Introduction
Ovarian cancer is one of the most prevalent cancers
and has the highest mortality rate among women with gynecological
malignancies (1). Without an
effective method of early detection and due to the highly invasive
property of ovarian cancer cells, the majority of patients suffer
distant metastasis at the time of diagnosis. It has been shown that
approximately 70–80% of patients with ovarian cancer of stage III
and stage IV die within 5 years of diagnosis, even when undergoing
aggressive cytoreductive surgery and combination chemotherapy
(2). Therefore, it is urgent to
elucidate the molecular mechanisms associated with ovarian cancer
metastasis and to identify new therapeutic approaches, in order to
achieve better treatment outcome.
In recent years, accumulating evidence has
demonstrated that epithelial-to-mesenchymal transition (EMT), which
is a morphologic conversion process that was first described in the
context of embryogenesis, is associated with the acquisition of
mesenchymal phenotypes and malignant characteristics in ovarian
cancer cells, representing mechanisms of escaping from apoptosis
and migrating through the extracellular environment (3–5). Loss
of the epithelial molecule E-cadherin and gain of mesenchymal
markers N-cadherin and vimentin have been considered as the most
important hallmarks of EMT (5).
Among the stimuli that trigger EMT, Snail family members, including
Snail, Slug, Twist, Zeb1 and SIP1, have been found to play an
important role in promoting EMT (4).
Ampelopsin
[(2R,3R)-3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydrochromen-4-one]
(Fig. 1A), also called
dihydromyricetin, is a type of flavonoid and is isolated from the
stems and leaves of Ampelopsis grossedentata. Numerous
pharmacological activities of ampelopsin have been reported, such
as anti-inflammatory (6),
antioxidant, and antimicrobial activity (7). In recent years, ampelopsin has been
described to possess anticancer activity in various types of
cancers. Ampelopsin was found to inhibit the growth and invasion of
breast cancer cells in vitro (8), and to inhibit the growth of prostate
cancer in vivo (9).
Ampelopsin also showed activity for inhibiting vascular endothelial
growth factor (VEGF) and basic fibroblast growth factor (bFGF),
suppressing angiogenesis in hepatocellular carcinoma (10). However, no evidence has been
