Introduction
Lung metastasis is the leading cause of
cancer-related death worldwide. Although treatment methods such as
surgery, irradiation and chemotherapy have improved, the prognosis
of patients with lung metastasis remains unsatisfactory, and new
therapeutic strategies are needed. Immunotherapy may represent a
new therapeutic strategy for lung metastasis (1,2). The
goal of immunotherapy for lung metastasis is to enhance the
weakened host immune response against tumors using specific and/or
non-specific immune stimulants.
α-galactosylceramide (GalCer) is a glycolipid that
binds to CD1d, and it is recognized by invariant natural killer T
(iNKT) cells. Injection with GalCer enhances the activation of NKT
cells, resulting in the rapid release of both Th1 and Th2
cytokines, thereby eliciting a downstream cascade of activation
that spreads to dendritic, NK and B cells (3–5). These
activation cascades powerfully induce adaptive immunity. Recent
studies have revealed the mechanism of GalCer-induced iNKT cell
activation in immune responses to tumors and microbes, as well as
in the suppression of autoimmune diseases (6–9).
Toll-like receptors (TLRs) recognize specific
molecular signatures called pathogen-associated molecular patterns
that are present in pathogens. Members of the TLR ligand class of
adjuvants, including Pam3Cys (TLR-2), poly-IC (TLR3),
lipopolysaccharide (LPS) (TLR4), imiquimod (TLR7) and CpG-ODN
(TLR9), induce antigen-presenting cell maturation and the
production of inflammatory cytokines, favoring effector T cell
responses and restricting Treg expansion (10), indicating that TLR signaling induces
the activation of immune responses. TLR3 agonists with variable
efficiency have been used previously as adjuvants in the treatment
of cancer, with the aim of inducing an IFN-mediated antitumor
immune response (11). TLR4
stimulation can overcome CD8+ T cell tolerance and
eradicate established tumors (12).
TLR7 and TLR9 ligands induce cancer cell death by enhancing
cytotoxic NK and CD8+ T cell activation of
anti-angiogenic factors and by promoting the production of
anti-angiogenic factors (13). The
antitumor effects of TLR activation in diverse cancer subtypes are
postulated to proceed via several parallel mechanisms, including
potentiating innate immune responses via activation of NK cells,
monocytes and macrophages; inducing the generation of tumoricidal
cytokines; inducing Th1 deviation of CD4+ T cells;
augmenting CTLs; and directly inducing apoptosis in TLR-expressing
tumor cells (14).
Thus, GalCer and TLR ligands have been used to treat
various types of cancers in the basic and clinical settings.
However, these therapies have had limited success against human
cancers in many studies. We recently reported that GalCer and a TLR
agonist synergistically enhanced the production of interferon-γ
(IFN-γ) and induced a robust immunological response in mice
(15–18). Facilitation of IFN-γ production
indicates the enhancement of Th1 immune responses in hosts
(6,19,20).
Therefore, combination therapy with GalCer and a TLR agonist
induces a robust Th1 immune response in tumor-bearing hosts and
suppresses tumor growth. In the present study, we demonstrated that
combination therapy with GalCer and a TLR ligand suppressed tumor
growth in a mouse lung-metastasis model.
Materials and methods
Mice
Male BALB/c mice at the age of ~8 weeks were
obtained from Japan SLC Inc. (Hamamatsu, Japan). All animal
procedures were conducted in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals and the Guidelines for the Care and Use of Animals
established by the Animal Ethics Committee of Gifu University.
Cell lines and reagents
CT26 cells (mouse colon carcinoma) used in the
present study were generously provided by Dr Hidekazu Shirota
(Laboratory of Experimental Immunology, Cancer and Inflammation
Program, the National Cancer Institute, Frederick, MD, USA).
Synthesized GalCer was obtained from Funakoshi, Co., Ltd. (Tokyo,
Japan). LPS from Escherichia coli O111:B4 was purchased from
Sigma-Aldrich (St. Louis, MO, USA). CFSE was purchased from
BioLegend (San Diego, CA, USA).
Tumor challenge and therapy
Mice were intravenously injected with
7.5×104 CT26 colon carcinoma cells and after 5 days,
GalCer (1 μg/mouse) was administered into the tumor-bearing mice.
