Introduction
The large conductance calcium and voltage activated
potassium (BKCa) channels have been shown to function as oncogenes
in certain cancers (1–4). BKCa channels generate vast amounts of
outward K+ currents and therefore are powerful
modulators of the transmembrane potential of a cell. BKCa channels
are overexpressed in many types of cancers via gene amplification,
alternative splicing or increased protein half-life (5–8). In
addition, neoplastic BKCa channels may possess augmented
sensitivity to Ca2+ and voltage and hence generate
K+ currents in environments where their normal
counterparts are silent (5). The
enhanced activity of BKCa channels shifts cellular transmembrane
potential to favor proliferative phenotypes (9). It is therefore plausible for BKCa
channels to be considered putative targets for anticancer
therapies. The contributions of BKCa channels to cancer cell
migration and invasion have been previously demonstrated; however,
their role in tumorigenesis has not been investigated (1,3,10).
The present study presents novel findings that an
inhibitor of BKCa channels, iberiotoxin (IbTX), selectively
decreased anchorage-independent growth and tumorigenicity in breast
cancer cells expressing β-catenin. Our data suggest that the
attenuated tumorigenicity is a result of depolarizing shifts in
cell transmembrane potential and subsequent downregulation of
β-catenin and (phospho)Akt and HER-2/neu protein levels.
Materials and methods
Cell culture
UACC893, MDA-MB-231, SK-BR-3 and MCF10A cells were
purchased from ATCC (Manassas, VA, USA). Cells were propagated in
DMEM:F12 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented
with 20% fetal bovine serum (FBS).
Membrane potential assays
Cells were pre-loaded for 30 min with 2 μmol/l of a
membrane potential-sensitive dye DiBAC4(3) (Invitrogen, Life
Technologies, Carlsbad, CA, USA) in buffer containing (mmol/l): 20
HEPES, 140 NaCl, 2 KCl, 1 MgCl2, 2 CaCl2, 10
glucose prior to seeding onto mouse laminin (Sigma-Aldrich)-coated
glass bottom 35-mm tissue culture dishes (MatTek Corporation,
Ashland, MA, USA). After 24 h, dishes were placed on a microscope
stage pre-heated to 37°C, and 10 nmol/l IbTX (Sigma-Aldrich) was
added. Immediately following IbTX, cells were observed every minute
for 20 min using the 488 nm laser of an LSM 510 Zeiss confocal
scanning microscope (Zeiss, Jena, Germany). Signals from 5 to 7
regions of interest were quantified and averaged using LSM 510
image browser (Zeiss). Data were fitted using non-linear regression
analysis and significant differences estimated by ANOVA and post
hoc Bonferroni’s t-test (SigmaPlot; Systat Software, Inc., San
Jose, CA, USA). A P-value ≤0.05 was considered to indicate a
statistically significant result.
Cell viability assays
Cells were seeded into 6-well cluster dishes at
100,000 cells/well in DMEM:F12 supplemented with 20% FBS in the
absence or presence of IbTX (2, 5, 10, 25 and 50 nmol/l) for 48 h.
Subsequently, cells were stained with trypan blue and counted using
Countess Automated Cell Counter (Invitrogen). Experiments were
repeated three times, and statistical differences were assessed
using the Student’s t-test.
Soft agar assays
Soft agar assays were performed as previously
described (17). Briefly, following
IbTX incubations as described for cell viability assays, cells were
re-suspended in 0.3% agar in DMEM:F12 tissue culture medium
supplemented with 20% FBS. Cell suspensions were seeded onto 0.5%
basal agar in 6-well cluster dishes at 1,000 cells/well. The cell
colonies were visualized 21 days later using crystal violet
solution (crystal violet 0.005% and citric acid 0.1%) and manually
counted. Experiments were repeated at least twice in
quadruplicate.