reported for the direct effect of ampelopsin on ovarian cancer cell
invasion and the mechanisms of this effect.
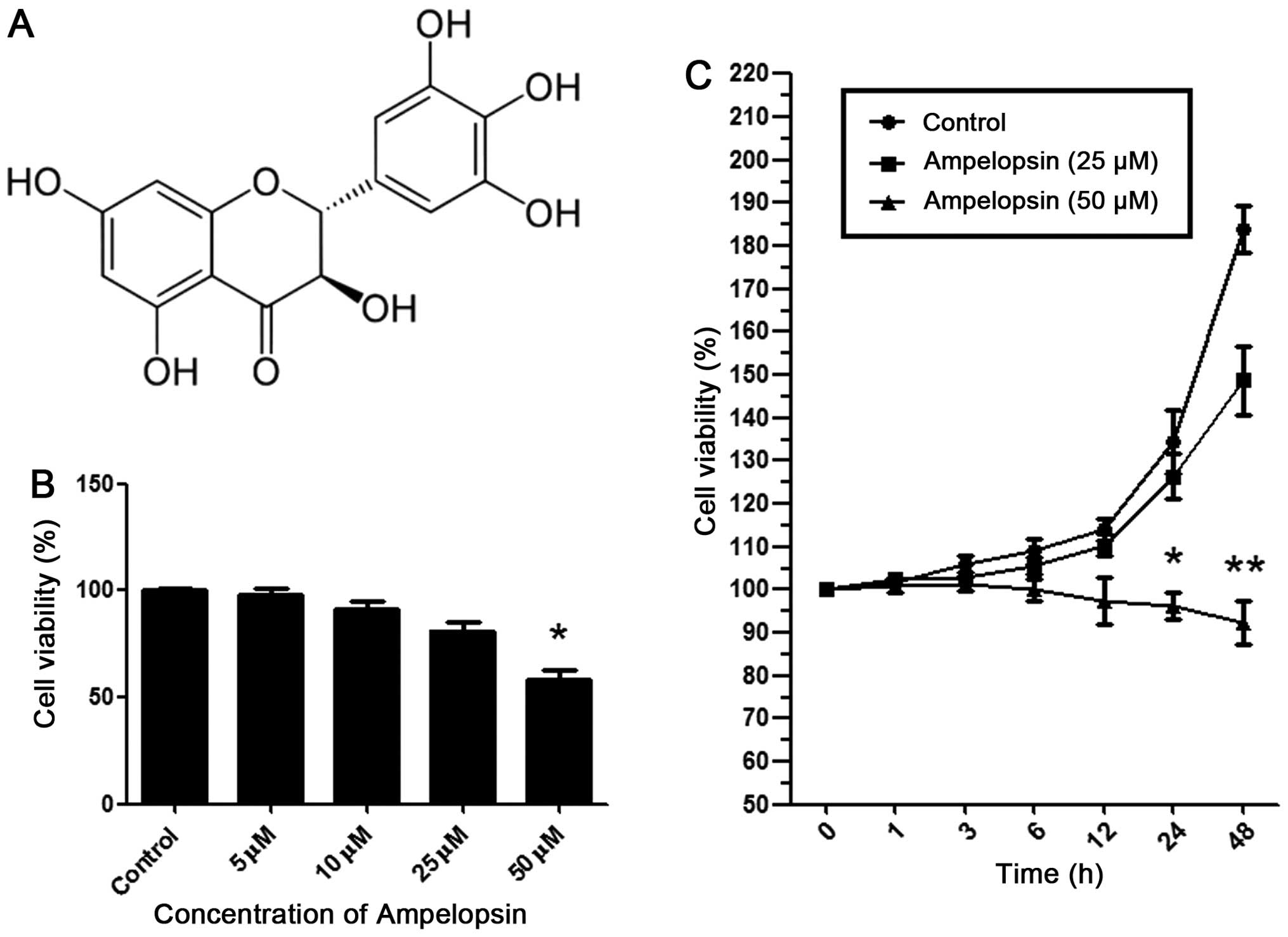 | Figure 1Ampelopsin inhibits the proliferation
of A2780 ovarian cancer cells. (A) Chemical structure of
ampelopsin. (B) After the A2780 cells were stimulated at 24 h with
ampelopsin of different concentration gradients (0, 5, 10, 25, and
50 μM), MTT assay was used to detect the viability of the A2780
cells. (C) After A2780 cells were stimulated with 0, 25 and 50 μM
ampelopsin for different time periods (0, 1, 3, 6, 12, 24 and 48
h), MTT assay was used to detect the viability of the A2780 cells.
*P<0.05; **P<0.01 vs. the control
group. Data shown are means ± SEM from 3 independent experiments in
duplicate. |
The present study was designed to investigate the
effects of ampelopsin on ovarian cancer cell migration and
invasion, as well as its influence on EMT.
Materials and methods
Reagents
Ampelopsin was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Rabbit monoclonal to E-cadherin and vimentin
antibodies were from Abcam (Cambridge, UK). Rabbit polyclonal to
N-cadherin, Snail and GAPDH were also from Abcam. Rabbit anti-mouse
antibodies for NF-κB (p65) and IκBα were both purchased from Cell
Signaling Technology Inc. (Danvers, MA, USA). BAY11-7082, a
selective inhibitor of NF-κB, was purchased from Sigma-Aldrich.
Cell culture
The A2780 cell line (human ovarian cancer cell line)
was obtained from the American Type Culture Collection (Manassas,
VA, USA), and was cultured in RPMI-1640 (HyClone Laboratories,
Inc., Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS) (HyClone) in an atmosphere containing 5% CO2.
Cell viability and proliferation
assay
Cell viability and proliferation activity were
assessed with the MTT colorimetric assay. A2780 cells were seeded
into 96-well plates (Corning Inc., Corning, NY, USA) at a
concentration of 5,000 cells/well. After stimulation with
ampelopsin of various concentrations (0, 5, 10, 25, and 50 μM) for
various time-points (0, 1, 3, 6, 12, 24, and 48 h), MTT (5 μg/ml,
20 μl) was added into each well for a 4-h incubation. Then, 80 μl
of dimethyl sulfoxide (DMSO) (Sigma-Aldrich) was added for another
15 min to fully solubilize the formazan (the metabolic product of
MTT). Finally, the liberated purple product was detected using a
microplate luminometer at 490 nm.