On the next day, LPS (1 μg/mouse) was injected intravenously. Five
days following LPS injection, mice were sacrificed, and their lungs
were removed to count superficial metastatic nodules.
Enzyme-linked immunospot (ELISPOT)
assay
Tumor-bearing mice were treated with LPS and GalCer,
either alone or in combination. Single-cell suspensions were
prepared from the mediastinal lymph nodes (MLNs) on day 1 after the
inoculation. A total of 2.0×105 lymphocytes/well were
stimulated for 14–16 h with 0, 0.1 or 1 μg/ml of AH-1 peptide
(Medical and Biological Laboratories Co., Ltd., Nagoya, Japan) in
96-well MultiScreen filter plates (Millipore, Billerica, MA, USA)
pre-coated with a monoclonal rat anti-IFN-γ antibody (R4-6A2; BD
Biosciences, Franklin Lakes, NJ, USA). The plates were washed and
then incubated with a biotinylated polyclonal goat anti-IFN-γ
antibody (R&D Systems, Minneapolis, MN, USA) followed by
incubation with streptavidin-alkaline phosphatase. Spots were
visualized by the addition of a 5-bromo-4-chloro-3-indolyl
phosphate solution (Sigma-Aldrich) and counted manually under a
microscope (magnification, ×40). The number of cytokine-secreting
cells was determined by a single observer in a blinded manner, and
all data were generated by analyzing 3 separate wells/sample.
Flow cytometry
Lymphocytes were isolated from the MLNs and lungs of
tumor-bearing mice 1 day after LPS injection with or without GalCer
treatment. Flow cytometry was used to evaluate the expression
levels of CD4, CD8, Foxp3, CD69 and CXCR3. The isolated cells were
stained with allophycocyanin (APC)-conjugated anti-mouse CD4 (clone
RM 4-5), fluorescein isothiocyanate (FITC)-conjugated anti-mouse
CD8 (clone 53-6.7), phycoerythrin (PE)-Cy7-conjugated anti-mouse
CD69 (clone H1.2F3), PE-conjugated anti-mouse Foxp3 (clone FJK-16s)
and APC-conjugated anti-mouse CXCR3 (clone CXCR3-173)
(eBiosocience). The stained cells were analyzed using a FACSCanto
II instrument (BD Biosciences, San Jose, CA, USA).
Quantitative reverse
transcription-polymerase chain reaction (RT-qPCR) analysis
RT-qPCR was used to quantify the mRNA expression
levels of various genes in the MLNs. Total RNA was isolated using
Isogen II reagent (Nippon Gene, Tokyo, Japan), and it was
transcribed into cDNA using a High-Capacity cDNA transcription kit
(Applied Biosystems, Foster City, CA, USA). Purified cDNA was used
as the template for RT-qPCR conducted by using pre-designed
primer/probe sets for IFN-γ, FasL, granzyme B, CXCL9, CXCL10 and
18S rRNA (Applied Biosystems) according to the manufacturer’s
instructions. 18S rRNA was used as an internal control. RT-qPCR was
conducted using a LightCycler Rapid Thermal Cycler system (Roche
Diagnostic Systems, Inc., Indianapolis, IN, USA).
Cytotoxicity assay
The cytotoxicity assay was performed as previously
described (21). For the in
vitro cytotoxicity assay, effector cells were obtained from the
MLNs of mice with lung metastasis that were stimulated with GalCer
and/or LPS. Effector cells were seeded on round-bottom 96-well
plates at an effector to target ratio of 20:1 with
1.5×105 high-labeled target cells. Control cells
consisting of 1.5×105 low-labeled CT26 tumor cells only
were incubated at 37°C for 4 h.
CD8+ T cell depletion
Mice were depleted of CD8+ T cells by an
intraperitoneally injection of 200 μg anti-CD8 mAb (clone 2.43;
BioXcell, West Lebanon, NH, USA), respectively, on days 4 and 7
after CT26 injection. Combination therapy was administered on day 5
and 6 following CT26 injection. Nine days after the combination
therapy, mice were sacrificed and their lungs were removed to count
superficial metastatic nodules.