Western immunoblotting
Whole cell lysates were isolated using RIPA buffer
containing (mmol/l) 150 NaCl, 50 Tris pH 7.4, 1 EDTA, 1% NP-40,
0.5% sodium deoxycholate, 0.1% SDS and protease and phosphatase
inhibitors. Twenty micrograms of protein was separated on 4–20%
Tris-HCl gels (Bio-Rad, Hercules, CA, USA), transferred on
nitrocellulose membranes, and blocked in 5% BSA for 1 h at room
temperature. Blots were then incubated with the rabbit
anti-HER-2/neu antibody (1:500 EMD; Millipore, Billerica, MA, USA),
rabbit β-catenin antibody (1:500), rabbit T308 phospho-Akt or total
Akt antibody (both from Cell Signaling Technology, Danvers, MA,
USA) overnight at 4°C. Signals were detected with IRDye 680
secondary antibodies (1:5,000) for 1 h at room temperature and
visualized using LI-COR Odyssey Imaging System (both from LI-COR,
Lincoln, NE, USA). Blots were subsequently incubated with mouse
GAPDH antibody (1:1,000; Sigma) and anti-mouse IRDye 800CW
secondary antibody (1:5,000; LI-COR) to ensure equal protein
loading. N=3.
RNA isolation and qPCR
Total mRNA was isolated using the mirVana miRNA
isolation kit (Ambion, Life Technologies, Carlbad, CA, USA).
Following isolation, the NanoDrop M-1000 was used to determine the
concentration and the quality of the total RNA. A reverse
transcription was performed using the SuperScript III First-Strand
Synthesis kit (Invitrogen, Life Technologies) with 700 ng of total
RNA. The gene expression was then determined with a SYBR-Green PCR
assay (Applied Biosystems, Life Technologies, Carlbad, CA, USA) and
run on the Applied Biosystems Model 7900 Genetic Analyzer. The data
were normalized to the endogenous control 18s rRNA and analyzed
using the program SDS 2.1 (Applied Biosystems, Life Technologies).
Comparison of gene expression between the breast cancer MDA-MB-231,
SK-BR-3 and UACC893 cells and the normal mammary MCF10A cells were
completed using the ΔΔCt method. Primer sequences are available
upon request.
Fluorescent immunocytochemistry
Cells seeded onto mouse laminin-coated glass bottom
35-mm tissue culture dishes for 24 h were fixed in 2%
paraformaldehyde for 30 min and permeabilized in 0.1% Triton X-100
