASEB J. 12:1063–1073.
1998.PubMed/NCBI
|
|
31
|
Arber N, Eagle CJ, Spicak J, et al:
Celecoxib for prevention of colorectal adenomas. N Engl J Med.
355:885–895. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baron JA, Sandler RS, Bresalier RS, et al:
A randomized trial of rofecoxib for the chemoprevention of
colorectal adenomas. Gastroenterology. 131:1674–1682. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bertagnolli MM, Eagle CJ, Zauber AG, et
al: Celecoxib for the prevention of sporadic colorectal adenomas. N
Engl J Med. 355:873–884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Enders GA: Cyclooxygenase-2 overexpression
abrogates the antiproliferative effects of TGF-beta. Br J Cancer.
97:1388–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen XL, Su BS, Sun RQ, Zhang J and Wang
YL: Relationship between expression and distribution of
cyclooxygenase-2 and bcl-2 in human gastric adenocarcinoma. World J
Gastroenterol. 11:1228–1231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han JA, Kim JI, Ongusaha PP, et al:
p53-mediated induction of Cox-2 counteracts p53- or genotoxic
stress-induced apoptosis. EMBO J. 21:5635–5644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: evolutionarily conserved mediators of immune
responses. Annu Rev Immunol. 16:225–260. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barkett M and Gilmore TD: Control of
apoptosis by Rel/NF-kappaB transcription factors. Oncogene.
18:6910–6924. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Karin M and Lin A: NF-kappaB at the
crossroads of life and death. Nat Immunol. 3:221–227. 2002.
View Article : Google Scholar : PubMed/NCBI
|