Statistical analysis
Results are expressed as means ± SEM. The
statistical significance of differences between the experimental
groups was analyzed using the Kruskal-Wallis test followed by the
Scheffe F-test. Significance was established at p<0.05.
Results
GalCer and LPS treatment suppresses the
growth of tumors in a mouse lung-metastasis model
We examined the antitumor effect of the combination
therapy with GalCer and LPS in a mouse lung-metastasis model
(Fig. 1A and B). Mice were
intravenously injected with 1×105 CT26 colon carcinoma
cells, and after 5 days, GalCer and LPS were administered to mice
presenting with lung metastasis. The mice were then sacrificed to
count the tumor metastatic nodules on the lung surface 5 days after
treatment. Compared with no treatment or LPS or GalCer monotherapy,
the combination therapy with GalCer and LPS significantly reduced
the number of nodules on the lungs. Next, the mRNA expression
levels of IFN-γ, FasL and granzyme B in the lungs of tumor-bearing
mice treated with LPS and/or GalCer were measured by performing
real-time RT-qPCR (Fig. 1C). The
combination treatment with LPS and GalCer significantly enhanced
the mRNA expression of IFN-γ and FasL in the lungs of the mice.
Combination therapy with GalCer and LPS
enhances tumor antigen-specific cellular immunity
To clarify the mechanism by which GalCer and LPS
augment antitumor activity, cells were isolated from the MLNs of
CT26 tumor-bearing mice. The cells were cultured with the AH-1
peptide, which is a CD8-restricted epitope expressed by CT26. We
observed that the number of IFN-γ-secreting MLN cells from the mice
treated with GalCer and LPS was significantly increased compared to
that of mice treated with GalCer or LPS alone (Fig. 2A). In contrast, the cells from the
lung tissue of mice exposed to any treatment secreted IFN-γ upon
stimulation with the AH-1 peptide (Fig.
2B). The MLN cells of mice treated with both GalCer and LPS
displayed increased tumor-specific cytotoxicity compared to the MLN
cells of the mice treated with GalCer or LPS alone (Fig. 2C). Thereby, the augmentation of the
antitumor effect by the combination therapy was induced by a tumor
antigen-specific cellular immune response in the MLNs.
Increased CD8+ T cell number
in the MLNs following GalCer and LPS stimulation
We subsequently examined the phenotype of the
lymphocytes in the MLNs of mice treated with GalCer and/or LPS. The
CD8+ T cell count in the MLNs from mice co-treated with
GalCer and LPS was significantly increased when compared to the
cell count in the non-treated mice or mice treated with GalCer or
LPS alone (Fig. 3A). There was no
significant difference in CD8+ T cell number
infiltrating the lungs (Fig. 3B).
The increase in CD8+ T cell number in the MLNs of the
mice co-treated with GalCer and LPS may contribute to the
enhancement of tumor antigen-specific immune responses in
tumor-bearing hosts.
Expression of chemokine mRNA and
chemokine receptors in MLNs stimulated by GalCer and LPS
GalCer and LPS combination therapy or GalCer
monotherapy enhanced chemokine mRNA expression in the MLNs
(Fig. 4A and B). Although GalCer
treatment induced mRNA expression of CXCL9 and CXCL10 in the MLNs,
GalCer monotherapy did not markedly enhance the antitumor activity
in the mouse lung-metastasis model. We hypothesized that the degree
of chemokine receptor expression differed between treatment with
GalCer alone and the co-treatment with GalCer and LPS. CXCR3 is the
chemokine receptor for CXCL9 and CXCL10, and is mainly expressed on
CD8+ T and NK cells. Therefore, we determined the number
of CD8 and CXCR3 double-positive T cells in the MLNs by performing
flow cytometric analysis. The expression of CXCR3 in the MLNs was
increased following GalCer and LPS combination therapy compared to
the expression in the MLNs following treatment with GalCer alone
(Fig. 4C). However, there was no
difference in the expression of CXCR3 on the spleen between the
combination therapy and the treatment of GalCer alone (Fig. 4D). Thus, GalCer treatment induced
the production of chemokines, and the combination therapy
maintained the CXCR3 expression in CD8+ T cells. The
enhancement of CXCL9 and CXCL10 expression in MLNs and CXCR3
expression on CD8+ T cells resulted in increased
CD8+ T cell numbers in the MLNs.
