Introduction
Non-invasive imaging methods are indispensable for
detecting and diagnosing tumors in internal organs, such as the
liver and brain. Among various imaging modalities, computed
tomography (CT) and magnetic resonance imaging (MRI) provide
anatomical information, whereas positron emission tomography (PET)
provides functional information regarding a disease. Since each
imaging modality has unique advantages and disadvantages (1,2),
appropriate complementary imaging technologies have been developed.
PET is widely used in nuclear medicine for the diagnosis of diverse
malignant tumors and monitoring tissue metabolism (3).
PET imaging is achieved by use of radiotracers, with
18F-fluoro-2-deoxyglucose (18F-FDG) being
commonly used in clinical practice for evaluation of the metabolic
status of abnormal and normal tissues (4). 18F-FDG is actively
transported into cells by the same transporters that incorporate
glucose (5), and is then
phosphorylated by hexokinase 2 (HK2) or glucokinase and converted
to FDG 6-phosphate, which is trapped in the cell immediately after
phosphorylation (5). Increased
glucose uptake is characteristic of malignant tumors, because
tumors have increased expression levels of glucose transporters and
intracellular enzymes, such as HK2, that are involved in glycolysis
(6).
The intracellular concentration of
18F-FDG in tumors typically represents the glycolytic
activity of viable tumor cells. However, the detection of primary
hepatocellular carcinoma (HCC) is less successful because
18F-FDG uptake varies among tumor cells at different
stages of differentiation (7). For
example, the detection rate of HCCs by 18F-FDG PET
varied from 50 to 70% in findings of previous studies (8–10).
However, 18F-FDG PET has been effective in the detection
of poorly differentiated HCC (10,11)
and has been considered valuable for detecting extrahepatic
metastasis and assessing the response to treatment after
molecular-targeted therapy (11).
HCC is one of the most common human cancers and its
incidence is on the increase worldwide according to epidemiological
data (12). The precise molecular
mechanism underlying HCC progression is poorly understood.
Therefore, it is essential to establish an animal model of HCC to
monitor tumor progression and the efficacy of therapeutic
intervention. In this respect, a xenograft model using
immune-deficient mice, such as athymic or severe combined immune
deficiency mice, is benificial, such as being readily and rapidly
established and that it can be used effectively and efficiently
when the appropriate cell lines are selected (13). However, as is the case for any
animal model, tumor progression and the tumor microenvironment may
not precisely reflect the human counterpart (14). Therefore, new models that can
precisely reflect the progression of malignancies in humans should
be identified.
Genetically modified mouse models (GMM), as well as
models based on treating mice with chemical carcinogens that mimic
the pathophysiological and molecular features of HCC in humans are
available (14). GMMs are generally
produced by the transgenic expression of oncogenes, such as c-myc
(15), H-Ras (16) and hepatitis B virus (HBV)-associated
genes (17,18) under the control of tissue-specific
promoters. Furthermore, several chemical carcinogens are known to
induce HCC, including diethylnitrosamine (DEN) (19), aflatoxin (20), thioacetamide (21) and carbon tetrachloride (22).
Among the chemical carcinogens that can induce HCC,
the carcinogenicity of DEN is due to alkylation of cellular DNA
(23) and generation of reactive
oxygen species (24). The mechanism
of HCC development following the administration of a single dose of
DEN depends on the dosage of DEN used and the gender and age of the
mice (25). Thus, DEN induced HCC
in 100% of male and in 30% of female mice. The gender disparity in
HCC incidence was caused by MyD88-dependent production of
interleukin-6 (26). In the present
study, we utilized murine models that resemble HCC in humans,
through transgenic mice expression of the oncogenic hepatitis B
virus X (HBx) and DEN-treated mice. We then evaluated primary HCC
and monitored HCC progression longitudinally using
18F-FDG small animal PET/CT and 3-T clinical MRI and
compared 18F-FDG uptake at different stages of tumor
differentiation.
Materials and methods
HCC tumor-bearing mouse models
The care, maintenance and treatment of animals in
these studies followed protocols approved by the Institutional
Animal Care and Use Committee of the Korea Institute of
Radiological and Medical Sciences.
Female and male C57BL/6 mice were purchased from The
Central Lab, Animal Inc., Seoul, Korea and transgenic mice
expressing the HBx (HBx-Tg model) were provided by Yu et al
(17). The mice were maintained at
22–24°C and 45–60% relative humidity, and had free access to food
and water, in an air-conditioned incubator. To establish the
chemical carcinogen-induced hepatic tumor model (DEN-model),
3-week-old male mice were injected intraperitoneally once with DEN
at 20 mg/kg body weight (Sigma Aldrich, St. Louis, MO, USA).