Scratch wound healing assay
A scratch wound healing assay was used to evaluate
the migratory ability of the A2780 cells. A2780 cells
(1×106/well, Corning Inc.) were cultured in 6-well
plates. Straight scratches of the same width were made in the
monolayer of A2780 cells with a pipette tip. After incubation with
ampelopsin of 25 μM for 24 h, images were captured to measure the
wound healing under a microscope.
Transwell assay
The effect of ampelopsin on the invasive ability of
the A2780 cells was detected with modified Boyden chambers with
8-μm pore filter inserts (Corning Inc.). The A2780 cells were
cultured in 24-well plates, and the upper chamber contained cells
in RPMI-1640 plus 1% FBS, while the lower chamber contained
RPMI-1640 plus 10% FBS. Cells (1×105/well) were
re-suspended in the upper chamber at 37°C in 5% CO2.
After a 24-h-incubation, the cells on the lower surface were fixed
with methanol for 30 min and stained with hexamethylpararosaniline,
while the cells remaining on the upper surface were wiped away.
Western blot analysis
After stimulation, the A2780 cells were collected
and lysed. The extracted protein concentration was measured using
BCA protein assay kit (Beyotime Biotechnology, China). Proteins of
equal amounts were separated via 10% SDS-polyacrylamide gel, and
transferred onto nitrocellulose (NC) membranes (Millipore,
Billerica, MA, USA). Blots were blocked and incubated with the
primary antibodies followed by incubation with the secondary
antibodies. Finally, the blots were visualized with
electrochemiluminescence (ECL) detection system (Millipore).
Statistical analysis
All data in the present study were evaluated with
predictive analytics software (PASW) statistics 18.0 (SPSS Inc.,
Chicago, IL, USA). The normally distributed data were analyzed by
one-way ANOVA and the non-parametric variables were analyzed by the
Mann-Whitney U test. Statistical significance was confirmed as
P<0.05.
Results
Ampelopsin inhibits the proliferation of
ovarian cancer cells
To clarify the specific role of ampelopsin in
ovarian cancer cell proliferation, various concentrations (0, 5,
10, 25, and 50 μM) of ampelopsin were added into the cultured A2780
cells. As shown in Fig. 1B, after a
24-h incubation at 50 μM, ampelopsin significantly inhibited the
cell viability as detected by MTT assay. However, at concentrations
below 50 μM (5, 10, and 25 μM), the inhibition was not significant.