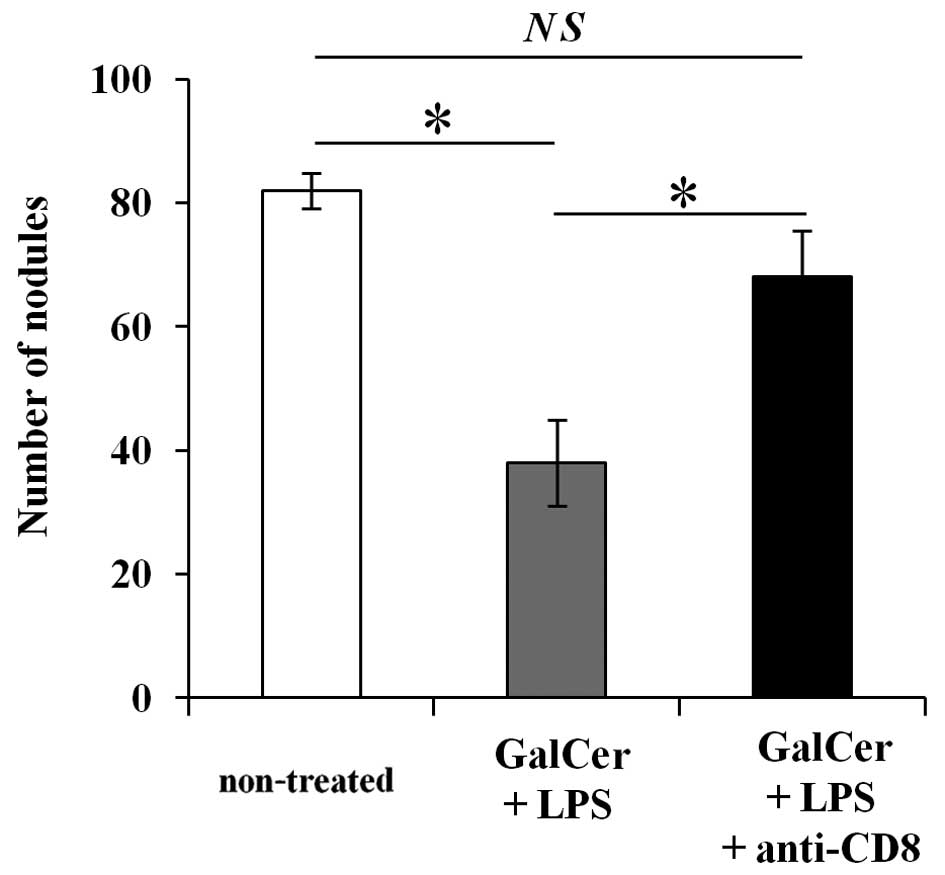
Increased CD8+ T cells are
essential for GalCer and LPS combination therapy
To determine whether the increase in CD8+
T cells are important for the observed inhibition of lung
metastasis by the combination therapy with GalCer and LPS, we
performed anti-CD8 Ab depletion experiment. As shown in Fig. 5, the depletion of CD8+ T
cells in the combination therapy restored tumor metastasis nodules.
These data indicated that the antitumor effect of the combination
therapy with GalCer and LPS was dependent on CD8+ T
cells.
Discussion
In the present study, we demonstrated that
co-treatment with GalCer and LPS enhanced tumor antigen-specific
immune responses in MLNs and suppressed tumor development in a
mouse model of lung metastasis. The increase in CD8+ T
cell numbers in MLNs contributed to the enhancement of tumor
antigen-specific immune responses. Moreover, our findings
illustrated that co-treatment with GalCer and LPS enhanced
chemokine and chemokine receptor expression in MLNs and stimulated
the recruitment of CD8+ T cells to the MLNs of
tumor-bearing mice.
Our previous studies revealed that co-treatment with
GalCer and a TLR agonist induced strong immune responses in
vitro and in vivo. In particular, GalCer and LPS
co-treatment strongly enhanced IFN-γ production in splenocytes
in vitro (18). In the
present study, co-treatment with GalCer and LPS enhanced mRNA
expression of IFN-γ in the lungs of tumor-bearing mice. It is well
known that the activation of IFN-γ production induces Th1 responses
in hosts (6,19,20).