for 4 min. Cells were blocked in phosphate-buffered saline (PBS)
supplemented with 5% FBS + 1% NDS for 30 min at 37°C and incubated
with the rabbit anti-BKCa antibody (Cell Signaling Technology)
diluted 1:500 in 0.1% blocking buffer for 30 min at 37°C. The
signal was detected using the donkey anti-rabbit DyLight 488
antibody (Jackson ImmunoResearch, West Grove, PA, USA) 1:1,000 in
0.1% blocking buffer for 15 min at 37°C. Cell nuclei were
counterstained with propidium iodide (5 mg/ml 1:10,000) in PBS for
5 min at room temperature. Cells were visualized with a Zeiss 510
laser confocal microscope, and images were reconstructed using LSM
Image Browser (both from Jena, Germany).
Results
BKCa channels contribute to resting
transmembrane potential
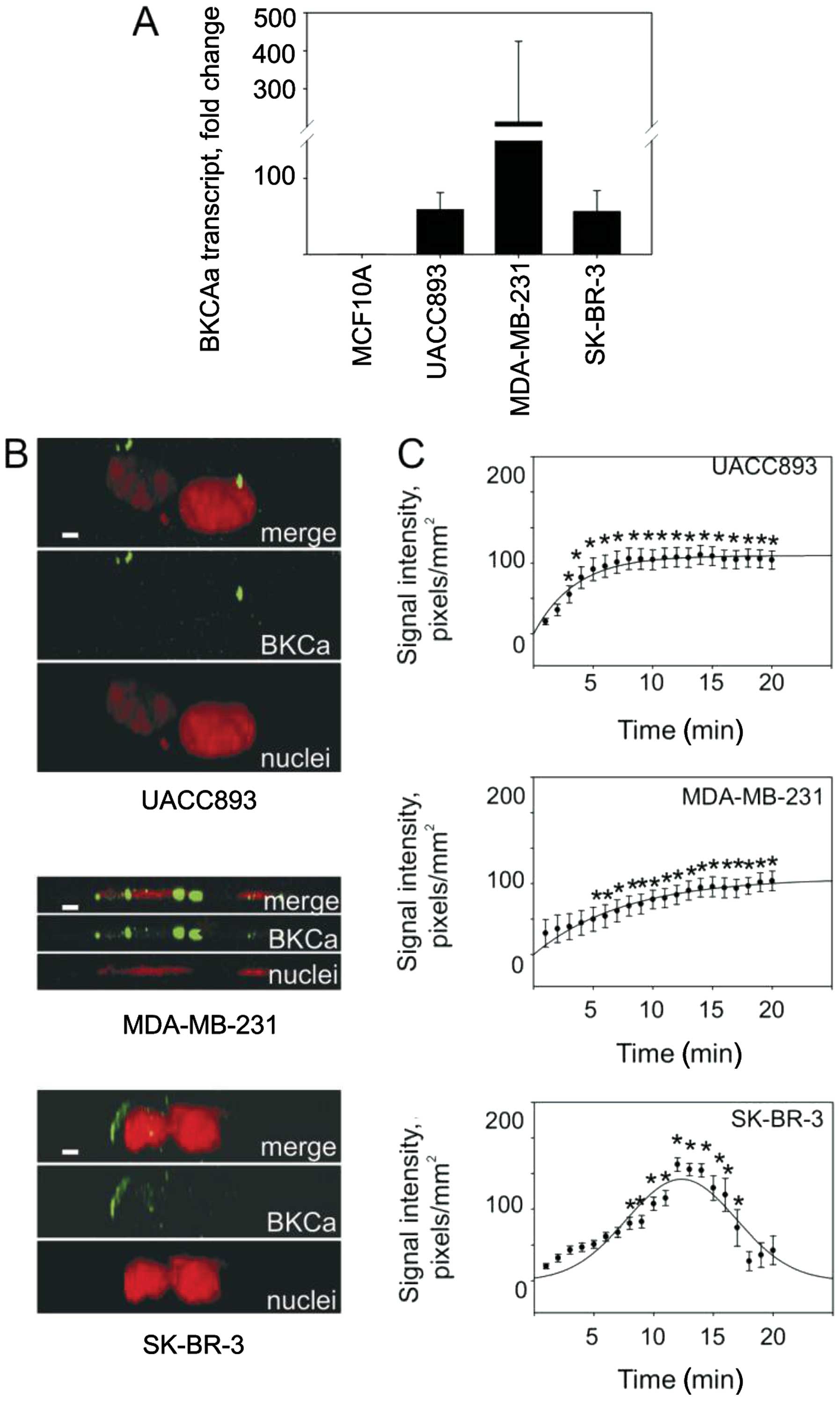
We began by using RT-PCR assays to establish the
levels of BKCa channel mRNA in several types of malignant and
non-malignant cell lines. We detected elevated levels of BKCa
channel mRNA in breast cancer cell lines (UACC893, SK-BR-3 and
MDA-MB-231) compared to these levels in the non-neoplastic MCF10A
cells (Fig. 1A). BKCa channel
proteins, detected using fluorescent immunocytochemistry, formed
typical clusters on plasma membranes in all breast cancer cell
models (Fig. 1B). Observations of
live cells loaded with a membrane potential sensitive dye revealed
that the selective BKCa channel inhibitor IbTX elicited cellular
depolarization indicative of a presence of functional channels.
Indeed, IbTX depolarized cells in all three models albeit to a
different magnitude (Fig. 1C). The
UACC893 cells were significantly depolarized within 4 min after
IbTX addition, after which depolarization plateaued for the
duration of the experiment. The MDA-MB-231 cells did not acquire a
plateau phase but rather steadily increased their transmembrane
potential throughout the 20-min observation period. SK-BR-3 cells
were depolarized only transiently. The emergence of depolarizing
phases, despite differences in duration, suggests that the BKCa
channels regulate the resting transmembrane potential of all three
breast cancer cell lines examined.