MRI
MRI scanning was performed using a clinical 3-T MR
unit (Magnetom Tim Trio, Siemens Medical Solutions, Erlangen,
Germany) with a wrist coil, and the mice were fixed in a prone
position. Fourteen mice were scanned at 19–21 months after DEN
treatment and 13 HBx-Tg mice were scanned at 11–19 months after
birth. Prior to scanning, the animals were anesthetized with 2%
isoflurane in oxygen. The imaging parameters for the T2-weighted
volumetric interpolated brain examination sequence were: repetition
time (TR) =1,620 msec, echo time (TE) =37 msec, 60-mm field of view
(FOV), 256×256 matrix size, 1-mm slice thickness, and number of
excitations =2. Tumor size was calculated by measuring the diameter
from MRI using Piview software (Mediface, Korea).
Small animal PET/CT study
Mice harboring HCC, as detected with 3-T MRI, were
fasted overnight prior to 18F-FDG PET/ CT scanning. The
mice were anesthetized with 2% isoflurane in oxygen and injected
into the tail vein with 7.4 MBq of 18F-FDG and then kept
in a chamber with 0.5% isoflurane for 1 h. PET/CT images were
obtained using an Inveon small animal PET/SPECT/CT system (Siemens
Medical Solutions). The CT scan was conducted, and then PET/CT
images were obtained using the following settings: CT scan total
rotation of 360°, estimated scan time of 180 sec; X-ray exposure
time of 300 msec, average frame of 1 and effective pixel size of
109.63 μm, with fluoroscopy scan time of 60 sec, X-ray voltage and
current of 70 kVp and 400 μA, respectively. PET images were scanned
using a 1,200 sec acquisition time and a 511-KeV energy level
(lower level was 350 KeV and the upper level, 650 KeV). During
acquisition of the PET images, the mice were anesthetized using 2%
isoflurane.
18F-FDG was obtained from the Korea
Institute of Radiological and Medical Sciences. The level of
18F-FDG uptake in tumor nodules was measured as max
standard uptake values (SUVmax), in which uptake
activity was normalized for body weight and injected activity.
Reconstruction of data sets, PET-CT fusion and image analysis were
performed using Inveon Research Workplace (IRW) software (Siemens
Medical Solutions).
Histopathological evaluation and
immunohistochemistry
To evaluate the histopathological changes of the
induced tumors, mice were euthanized and the liver tissues
containing visible cancer masses were dissected. The specimens were
washed with cold phosphate-buffered saline, fixed in 10% neutral
buffered formalin and then embedded in paraffin. The tissue blocks
were cut into 5-μm sections and subjected to hematoxylin and eosin
(H&E) staining for light microscopy. Tumor regions with high or
low 18F-FDG accumulations were dissected, removed and
processed separately.
Immunohistochemistry (IHC) was performed to
determine the level of HK2 (HK2 monoclonal antibody, Thermo
Scientific, Waltham, MA, USA) and Glut1 (Glut1; anti-GLUT1
antibody, Abcam, Cambridge, UK). Tissue sections were
deparaffinized, rehydrated and incubated with 3% hydrogen peroxide
for 20 min to block the endogenous peroxidase activity. Blocking
was performed with 10% goat serum for 10 min. The sections were
incubated with primary antibody at 4°C overnight and then with the
appropriate secondary and tertiary antibodies for 30 min at room
temperature. After washing, signals were detected using
diaminobenzidine. Sections incubated without primary antibody
served as negative controls. After examination of stained sections,
the relative intensity of the immunoreactivity was classified into
3 grades: + (weak signal), ++ (medium signal) and +++ (fairly
strong signal).
Results
Detection of induced HCC and multiple
tumor lesions
The development of HCC in the DEN- or HBx-Tg model
was observed using a clinical 3-T MRI capable of detecting tumors
>1 mm in diameter. Lesions of this size were readily detected
using MRI, but not with the small animal 18F-FDG PET due
to a resolution difference between the two imaging systems. Using
MRI, tumors were detected in mice aged 6.5–21 months and 11–17
months in the DEN and HBx-Tg models, respectively.