Subsequently, 25 and 50 μM of ampelopsin were selected to stimulate
the cells for different times (0, 1, 3, 6, 12, 24 and 48 h). As
shown in Fig. 1C, ampelopsin of 50
μM significantly inhibited the proliferation after a 24-h
stimulation, while ampelopsin of 25 μM did not inhibit the
proliferation after a 48-h stimulation. As a result, we chose 25 μM
of ampelopsin for the subsequent migration and invasion experiments
so that the influence of proliferation was excluded.
Ampelopsin inhibits the migration and
invasion of ovarian cancer cells
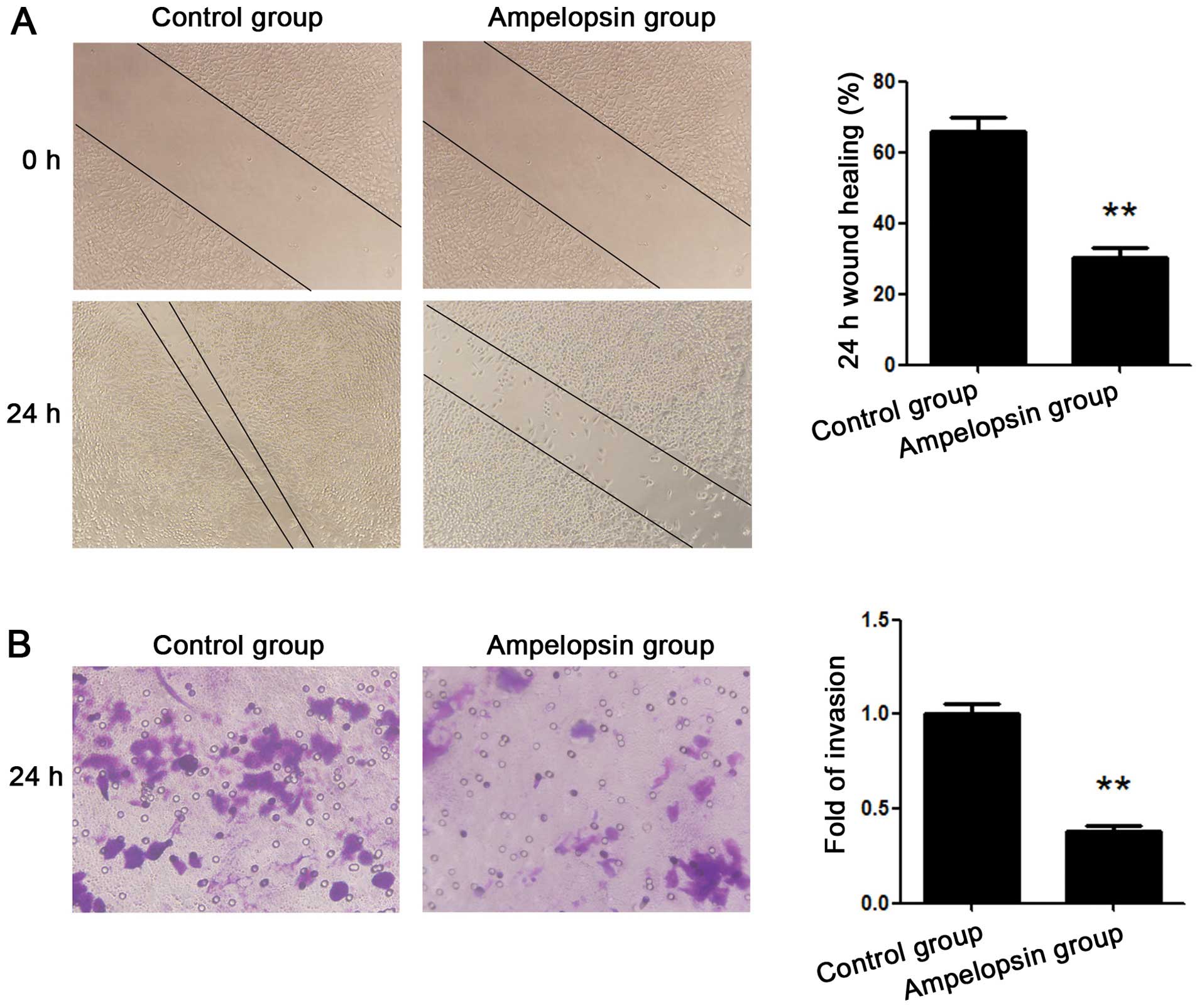
A wound healing assay was used to assess the
migration of the ovarian cancer cells, while a Transwell assay was
applied to evaluate the invasion of the ovarian cancer cells. A2780
ovarian cancer cells were treated with ampelopsin at the
concentration of 25 μM for 24 h. As shown in Fig. 2A, the results of the wound healing
assay demonstrated that healing over the scratch was significantly
reduced after treatment with ampelopsin. As shown in Fig. 2B, the results of the Transwell assay
demonstrated that the number of invading cells migrating from the
upper to the lower surface was also significantly reduced after
treatment with ampelopsin.
Effects of ampelopsin on expression of
EMT markers in the ovarian cancer cells
EMT is thought to play an important role in the
process of cancer cell migration and invasion. We thus assessed the
effect of ampelopsin on EMT marker expression in the A2780 ovarian
cancer cells by western blot analysis. Various concentrations of
ampelopsin (5, 10, 25, and 50 μM) were respectively added to the
cells, and the cancer cells were cultured for another 12 h. As
shown in Fig. 3A, ampelopsin
treatment significantly increased the expression of epithelial
marker E-cadherin and decreased the expression of mesenchymal
markers N-cadherin and vimentin in the A2780 cells. The results
above suggest that ampelopsin may alter the expression of EMT
markers in a concentration-dependent manner and indicate that the
inhibitory effect of ampelopsin on A2780 cell invasion and
migration may be associated with EMT.
NF-κB pathway is involved in the
anti-metastatic mechanism of ampelopsin
As a transcription factor, NF-κB shows a
significantly increased expression in ovarian cancer, and plays an
important role in the aggressiveness of tumors (11,12).
NF-κB is tightly masked by its inhibitor protein IκB and thereby
sequestered in the cytoplasm (13).