BKCa channel inhibition selectively
modulates anchorage-independent growth
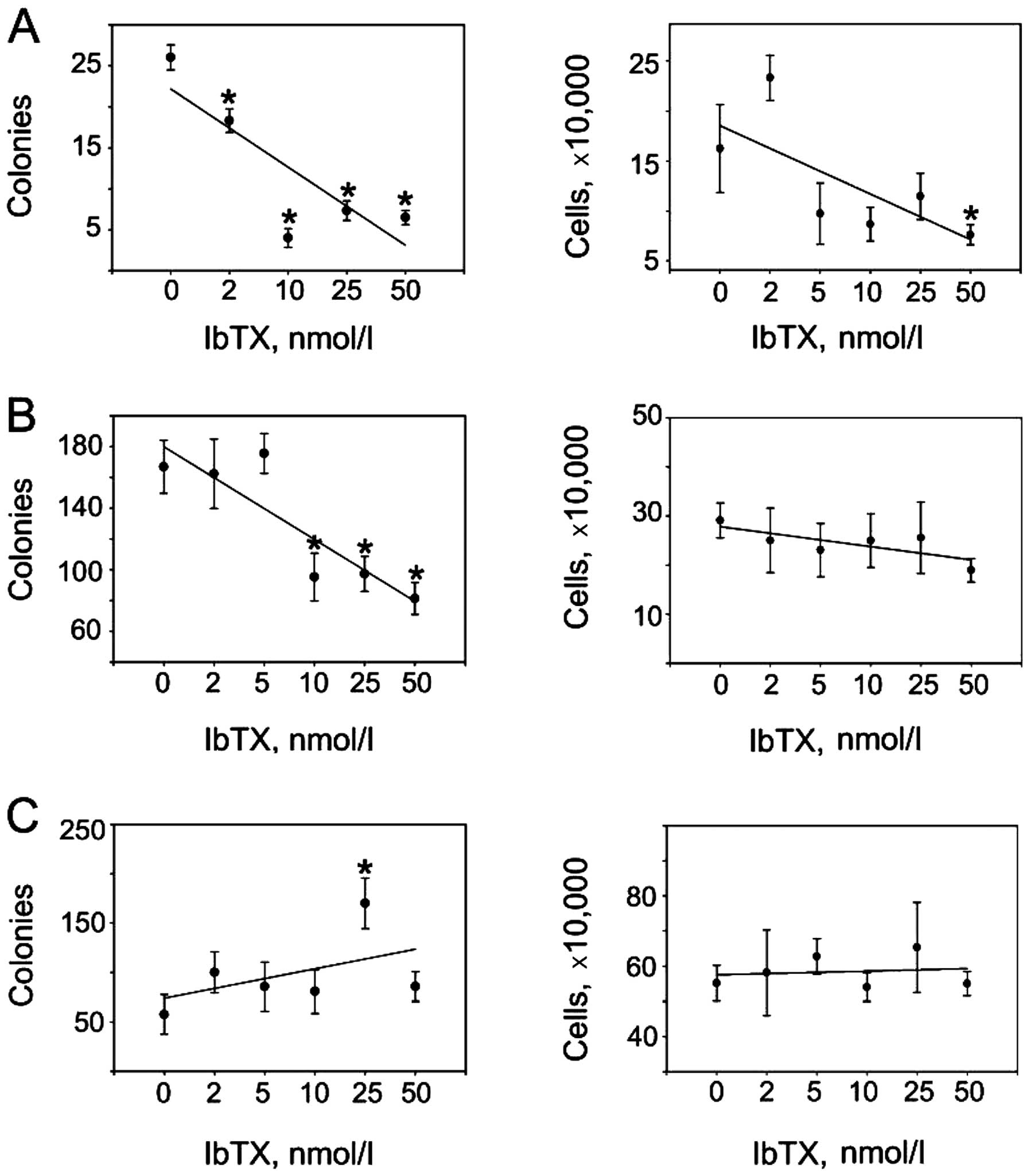
Given that BKCa channels were expressed and
functional in our cancer cell lines, we asked whether
pharmacological inhibition of these channels could lessen malignant
phenotypes by impacting the ability of cells to proliferate,
survive and/or form tumors in vitro. We found IbTX to
attenuate de novo cell colony development in the UACC893
cells (Fig. 2A, left upper panel)
with minimal inhibitory effects on anchorage-dependent cell
proliferation (Fig. 2A, right upper
panel). A similar dynamic was observed in the MDA-MB-231 cells
where clonogenic growth (Fig. 2B,
left middle panel) but not anchorage-dependent proliferation
(Fig. 2B, right middle panel) was
inhibited by IbTX. On the contrary, IbTX was ineffective in
modulating colony formation (Fig.