The incidence of lesions was 50% (7/14 mice) in the
DEN model and 38% (5/13 mice) in the HBx-Tg model. Eighteen and 11
multiple tumors developed in seven DEN- and five HBx-Tg model mice,
respectively. Approximately 1–8 nodules of varying size were
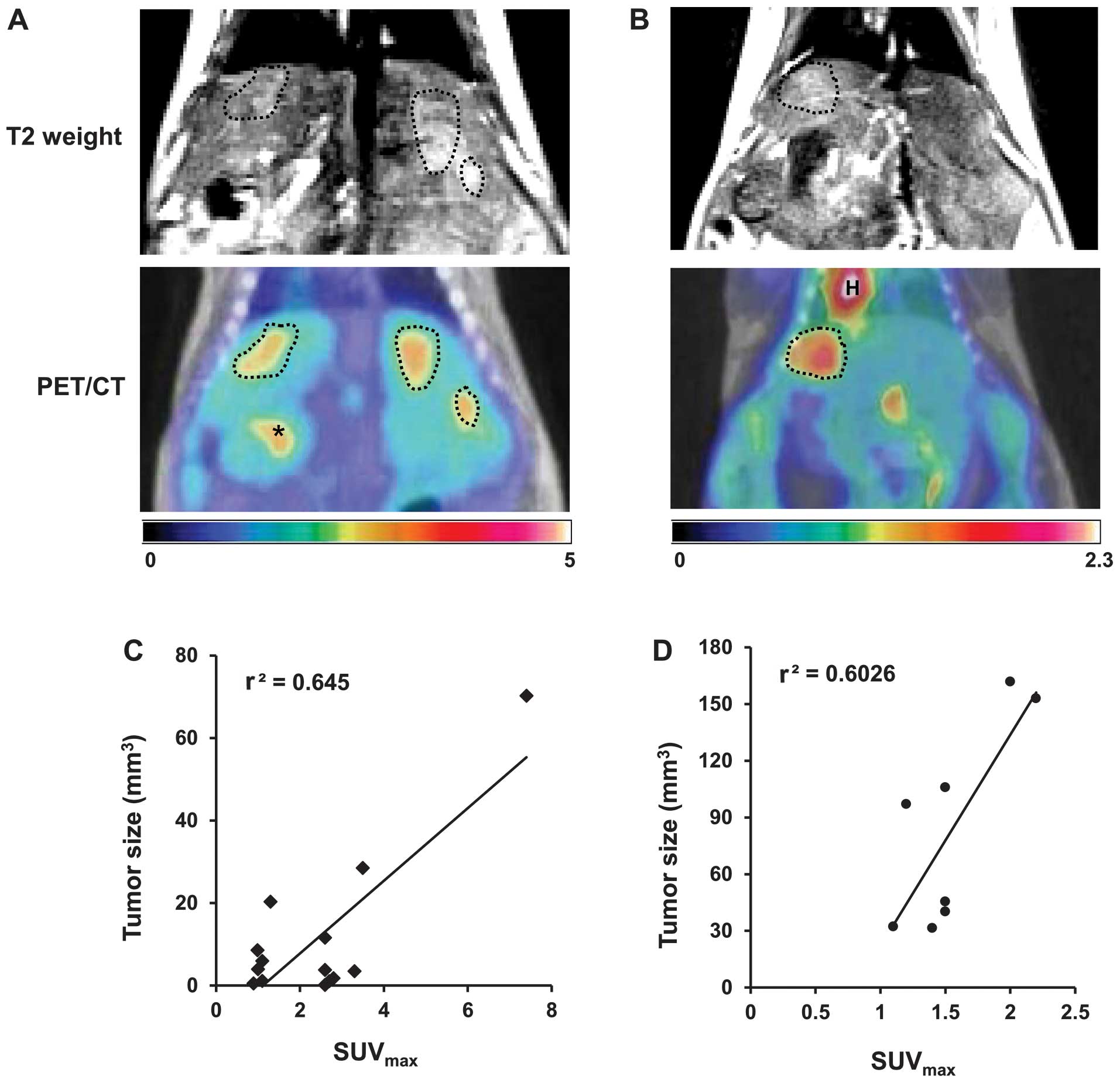
detected in each mouse. For example, three nodules induced by DEN
throughout the liver (Fig. 1A) and
one nodule by HBx oncogene (Fig.
1B) were visualized by MRI and 18F-FDG PET/CT. There
was a correlation between tumor size and SUVmax in the
DEN-model (r2=0.645) and HBx-Tg model
(r2=0.6026) (Fig. 1C and
D, respectively).
Longitudinal monitoring of tumor growth
using 18F-FDG PET/CT
Tumor progression was monitored longitudinally and
non-invasively using 18F-FDG PET/CT. Tumors in DEN-model
mice were first detected 6.5 months after DEN treatment and were
longitudinally monitored until 10 months (Fig. 2A). In the HBx-Tg model mice, tumor
nodules were observed at 11 months after birth and were followed up
to 20 months (Fig. 2B). The
increased size of tumor nodules was also assessed by the enhanced
18F-FDG uptake in PET (Fig.