Various stimulation signals may phosphorylate IκBα and trigger a
ubiquitination-mediated degradation of IκBα (13), which allows phosphorylation of p65
and translocation of NF-κB from the cytoplasm to the nucleus where
it binds with the promoter of its target genes. A previous study of
pancreatic carcinoma cells demonstrated that blockage of NF-κB
signaling may render cells resistant to TGF-β-induced EMT and then
suppress migration and invasion (14). Thus, in the present study, we aimed
to ascertain whether ampelopsin exerts its anti-metastatic effects
and alters the expression of EMT markers via the NF-κB pathway.
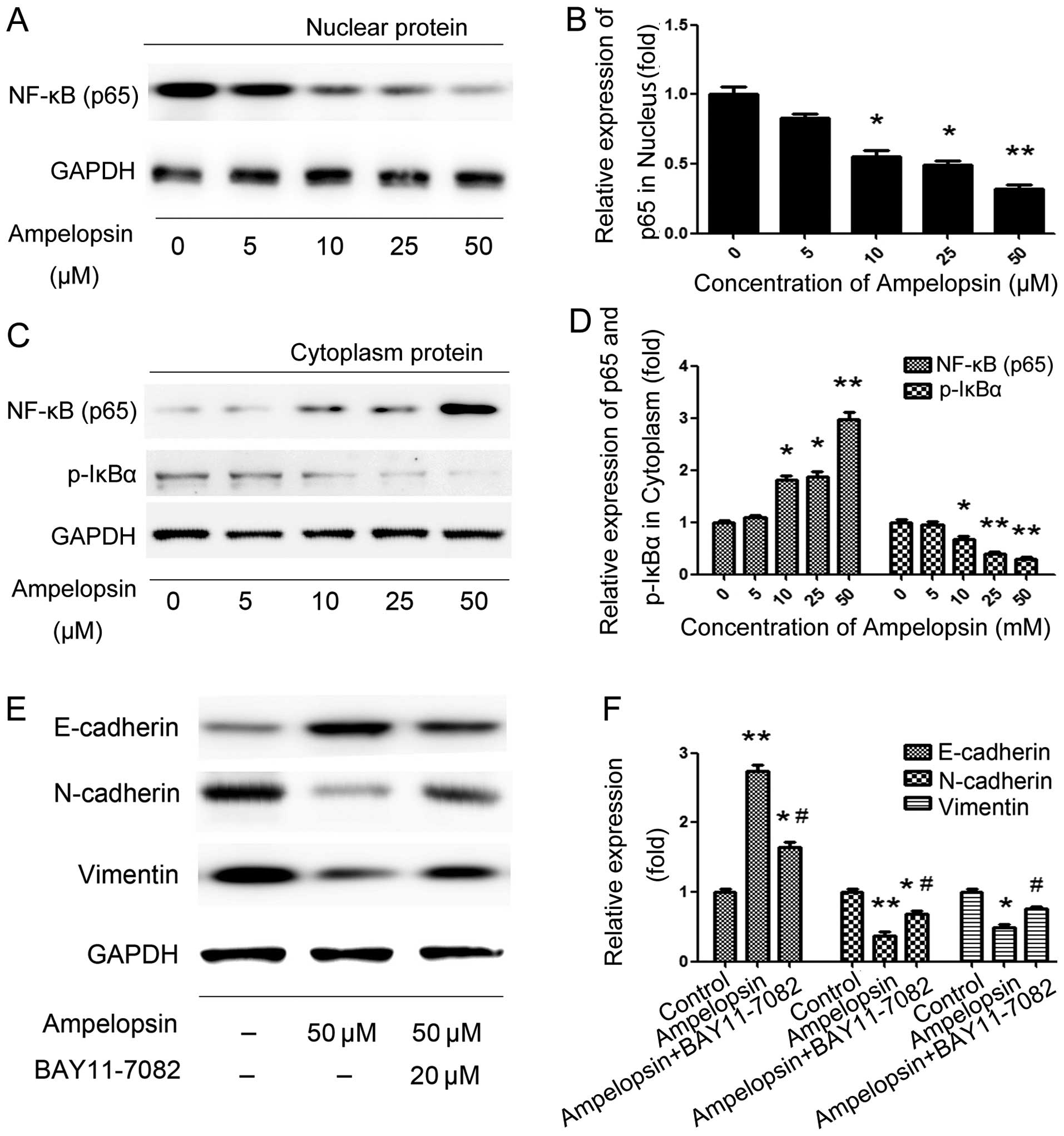
In order to determine the effects of ampelopsin on
NF-κB, we first stimulated the cells with different concentrations
of ampelopsin (5, 10, 25 and 50 μM) for 12 h. Then cytosol and
nuclear proteins were extracted, respectively, followed by the
detection of the expression of p65 and IκBα. As shown in Fig. 4A and B, ampelopsin significantly
increased the expression of p65 in the cytosol but decreased its
expression in the nucleus in a concentration-dependent manner.