2C, left lower panel) or proliferation (Fig. 2C, right lower panel) of SK-BR-3
cells. Thus, IbTX appeared to specifically reduce the tumorigenic
features of the neoplastic phenotype in selected breast cancer
models.
BKCa channel inhibition modulates
oncogenic pathways
Both UACC893 and SK-BR-3 cells express the HER-2/neu
gene. Thus, it was possible that disparate trends in their
tumorigenic potential following IbTX treatment may have resulted
from IbTX-induced modulations of oncoprotein levels. HER-2/neu
levels decreased in the UACC893 cells at growth-inhibiting IbTX
concentrations (Fig. 3A). By
contrast, SK-BR-3 cells, which express both the long and short
isoforms of HER-2/neu, demonstrated increased HER-2 levels after
IbTX addition (Fig. 3A) (11). However, changes in HER-2/neu
oncoprotein expression cannot account for the attenuated
tumorigenic ability of triple-negative MDA-MB-231 cells, thus
suggesting alternative mechanisms of growth regulation.
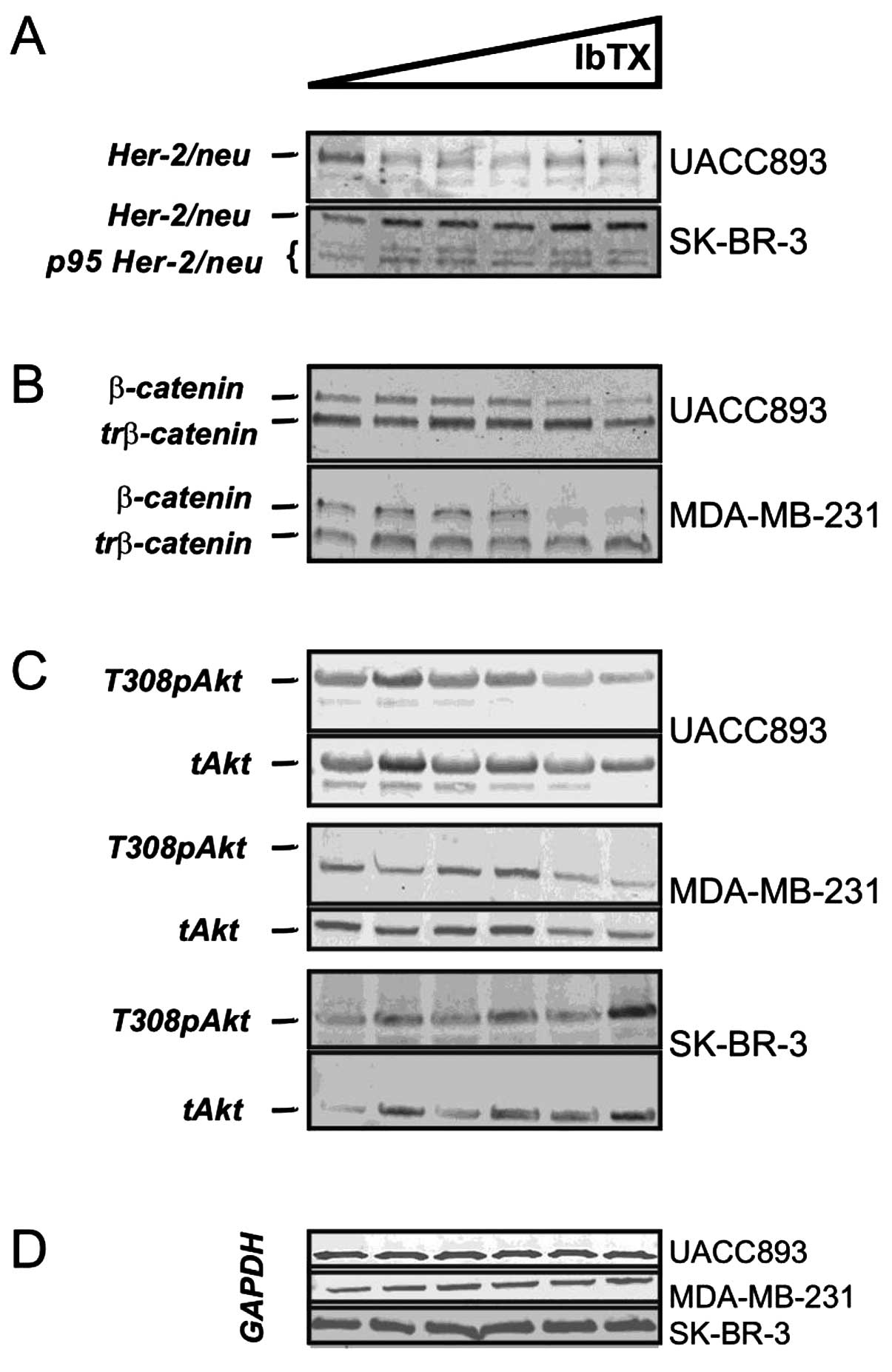 | Figure 3IbTX differentially regulates
oncogenic pathways. (A) IbTX attenuated the HER-2/neu levels in the
UACC893 cells (HER-2/neu, UACC893). SK-BR-3 cells
express full length (HER-2/neu, SK-BR-3) and
truncated (p95 HER-2/neu, SK-BR-3) oncoprotein
isoforms with both being increased by IbTX. (B) IbTX downregulated
the full length (β-catenin) but not truncated
(trβ-catenin) β-catenin isoforms in the UACC893 and
MDA-MB-231 cells. (C) In the UACC893 cells, IbTX attenuated
phosphorylated (T308pAkt, UACC893) but not total Akt
(Akt, UACC893). Both phosphorylated (T308pAkt,
MDA-MB-231) and total (Akt, MDA-MB-231) Akt
were decreased in the MDA-MB-231 cells with IbTX. In SK-BR-3 cells,
phosphorylated (T308pAkt, SK-BR-3) and total
(Akt, SK-BR-3) Akt increased at high IbTX
concentrations. (D) GAPDH was used in the UACC893, MDA-MB-231 and
SK-BR-3 cell immunoblotting to ensure equal protein loading. IbTX,
iberiotoxin. |
Upon further investigation, we found that cells with
reduced tumorigenicity expressed β-catenin (Fig. 3B, UACC893 and MDA-MB-231).
IbTX-resistant SK-BR-3 cells were β-catenin-negative (data not
shown); these findings are consistent with earlier reports
(12). In UACC893 and MDA-MB-231