2).
Histopathological evaluation for
verification of HCC
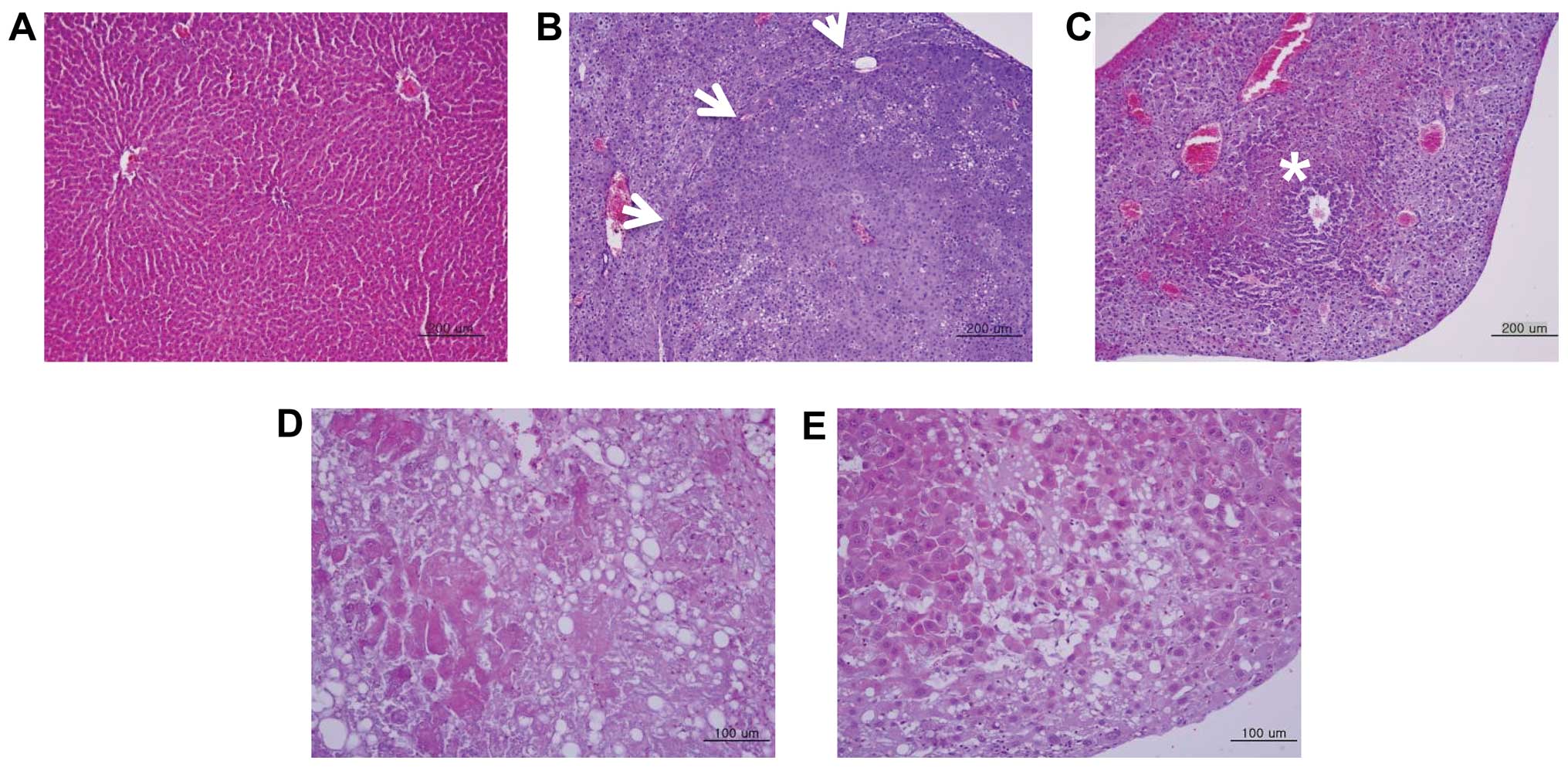
The sizes of the HCC nodules varied in the two
models. The tumor nodules were either encapsulated by connective
tissue or not well circumscribed (Fig.