Meanwhile, the phosphorylation of IκBα in the cytosol was decreased
after ampelopsin treatment. Subsequently, to further determine the
effect of NF-κB on the expression of EMT markers, we blocked the
NF-κB pathway by its inhibitor BAY11-7082 (20 μM), which was chosen
to pretreat the cells for 2 h before ampelopsin. As shown in
Fig. 4C, BAY11-7082 significantly
reversed ampelopsin-induced E-cadherin expression and N-cadherin
and vimentin expression. The results above indicate that NF-κB
activation is critical for EMT and ampelopsin exerted its effect on
cancer cell migration and invasion, as well as EMT, through
suppressing the NF-κB pathway.
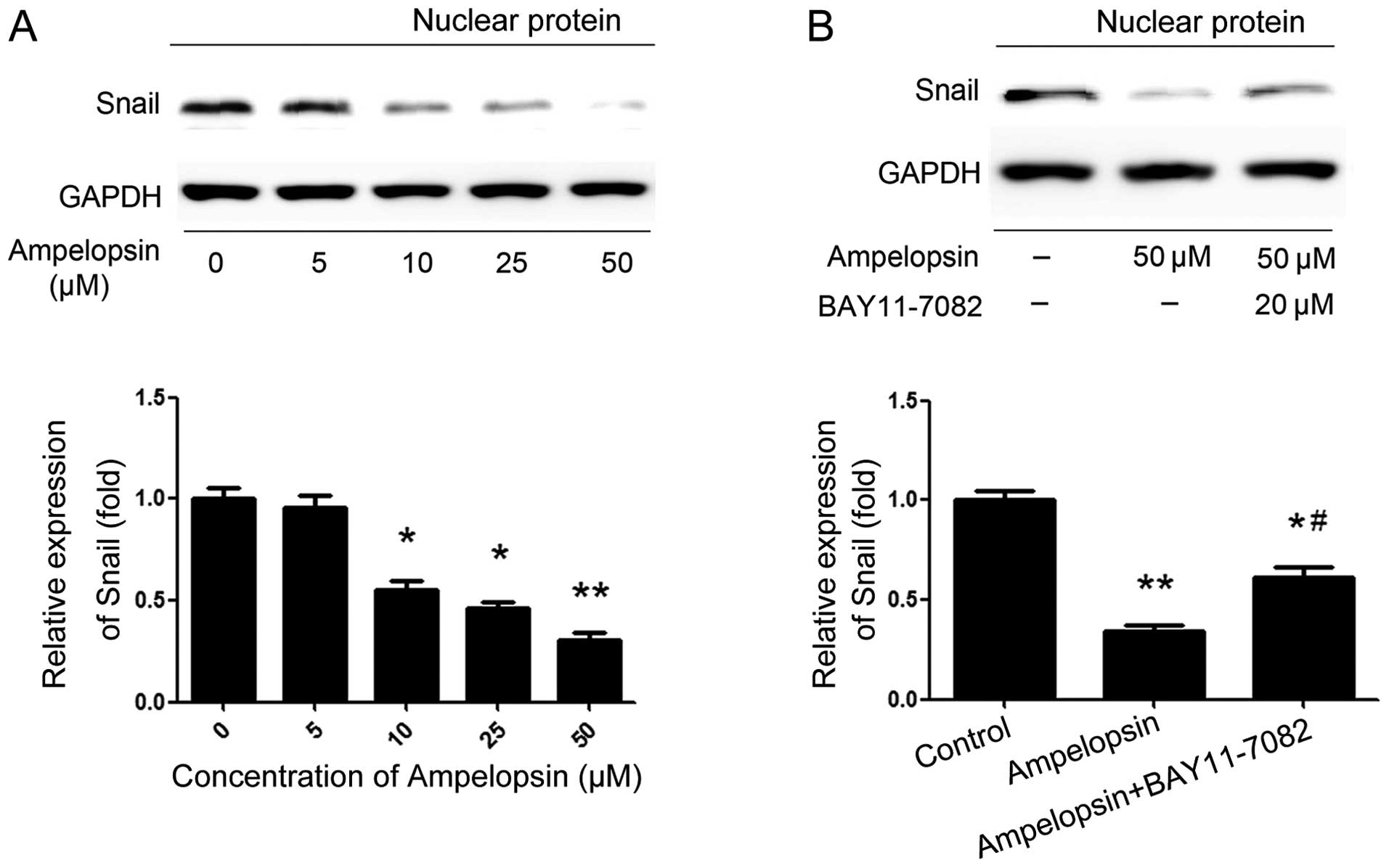
Ampelopsin induces Snail upregulation via
NF-κB activation
As a complex multistep process occurring during
tumor progression, EMT is governed by a variety of regulatory
networks, in particular, the Snail family (15). Among the family members, Snail was
the first described transcriptional repressor of E-cadherin and is
the most extensively studied transcription factor. Thus, the
effects of ampelopsin on the expression of Snail and the
relationship between NF-κB and Snail were investigated. We choose
the concentration gradient of ampelopsin (5, 10, 25, and 50 μM) to
stimulate the ovarian cancer cells for 24 h. As shown in Fig. 5A, ampelopsin obviously suppresses
the expression of Snail along with the increase in concentration,
reaching a peak activity at the concentration of 50 μM. Then, the
cells were also pretreated with BAY11-7082 (20 μM) for 2 h. As
shown in Fig. 5B, pretreatment with
the NF-κB inhibitor BAY11-7082 significantly abrogated the
inhibitory effect of ampelopsin. These results indicate that NF-κB
is a key regulator of Snail, and ampelopsin suppresses the
expression of Snail through blocking the NF-κB pathway at least in
part.
Discussion
Metastasis is considered to be a primary cause of
mortality among most ovarian cancer patients. Thus, understanding
the molecular mechanisms of metastasis and searching for effective
approaches to inhibit metastasis are the most important issues in
cancer research. As a type of flavonoid extracted from