cells, both long and truncated isoforms of β-catenin were
identified (Fig. 3B, β-catenin and
trβ-catenin), although IbTX decreased expression of only the long
isoform (90 kDa) (13). The protein
kinase Akt is known to phosphorylate β-catenin and regulate its
activity (14). We therefore
examined Akt levels in UACC893 and MDA-MB-231 cells treated with
IbTX. In the UACC893 cells, T308 phospho-Akt was diminished with
increasing concentrations of IbTX; in MDA-MB-231 cells both total
and phospho-Akt were downregulated, concordant with β-catenin
levels. In contrast, SK-BR-3 cells demonstrated fluctuating levels
of phospho- and total-Akt that increased at higher IbTX
concentrations (Fig. 3C). Hence,
β-catenin-dependent pathway(s) may mediate the inhibitory actions
of IbTX with regard to clonogenic potential in breast cancer
cells.
Discussion
BKCa channels have previously been proposed as
targets for anticancer therapies in brain and prostate cancers due
to their presumptive pro-oncogenic roles. However, their
contributions to the pathogenesis of hormone-independent breast
cancers are not well understood (1). The present study implies that BKCa
channels may exert either pro- or anti-growth effects, depending on
the particular molecular composition of the cancer cell type in
question.
The growth-modulating roles of BKCa channels became
apparent in our experiments when we explored anchorage-independent
cell growth. These findings are in accord with earlier reports that
found the BKCa channels modulated the tumorigenic potential of
subpopulations of cancer cells (15). Molecular studies provided insights
into the mechanisms used by BKCa channels to differentially
regulate the tumorigenic properties of breast cancer cells. IbTX is
a potent and selective BKCa channel inhibitor with minimal
off-target effects at concentrations within the inhibitor’s
selectivity range (16). In
addition, cells with reduced tumorigenicity, i.e. UACC893 and
MDA-MB-231, uniformly developed sustained transmembrane
depolarization when IbTX was present. It is therefore plausible
that suppression of oncogene(s) levels, and hence tumor formation,
are secondary events occurring subsequent to sustained increases in
transmembrane potential.