3B and C). Following histopathological examination, tumor
nodules were identified as either hepatocellular adenoma (HCA) or
carcinoma. HCA was developed in the DEN- and HBx-Tg model mice and
showed characteristics typical of adenoma, including irregular
hepatic cord cells and vacuolated cytoplasm (arrows, Fig. 3B). HCC was also evident in the two
models. In HCCs, the architecture of the hepatocytes appeared to be
irregular and the tumor nodules contained pleomorphic cells
(Fig. 3C–E). The cellular
arrangement of HCC that developed in HBx-Tg mice was either that of
a poorly differentiated (Fig. 3D)
or well-differentiated carcinoma (Fig.
3E). We found that HCCs from high 18F-FDG uptake
tumor nodules were poorly differentiated, while one from low
18F-FDG uptake was well-differentiated. To determine the
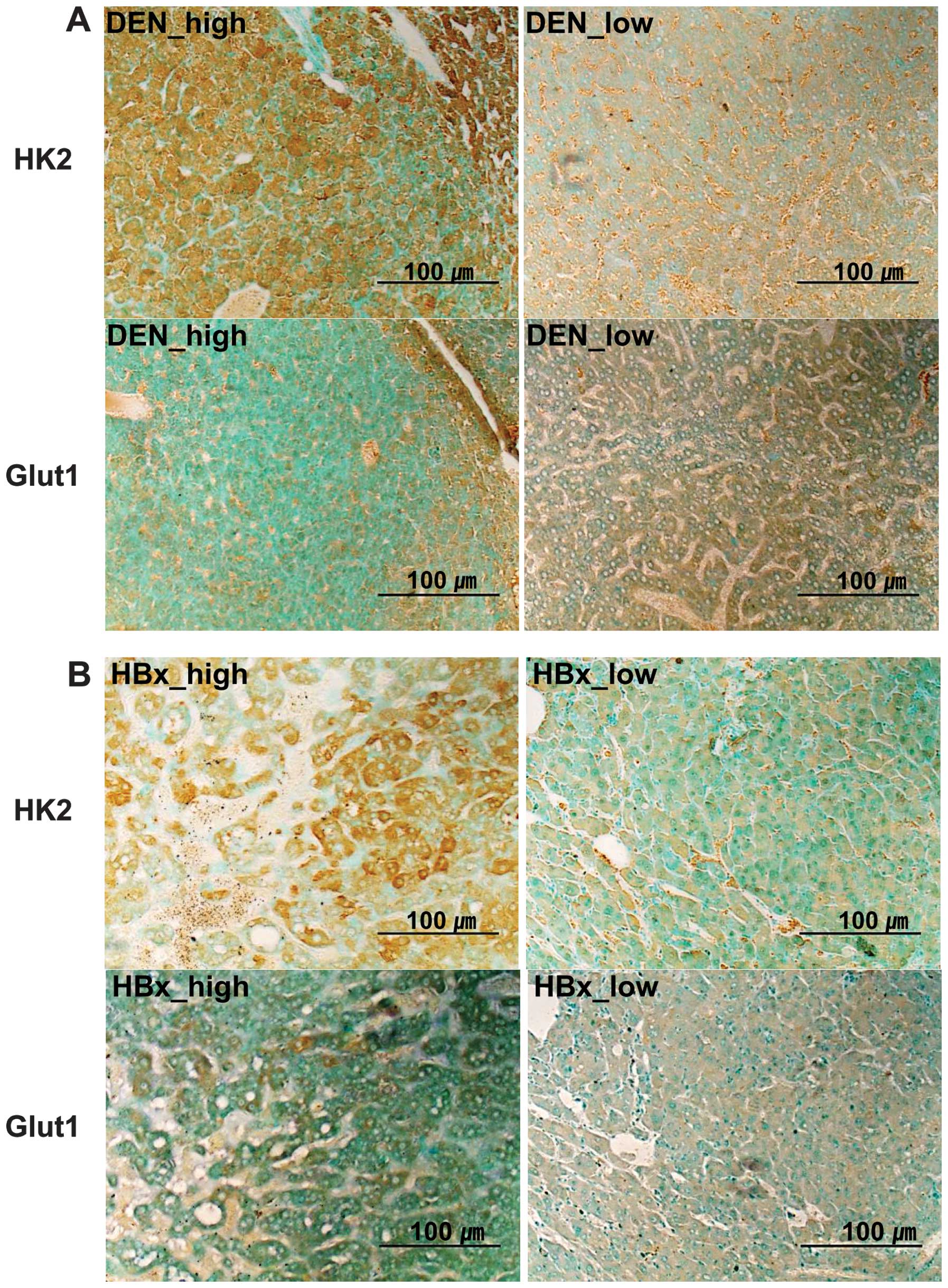
level of Glut1 and HK2 protein expression, IHC was performed using
high and low 18F-FDG uptake tumors. A stronger
expression of HK2 protein was noted in tumors with high
18F-FDG uptake, as compared with that in tumors with low
18F-FDG uptake (Fig. 4),
in the DEN and HBx-Tg models. On the other hand, comparable
expression levels of Glut1 were observed in the two types of tumors
(Table I and Fig. 4).