Upon CD8 activation, NK and NKT cells can produce IFN-γ, and these
cells exert cytotoxic activity against pathogens, virus-infected
and tumor cells (22–24). Many basic and clinical studies have
evaluated IFN-γ production by host immune cells stimulated with
tumor antigens to determine the effects of various therapies in
tumor-bearing hosts (25–27). Therefore, the enhancement of IFN-γ
production leads to the induction of antitumor immunity in
tumor-bearing hosts. In the present study, GalCer and LPS
combination therapy significantly decreased the number of distinct
lung metastatic nodules (Fig. 1A and
B). Moreover, the results from the ELISPOT assay demonstrated
that the combination therapy enhanced tumor antigen-specific Th1
immune responses in tumor draining lymph nodes (Fig. 2A). However, antigen-specific
IFN-γ-producing cells did not increase in the lung after
combination therapy (Fig. 2B). In
the lung, alveolar macrophages are made up of a majority of
pulmonary lymphocytes. Therefore, the ratio of CD8+ T
cells in the lung cells using ELISPOT assay was extremely lower
than that in the MLNs. As a result, we may not well detect the
production of IFN-γ in lung single cell suspension. Moreover,
tumor-specific cytotoxicity increased in the tumor-bearing mice
treated with GalCer and LPS. Thus, the enhancement of tumor
antigen-specific immune responses may have decreased lung
metastasis in this model. Furthermore, the expression of IFN-γ and
FasL was enhanced by the combination therapy in the lungs, and the
increase in cytotoxic ability in the lungs led to the suppression
of tumor growth. In addition, CD8+ T cell numbers were
significantly elevated in the MLNs of mice treated with both GalCer
and LPS (Fig. 3), and this increase
may have been involved in the enhancement of antigen-specific
immune responses in the MLNs.
In previous studies, various cytokines and
chemokines induced by GalCer or LPS treatment contributed to the
induction of antitumor immunity (12,28,29).
CXCL9 and CXCL10 chemokines are also important for antitumor immune
responses (30–32). CXCL9 and CXCL10 mRNA expression was
enhanced by GalCer monotherapy or by the combination therapy
(Fig. 4A and B). CXCL9 and CXCL10
are IFN-γ inducible antiangiogenic chemokines, and they recruit
CXCR3+ cells (33–35).
In our previous report, co-treatment with GalCer and LPS markedly
induced IFN-γ production in murine splenocytes (18). The enhancement of IFN-γ production
by GalCer and LPS appears to be involved in the upregulation of
CXCL9 and CXCL10 expression. Furthermore, CXCR3 is the receptor for
CXCL9 and CXCL10, and it is expressed on T and NK cells. The
expression of CXCR3 is induced by GalCer or LPS via IFN-γ
stimulation (34). The expression
of CXCR3 was higher in the CD8+ T cells treated with
GalCer and LPS than in the CD8+ T cells treated with
GalCer alone. The number of CXCR3+/CD8+ cells
in the mice treated with GalCer alone was lower than that in the
mice treated with GalCer and LPS (Fig.
4C). Combination therapy with GalCer and LPS increased
chemokine (CXCL9 and CXCL10) expression without affecting the CXCR3
expression. As a result, CD8+ T cells accumulated in the
MLNs. The depletion of CD8+ T cells restored the number
of lung nodules (Fig. 5). Thus,
CD8+ T cells play a critical role in the development of
the antitumor effect induced by GalCer and LPS combination therapy.
In a previous study, CD8+ T cells contributed to optimal
tumor antigen-specific immune response. The progression of tumor
growth and metastasis was induced by CD8+ T cell
depletion (36). The increased
CD8+ T cells in MLNs may be essential for GalCer and LPS
combination therapy.
In conclusion, the combination therapy with GalCer
and LPS induced a tumor antigen-specific immune response and
antitumor activity in an established lung metastasis model. The
induction of the tumor antigen-specific immune responses was
depended on the upregulation of chemokine mRNA expression by GalCer
and the maintenance of CXCR3 expression by the combination therapy.
Our findings may provide insight for the design of new techniques
to prevent lung metastasis.