Notably, we also observed that BKCa channel
downregulation via siRNAs did not recapitulate the inhibitory
actions of IbTX on in vitro tumorigenesis despite
satisfactory transfection levels (data not shown). These findings
lend further credence to the importance of transmembrane potential
for tumorigenesis as siRNAs are far less efficient in inhibiting
K+ currents compared to IbTX (17). TEA, a non-selective blocker of
K+ channels and depolarizing agent, has been shown to
hamper in vitro endometrial tumorigenesis akin to IbTX in
breast cancer models (18). A
single discrepancy, namely the enhanced tumorigenic activity upon
TEA washout that did not occur with IbTX, was due to the lower
dissociation constant of IbTX compared to TEA, thus ensuring IbTX
retention in soft agar (18).
Hence, inhibitors with dissimilar chemical and pharmacological
profiles that share similar depolarizing actions can equivalently
modulate anchorage-independent growth in disparate cancer models,
suggesting that transmembrane potential mediates their inhibitory
effects.
In the present study, tumor cell lines that showed
suppressed in vitro colony growth in the presence of IbTX
shared positivity for β-catenin. Thus, it is possible that a
canonical Wnt pathway could mediate the anti-tumorigenic actions of
IbTX. BKCa/β-catenin complexes have been reported in cells of
neural origin and in overexpression systems where β-catenin
determines BKCa channel surface expression and clustering (19). However, in our experiments, IbTX
downregulated β-catenin levels without significantly affecting the
expression patterns of the BKCa channels. The observed differences
were not due to the presence of BKCa splice variants with
alterations in the β-catenin binding S10 domain (19). Moreover, β-catenin-negative SK-BR-3
cells not only have BKCa channels present on the cell surface, but
also respond to IbTX with β-catenin-independent increases in the
expression of BKCa channels. These findings imply that breast
cancer cells and neural cells/heterologous systems utilize
disparate mechanisms to sustain BKCa channel expression. Canonical
Wnt signaling may be a prerequisite for the antitumor effects of
BKCa channel inhibitors; however, mechanisms other than BKCa/Wnt
interactions may also mediate these effects. Similarly,
differential regulation of HER-2/neu oncoprotein levels by IbTX
cannot be ascribed solely to previously reported HER-2neu/β-catenin
complexes at least in SK-BR-3 cells (20).
In conclusion, the present study suggests that: i)
BKCa channels function as oncogenes in β-catenin-positive breast
cancer cells; ii) they direct their oncogenic input towards
sustaining the tumorigenic ability of cancer cells; and iii)
inhibitors of BKCa channels may modulate in vitro
tumorigenesis via transmembrane depolarization.
Acknowledgements
This study was supported in part by the Biological
Sciences Funding Program of the Office of Vice-President for
Research of the University of Iowa, NIH R01CA99908 (K.K.L.) and NIH
R01-CA133114 (N.A.B.), and the Department of Obstetrics and
Gynecology Research Development Fund (K.K.L.). We thank Kristina W.
Thiel for assistance in the manuscript preparation. V.P.K. is
founder and CEO of HiBiotechnology, L.L.C. K.K.L. is a co-founder
of Immortagen, L.L.C.
References
|
1
|
Khaitan D, Sankpal UT, Weksler B, Meister
EA, Romero IA, Couraud PO and Ningaraj NS: Role of KCNMA1 gene in
breast cancer invasion and metastasis to brain. BMC Cancer.
9:2582009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tajima N, Itokazu Y, Korpi ER, Somerharju
P and Käkelä R: Activity of BKCa channel is modulated by
membrane cholesterol content and association with
Na+/K+-ATPase in human melanoma IGR39 cells.
J Biol Chem. 286:5624–5638. 2011. View Article : Google Scholar :
|
|
3
|
Mazar J, DeYoung K, Khaitan D, Meister E,
Almodovar A, Goydos J, Ray A and Perera RJ: The regulation of
miRNA-211 expression and its role in melanoma cell invasiveness.