 | Table ISummary of HCC models by
immunohistochemical analysis. |
Table I
Summary of HCC models by
immunohistochemical analysis.
| Models | 18F-FDG
uptakea | Tumor type |
Differentiation-grade | HK2 | Glut1 |
|---|
| DEN | High | HCA | −b | +++ | + |
| Low | Not yet cancer
status | −b | ++ | + |
| HBx-Tg | High | HCC | Poor | +++ | + |
| Low | HCC | Well | + | + |
Discussion
Molecular imaging methods such as MRI, PET or
optical imaging may be applicable for the non-invasive detection of
induced tumors and monitoring of therapeutic effects. Early
diagnosis using imaging methods also provides information
associated with the mechanism of tumor development and progression
and may be used for monitoring the tumors developed in internal
organs, including the liver, colon or brain, as well as metastatic
tumors (23,27).
Schmid et al (28) previously reported on the growth
kinetics of DEN-induced liver tumors in mice analyzed using 7-T MRI
without contrast agent. They identified that 18F-FDG PET
is not suitable for detection of liver tumors because of the
non-specific signals obtained from highly perfused livers. However,
18F-FDG PET/CT imaging was previously successfully
applied in the study of liver tumors in a c-myc transgenic
murine model to monitor the effects of DEN as an enhancer of tumor
growth and metastatic spread (29).
Recently, Heijink et al (30) investigated the use of
18F-FDG PET for the longitudinal monitoring of
Apc mutant mice that had developed multiple colorectal
adenomas. Authors of that study were able to detect abdominal hot
spots reflecting metabolically active intestinal adenomas with
diameters <2 mm.
In the present study, we induced HCC in mice by DEN
treatment or by transgenic expression of the HBx oncoprotein. We
determined the location, boundary, number and size of multiple
tumor nodules using a 3-T clinical MRI unit and 18F-FDG
small animal PET/CT imaging. Our data demonstrated that multimodal
imaging techniques can be successfully applied to evaluate and
monitor tumor progression and that 18F-FDG PET allows
longitudinal monitoring of tumor development.
Lee et al (31) previously applied functional genomics
to identify a best-fit murine model of human HCC from seven mouse
models, including two chemically induced, four transgenic and one
knockout model. Those authors concluded that the gene expression
patterns of HCC induced in c-myc- and transforming growth
factor-α-overexpressing transgenic mice and DEN-treated mice were
similar to those of HCC in humans with poor prognosis. Thus, the
present study made use of mouse models of HCC that mimic human HCC.
Moreover, HCCs induced in an HBx-Tg mouse model were analogous to
human HCCs with respect to histological findings (17).
DEN induces liver (19), gastrointestinal (32) and hematopoietic tumors (33) by alkylation of genomic DNA (23) and generation of reactive oxygen
species (24). Since HCCs induced
by DEN express α-fetoprotein (AFP), transgenic mice-expressing
reporter genes such as luciferase or HSV1-thymidine kinase under
the control of the AFP enhancer/promoter have previously been
applied in the study of DEN-induced hepatocarcinogenesis (34,35).
Kim et al (36) detected
DEN-induced AFP-positive HCC using systemic administration of a
recombinant adenovirus-expressing luciferase controlled by the AFP
promoter/enhancer.
HBV virus is a small hepatotropic DNA virus and its
carcinogenic effects on the liver are attributed to the
non-structural X protein (37) that
functions as a transcriptional activator of proteins, triggering
signaling cascades required for hepatocyte proliferation. Moreover,
the transgenic expression of HBx has been reported to induce liver
cancer in mice (17) and to promote
DEN-mediated hepatocarcinogenesis caused by the proliferation of
altered hepatocytes (38). The
present study is the first, to the best of our knowledge, to
characterize HBx-induced HCC using MRI and 18F-FDG
PET/CT, although HCCs induced by the expression of a c-myc
transgene in mice have also been characterized using
18F-FDG PET/CT or MRI (29,39).
Yu et al (17) reported that
grossly defined HCC was observed from the age of 11–18 months by
histologic investigation after sacrifice of HBx-Tg mice. However,
we readily and non-invasively detected HBx gene expression-induced
HCC by 18F-FDG PET from the age of 11 months (Fig. 1 and 2B). We have also demonstrated a
correlation between tumor sizes measured by MRI and
SUVmax of 18F-FDG PET/CT in chemical- and
oncogene-induced HCC models.