Acknowledgements
The authors thank Dr H.S. for kindly providing CT26
tumor cell lines in the present study. This study was supported by
a Grant-in-Aid for Scientific Research (C) (24890080) from the
Ministry for Education, Culture, Sports, Science and Technology of
Japan.
References
|
1
|
Saga K, Tamai K, Yamazaki T and Kaneda Y:
Systemic administration of a novel immune-stimulatory pseudovirion
suppresses lung metastatic melanoma by regionally enhancing IFN-γ
production. Clin Cancer Res. 19:668–679. 2013. View Article : Google Scholar
|
|
2
|
Purwar R, Schlapbach C, Xiao S, et al:
Robust tumor immunity to melanoma mediated by
interleukin-9-producing T cells. Nat Med. 18:1248–1253. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kronenberg M: Toward an understanding of
NKT cell biology: progress and paradoxes. Annu Rev Immunol.
23:877–900. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kronenberg M and Gapin L: The
unconventional lifestyle of NKT cells. Nat Rev Immunol. 2:557–568.
2002.PubMed/NCBI
|
|
5
|
Taniguchi M, Harada M, Kojo S, Nakayama T
and Wakao H: The regulatory role of Vα14 NKT cells in innate and
acquired immune response. Annu Rev Immunol. 21:483–513. 2003.
View Article : Google Scholar
|
|
6
|
Paget C, Chow MT, Duret H, Mattarollo SR
and Smyth MJ: Role of γδ T cells in α-galactosylceramide-mediated
immunity. J Immunol. 188:3928–3939. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tessmer MS, Fatima A, Paget C, Trottein F
and Brossay L: NKT cell immune responses to viral infection. Expert
Opin Ther Targets. 13:153–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tupin E, Kinjo Y and Kronenberg M: The
unique role of natural killer T cells in the response to
microorganisms. Nat Rev Microbiol. 5:405–417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu L and Van Kaer L: Natural killer T
cells and autoimmune disease. Curr Mol Med. 9:4–14. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perret R, Sierro SR, Botelho NK, Corgnac
S, Donda A and Romero P: Adjuvants that improve the ratio of
antigen-specific effector to regulatory T cells enhance tumor
immunity. Cancer Res. 73:6597–6608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salaun B, Coste I, Rissoan MC, Lebecque SJ
and Renno T: TLR3 can directly trigger apoptosis in human cancer
cells. J Immunol. 176:4894–4901. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davis MB, Vasquez-Dunddel D, Fu J,
Albesiano E, Pardoll D and Kim YJ: Intratumoral administration of
TLR4 agonist absorbed into a cellular vector improves antitumor
responses. Clin Cancer Res. 17:3984–3992. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spaner DE and Masellis A: Toll-like
receptor agonists in the treatment of chronic lymphocytic leukemia.
Leukemia. 21:53–60. 2007. View Article : Google Scholar
|
|
14
|
Ochi A, Graffeo CS, Zambirinis CP, et al:
Toll-like receptor 7 regulates pancreatic carcinogenesis in mice
and humans. J Clin Invest. 122:4118–4129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ito H, Koide N, Morikawa A, et al:
Augmentation of lipopolysaccharide-induced nitric oxide production
by α-galactosylceramide in mouse peritoneal cells. J Endotoxin Res.
11:213–219. 2005.
|
|
16
|
Ito H, Koide N, Hassan F, et al: Lethal
endotoxic shock using α-galactosylceramide sensitization as a new
experimental model of septic shock. Lab Invest. 86:254–261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohtaki H, Ito H, Ando K, et al: Vα14 NKT
cells activated by alpha-galactosylceramide augment
lipopolysaccharide-induced nitric oxide production in mouse
intra-hepatic lymphocytes. Biochem Biophys Res Commun. 378:579–583.