Ampelopsis grossedentata, ampelopsin exhibits multiple
functions in inflammation and oxidation. In recent years, more and
more evidence suggests that ampelopsin has the ability to inhibit
cell proliferation, migration and invasion in breast and prostate
cancer. However, although the previous research (8–10) shed
light on the action of ampelopsin’s anticarcinogenesis, direct
evidence involving ovarian cancer and the detailed molecular
mechanisms have not been clearly elucidated.
As shown in a previous study (9), ampelopsin exhibits potent activity in
inhibiting the proliferation of cancer cells by inducing apoptosis
and downregulating Bcl-2. Thus, in the present study, we firstly
detected the effect of ampelopsin on the viability of cultured
cancer cells, and the results revealed that ampelopsin of 50 μM
significantly suppressed ovarian cancer cell proliferation after 24
and 48 h of incubation, while there was no difference at 12 h. As a
result, in the following experiments involving EMT markers, we
selected 12 h as the stimulation time-point and 25 μM as the
stimulation concentration, so that the influence of proliferation
was excluded. Subsequently, the wound healing and Transwell assays
were respectively applied, and the results demonstrated that
migration and invasion of ovarian cancer cells were both suppressed
after incubation with ampelopsin, which indicated the
anti-metastatic activity of ampelopsin.
EMT, a developmental reprogramming process through
which polarized, immotile epithelial cells undergo
transdifferentiation into motile mesenchymal cells, is
characterized by loss of epithelial markers, such as E-cadherin,
and in turn, acquisition of mesenchymal markers, such as N-cadherin
and vimentin. Previous studies have demonstrated that, during
ovarian cancer progression, EMT plays an important role in inducing
matrix metalloproteinase production and in promoting dissemination
of tumor cells, thereby increasing cell invasion and contributing
to the poor outcome of ovarian cancer patients (16–18).
In addition, evidence suggests that EMT has been found to give rise
to resistance to chemotherapeutic drugs in ovarian cancer (19,20).