PLoS One. 5:e137792010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bloch M, Ousingsawat J, Simon R, Schraml
P, Gasser TC, Mihatsch MJ, Kunzelmann K and Bubendorf L: KCNMA1
gene amplification promotes tumor cell proliferation in human
prostate cancer. Oncogene. 26:2525–2534. 2007. View Article : Google Scholar
|
|
5
|
Liu X and Chang Y, Reinhart PH, Sontheimer
H and Chang Y: Cloning and characterization of glioma BK, a novel
BK channel isoform highly expressed in human glioma cells. J
Neurosci. 22:1840–1849. 2002.PubMed/NCBI
|
|
6
|
Lu R, Alioua A, Kumar Y, Eghbali M,
Stefani E and Toro L: MaxiK channel partners: physiological impact.
J Physiol. 570:65–72. 2006. View Article : Google Scholar
|
|
7
|
Tian L, McClafferty H, Chen L and Shipston
MJ: Reversible tyrosine protein phosphorylation regulates large
conductance voltage- and calcium-activated potassium channels via
cortactin. J Biol Chem. 283:3067–3076. 2008. View Article : Google Scholar
|
|
8
|
So EC, Wu KC, Liang CH, Chen JY and Wu SN:
Evidence for activation of BKCa channels by a known
inhibitor of focal adhesion kinase, PF573228. Life Sci. 89:691–701.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sundelacruz S, Levin M and Kaplan DL: Role
of membrane potential in the regulation of cell proliferation and
differentiation. Stem Cell Rev. 5:231–246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wondergem R and Bartley JW: Menthol
increases human glioblastoma intracellular Ca2+, BK
channel activity and cell migration. J Biomed Sci. 16:902009.
View Article : Google Scholar
|
|
11
|
Arribas J, Baselga J, Pedersen K and
Parra-Palau JL: p95HER2 and breast cancer. Cancer Res.
71:1515–1519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He B, You L, Uematsu K, Xu Z, Lee AY,
Matsangou M, McCormick F and Jablons DM: A monoclonal antibody
against Wnt-1 induces apoptosis in human cancer cells. Neoplasia.
6:7–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyoshi K and Hennighausen L: β-Catenin: a
transforming actor on many stages. Breast Cancer Res. 5:63–68.
2003. View
Article : Google Scholar
|
|
14
|
Fang D, Hawke D, Zheng Y, Xia Y,
Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T and Lu Z:
Phosphorylation of β-catenin by AKT promotes β-catenin
transcriptional activity. J Biol Chem. 282:11221–11229. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park JH, Park SJ, Chung MK, Jung KH, Choi
MR, Kim Y, Chai YG, Kim SJ and Park KS: High expression of
large-conductance Ca2+-activated K+ channel
in the CD133+ subpopulation of SH-SY5Y neuroblastoma
cells. Biochem Biophys Res Commun. 396:637–642. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Candia S, Garcia ML and Latorre R: Mode of
action of iberiotoxin, a potent blocker of the large conductance
Ca(2+)-activated K+ channel. Biophys J. 63:583–590. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shimazu K, Takeda K, Yu ZX, Jiang H, Liu
XW, Nelson PG and Guroff G: Multiple acute effects on the membrane
potential of PC12 cells produced by nerve growth factor (NGF). J
Cell Physiol. 203:501–509. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schickling BM, Aykin-Burns N, Leslie KK,
Spitz DR and Korovkina VP: An inhibitor of K+ channels
modulates human endometrial tumor-initiating cells. Cancer Cell
Int. 11:252011. View Article : Google Scholar
|
|
19
|
Bian S, Bai JP, Chapin H, Le Moellic C,
Dong H, Caplan M, Sigworth FJ and Navaratnam DS: Interactions
between β-catenin and the HSlo potassium channel regulates HSlo
surface expression. PLoS One. 6:e282642011. View Article : Google Scholar
|
|
20
|
Schroeder JA, Adriance MC, McConnell EJ,
Thompson MC, Pockaj B and Gendler SJ: ErbB-β-catenin complexes are
associated with human infiltrating ductal breast and murine mammary
tumor virus (MMTV)-Wnt-1 and MMTV-c-Neu transgenic carcinomas. J
Biol Chem. 77:22692–22698. 2002. View Article : Google Scholar
|