2009. View Article : Google Scholar
|
|
18
|
Ando T, Ito H, Ohtaki H and Seishima M:
Toll-like receptor agonists and alpha-galactosylceramide
synergistically enhance the production of interferon-gamma in
murine splenocytes. Sci Rep. 3:25592013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hayakawa Y, Takeda K, Yagita H, et al:
Critical contribution of IFN-γ and NK cells, but not
perforin-mediated cytotoxicity, to anti-metastatic effect of
α-galactosylceramide. Eur J Immunol. 31:1720–1727. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marth C, Fiegl H, Zeimet AG, et al:
Interferon-γ expression is an independent prognostic factor in
ovarian cancer. Am J Obstet Gynecol. 191:1598–1605. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakagawa Y, Watari E, Shimizu M and
Takahashi H: One-step simple assay to determine antigen-specific
cytotoxic activities by single-color flow cytometry. Biomed Res.
32:159–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seki S, Nakashima H, Nakashima M and
Kinoshita M: Antitumor immunity produced by the liver Kupffer
cells, NK cells, NKT cells, and CD8+ CD122+ T
cells. Clin Dev Immunol. 2011:8683452011. View Article : Google Scholar
|
|
23
|
Smith SM and Dockrell HM: Role of
CD8+ T cells in mycobacterial infections. Immunol Cell
Biol. 78:325–333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Exley MA, Lynch L, Varghese B, Nowak M,
Alatrakchi N and Balk SP: Developing understanding of the roles of
CD1d-restricted T cell subsets in cancer: reversing tumor-induced
defects. Clin Immunol. 140:184–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moreno M, Molling JW, von
Mensdorff-Pouilly S, et al: IFN-γ-producing human invariant NKT
cells promote tumor-associated antigen-specific cytotoxic T cell
responses. J Immunol. 181:2446–2454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang D, Precopio M, Lan T, et al:
Antitumor activity and immune response induction of a dual agonist
of Toll-like receptors 7 and 8. Mol Cancer Ther. 9:1788–1797. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lim JY, Gerber SA, Murphy SP and Lord EM:
Type I interferons induced by radiation therapy mediate recruitment
and effector function of CD8+ T cells. Cancer Immunol
Immunother. 63:259–271. 2014. View Article : Google Scholar
|
|
28
|
Choi DH, Kim KS, Yang SH, et al: Dendritic
cell internalization of α-galactosylceramide from CD8 T cells
induces potent antitumor CD8 T-cell responses. Cancer Res.
71:7442–7451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang DH, Osman K, Connolly J, et al:
Sustained expansion of NKT cells and antigen-specific T cells after
injection of α-galactosyl-ceramide loaded mature dendritic cells in
cancer patients. J Exp Med. 201:1503–1517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Andersson A, Yang SC, Huang M, et al: IL-7
promotes CXCR3 ligand-dependent T cell antitumor reactivity in lung
cancer. J Immunol. 182:6951–6958. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wong JL, Berk E, Edwards RP and Kalinski
P: IL-18-primed helper NK cells collaborate with dendritic cells to
promote recruitment of effector CD8+ T cells to the
tumor microenvironment. Cancer Res. 73:4653–4662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang P, Yang X, Xu W, Li K, Chu Y and
Xiong S: Integrating individual functional moieties of CXCL10 and
CXCL11 into a novel chimeric chemokine leads to synergistic
antitumor effects: a strategy for chemokine-based
multi-target-directed cancer therapy. Cancer Immunol Immunother.
59:1715–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wallace KL, Marshall MA, Ramos SI, et al:
NKT cells mediate pulmonary inflammation and dysfunction in murine
sickle cell disease through production of IFN-γ and CXCR3
chemokines. Blood. 114:667–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Müller M, Carter S, Hofer MJ and Campbell
IL: Review: the chemokine receptor CXCR3 and its ligands CXCL9,
CXCL10 and CXCL11 in neuroimmunity - a tale of conflict and
conundrum. Neuropathol Appl Neurobiol. 36:368–387. 2010. View Article : Google Scholar
|
|
35
|
Rosenblum JM, Shimoda N, Schenk AD, et al:
CXC chemokine ligand (CXCL) 9 and CXCL10 are antagonistic
costimulation molecules during the priming of alloreactive T cell
effectors. J Immunol. 184:3450–3460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takeshima T, Chamoto K, Wakita D, et al:
Local radiation therapy inhibits tumor growth through the
generation of tumor-specific CTL: its potentiation by combination
with Th1 cell therapy. Cancer Res. 70:2697–2706. 2010. View Article : Google Scholar : PubMed/NCBI
|