In humans, detection of HCC using 18F-FDG
PET varies depending on the histological differentiation grade.
Poorly differentiated HCC, which is associated with poorer
survival, exhibited a higher uptake of 18F-FDG than
low-grade HCC, which is associated with a higher expression of HK2
(10,11,40).
In the present study, the differentiation grade of HCC in the
HBx-Tg model as determined using H&E staining was shown for the
first time shown to correlate with 18F-FDG uptake
(Table I).
The present findings indicate the potential of
non-invasive multimodal imaging to study the pathogenesis of HCC
and develop highly sensitive and specific diagnostic techniques.
The animal models described in this study should also yield key
insights into the molecular basis of HCC and improve diagnosis and
therapy.
Acknowledgements
This study was supported by a Korea Science and
Engineering Foundation (KOSEF) grant funded by the Korean
government (MEST) (NRF-2012M2A2A7013480). The authors would like to
thank Kyungho Min for assistance with histological analysis.
References
|
1
|
Weissleder R: Scaling down imaging:
Molecular mapping of cancer in mice. Nat Rev Cancer. 2:11–18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kang JH and Chung JK: Molecular-genetic
imaging based on reporter gene expression. J Nucl Med. 49:164–179.
2008. View Article : Google Scholar
|
|
3
|
Kelloff GJ, Hoffman JM, Johnson B, et al:
Progress and promise of FDG-PET imaging for cancer patient
management and oncolytic drug development. Clin Cancer Res.
11:2785–2808. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Southworth R, Parry CR, Parkes HG, Medina
RA and Garlick PB: Tissue-specific differences in 2-f
luoro-2-deoxyglucose metabolism beyond FDG-6-P: a 19F NMR
spectroscopy study in the rat. NMR Biomed. 16:494–502. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okazumi S, Isono K, Enomoto K, et al:
Evaluation of liver tumours using fluorine-18-fluorodeoxyglucose
PET: characterization of tumor and assessment of effect of
treatment. J Nucl Med. 33:333–339. 1992.PubMed/NCBI
|
|
7
|
Khandani AH and Wahl RL: Applications of
PET in liver imaging. Radiol Clin North Am. 43:849–860. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trojan J, Schroeder O, Raedle J, et al:
Fluorine-18 FDG positron emission tomography for imaging of
hepatocellular carcinoma. Am J Gastroenterol. 94:3314–3319. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Delbeke D, Martin WH, Sandler MP, Chapman
WC, Wright JK Jr and Pinson CW: Evaluation of benign vs malignant
hepatic lesions with positron emission tomography. Arch Surg.
133:510–515. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan MA, Combs CS, Brunt EM, et al:
Positron emission tomography scanning in the evaluation of
hepatocellular carcinoma. J Hepatol. 32:792–797. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kudo M: Diagnostic imaging of
hepatocellular carcinoma: Recent Progress. Oncology. 81:73–85.
2011. View Article : Google Scholar
|
|
12
|
Paradis V: Histopathology of
hepatocellular carcinoma. Recent Results. Cancer Res. 190:21–32.
2013.
|
|
13
|
Kelland LR: Of mice and men: values and
liabilities of the athymic nude mouse model in anticancer drug
development. Eur J Cancer. 40:827–836. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frese KK and Tuveson DA: Maximizing mouse
cancer models. Nat Rev Cancer. 7:645–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thorgeirsson SS and Santoni-Rugiu E:
Transgenic mouse models in carcinogenesis: interaction of c-myc
with transforming growth factor alpha and hepatocyte growth factor
in hepatocarcinogenesis. Br J Clin Pharmacol. 42:43–52. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harada N, Oshima H, Katoh M, Tamai Y,
Oshima M and Taketo MM: Hepatocarcinogenesis in mice with
beta-catenin and Ha-Ras gene mutations. Cancer Res. 64:48–54. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu DY, Moon HB, Son JK, et al: Incidence
of hepatocellular carcinoma in transgenic mice expressing the
hepatitis B virus X-protein. J Hepatol. 31:123–132. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng Y, Chen WL, Louie SG, Yen TS and Ou
JH: Hepatitis B virus promotes hepatocarcinogenesis in transgenic
mice. Hepatology. 45:16–21. 2007. View Article : Google Scholar
|
|
19
|
Finnberg N, Stenius U and Högberg J:
Heterozygous p53-deficient (+/−) mice develop fewer p53-negative
preneoplastic focal liver lesions in response to treatment with
diethylnitrosamine than do wild-type (+ / +) mice. Cancer Lett.
207:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McGlynn KA, Hunter K, LeVoyer T, et al:
Susceptibility to aflatoxin B-1-related primary hepatocellular
carcinoma in mice and humans. Cancer Res. 63:4594–4601.
2003.PubMed/NCBI
|
|
21
|
Salguero Palacios R, Roderfeld M, Hemmann
S, et al: Activation of hepatic stellate cells is associated with
cytokine expression in thioacetamide-induced hepatic fibrosis in
mice. Lab Invest. 88:1192–1203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weisburger EK: Carcinogenicity studies on
halogenated hydrocarbons. Environ Health Perspect. 21:7–16. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Teoh NC, Dan YY, Swisshelm K, et al:
Defective DNA strand break repair causes chromosomal instability
and accelerates liver carcinogenesis in mice. Hepatology.
47:2078–2088. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qi Y, Chen X, Chan CY, et al:
Two-dimensional differential gel electrophoresis/analysis of
diethylnitrosamine induced rat hepatocellular carcinoma. Int J
Cancer. 122:2682–2688. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rao KV and Vesselinovitch SD: Age- and
sex-associated diethylnitrosamine dealkylation activity of the
mouse liver and hepatocarcinogenesis. Cancer Res. 33:1625–1627.
1973.PubMed/NCBI
|
|
26
|
Naugler WE, Sakurai T, Kim S, et al:
Gender disparity in liver cancer due to sex differences in
myd88-dependent IL-6 production. Science. 317:121–124. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sell S: Mouse models to study the
interaction of risk factors for human liver cancer. Cancer Res.
63:7553–7562. 2003.PubMed/NCBI
|
|
28
|
Schmid A, Rignall B, Pichler BJ and
Schwarz M: Quantitative analysis of the growth kinetics of
chemically induced mouse liver tumors by magnetic resonance
imaging. Toxicol Sci. 126:52–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hueper K, Elalfy M, Laenger F, et al:
PET/CT imaging of c-myc transgenic mice identifies the genotoxic
n-nitroso-diethylamine as carcinogen in a short-term cancer
bioassay. PLoS One. 7:e304322012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heijink DM, Kleibeuker JH, Nagengast WB,
et al: Total abdominal 18F-FDG uptake reflects
intestinal adenoma burden in Apc mutant mice. J Nucl Med.
52:431–436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JS, Chu IS, Mikaelyan A, et al:
Application of comparative functional genomics to identify best-fit
mouse models to study human cancer. Nat Genet. 36:1306–1311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Binato M, Kruel Schmidt M, Silveira
Volkweis B, et al: Mouse model of diethylnitrosamine-induced
gastric cancer. J Surg Res. 148:152–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gray R, Peto R, Brantom P and Grasso P:
Chronic nitrosamine ingestion in 1040 rodents: the effect of the
choice of nitrosamine, the species studied and the age of starting
exposure. Cancer Res. 51:6470–6491. 1991.PubMed/NCBI
|
|
34
|
Lu X, Guo H, Molter J, et al:
Alpha-fetoprotein-thymidine kinase-luciferase knock-in mice: a
novel model for dual modality longitudinal imaging of tumorigenesis
in liver. J Hepatol. 55:96–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park JH, Kim KI, Lee YJ, et al:
Non-invasive monitoring of hepatocellular carcinoma in transgenic
mouse with bioluminescent imaging. Cancer Lett. 310:53–60.
2011.PubMed/NCBI
|
|
36
|
Kim KI, Park JH, Lee YJ, et al: In vivo
bioluminescent imaging of α-fetoprotein-producing hepatocellular
carcinoma in the diethylnitrosamine-treated mouse using recombinant
adenoviral vector. J Gene Med. 14:513–520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koike K: Hepatitis B virus X gene is
implicated in liver carcinogenesis. Cancer Lett. 286:60–68. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Madden CR, Finegold MJ and Slagle BL:
Hepatitis B virus X protein acts as a tumor promoter in development
of diethyl-nitrosamine-induced preneoplastic lesions. J Virol.
75:3851–3858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Freimuth J, Gassler N, Moro N, et al:
Application of magnetic resonance imaging in transgenic and
chemical mouse models of hepatocellular carcinoma. Mol Cancer.
9:942010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee JD, Yang WI, Park YN, et al: Different
glucose uptake and glycolytic mechanisms between hepatocellular
carcinoma and intrahepatic mass-forming cholangiocarcinoma with
increased (18)F-FDG uptake. J Nucl Med. 46:1753–1759.
2005.PubMed/NCBI
|