Therefore, in the present study, we aimed to ascertain the specific
roles of ampelopsin in the metastasis of ovarian cancer cells, as
well as the underlying relationship between ampelopsin and EMT. The
results demonstrated that incubation with ampelopsin for 12 h
concentration-dependently promoted the expression of epithelial
marker E-cadherin, and inhibited mesenchymal markers N-cadherin and
vimentin, which indicated that EMT was promoted by ampelopsin in
ovarian cancer cells.
NF-κB, a pleiotropic transcription factor, plays
important roles in pathological processes associated with cancer
development, such as proliferation, migration, invasion,
angiogenesis, drug resistance and inflammation (21–23).
It was also found in previous studies that the induction of EMT was
closely associated with the activation of NF-κB. Cichon and Radisky
found that ROS-induced EMT in mammary epithelial cells was mediated
by NF-κB (24), while Liu and
colleagues found that triptolide reversed hypoxia-induced EMT in
pancreatic cancer by NF-κB downregulation (25). In addition, it was also reported in
several other studies that EMT was induced by many factors via the
NF-κB signaling pathway in hypopharyngeal cancer (26), tongue squamous cell carcinoma
(27), and prostate cancer
(28). In the present study, our
results demonstrated that the expression of p65 in the cytosol was
increased while its expression in the nucleus was decreased, which
indicated that the inhibition of NF-κB nuclear translocation
mediated the most important biological effects of NF-κB. The
phosphorylation of IκBα in the cytosol, which is the major
inhibitor of NF-κB, was detected simultaneously, and its expression
was decreased after ampelopsin treatment. Subsequently, the
application of BAY11-7082 significantly reversed ampelopsin’s
effects on E-cadherin, N-cadherin and vimentin expression, which
further proved the important role of NF-κB in the induction of EMT
by ampelopsin.
Finally, we detected the effect of ampelopsin and
NF-κB on transcription factor Snail. Snail, a zinc finger protein,
is the most important member of the Snail superfamily. Previous
studies have confirmed that it mediates EMT through downregulation
of epithelial marker E-cadherin and upregulation of mesenchymal
markers N-cadherin and vimentin, through binding with several boxes
in the promotor region (29). Snail
is overexpressed in several types of cancers, especially in ovarian
cancer, and has also been associated with tumor progression
(30). Notably, Snail was also
found to confer migration and invasion properties to cancer cells
and promote carcinoma metastasis. The localization, expression and
activity of Snail may be regulated by various factors, and the
NF-κB signaling pathway is the most important and hackneyed
mediator (31,32). The results also revealed that
ampelopsin concentration-dependently increased the expression of
Snail in the nucleus, while BAY11-7082 significantly reversed the
effects, further indicating that NF-κB was upstream of Snail.
In summary, the present study firstly demonstrated
that ampelopsin inhibited EMT and reduced the invasion of ovarian
cancer cells. Moreover, the effect of ampelopsin was mediated by
the NF-κB/Snail signaling pathway. According to the results of the
present study, the invasive ability of ovarian cancer cells may be
restrained by ampelopsin by inhibiting the NF-κB/Snail signaling
pathway and EMT. Further in vivo studies should be
performed.
Acknowledgements
This study was supported by the Natural Science
Foundation of Shandong Province (no. 2012ZRE27087).
References
|
1
|
Haslehurst AM, Koti M, Dharsee M, Nuin P,
Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, Davey S,
Squire J, Park PC and Feilotter H: EMT transcription factors snail
and slug directly contribute to cisplatin resistance in ovarian
cancer. BMC Cancer. 12:912012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holschneider CH and Berek JS: Ovarian
cancer: Epidemiology, biology, and prognostic factors. Sem Surg
Oncol. 19:3–10. 2000. View Article : Google Scholar
|
|
3
|
Elloul S, Vaksman O, Stavnes HT, Trope CG,
Davidson B and Reich R: Mesenchymal-to-epithelial transition
determinants as characteristics of ovarian carcinoma effusions.
Clin Exp Metastasis. 27:161–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan H, Kajiyama H, Ito S, Yoshikawa N,
Hyodo T, Asano E, Hasegawa H, Maeda M, Shibata K, Hamaguchi M,
Kikkawa F and Senga T: Alx1 induces snail expression to promote
epithelial-to-mesenchymal transition and invasion of ovarian cancer
cells. Cancer Res. 73:1581–1590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosano L, Cianfrocca R, Spinella F, Di
Castro V, Nicotra MR, Lucidi A, Ferrandina G, Natali PG and Bagnato
A: Acquisition of chemoresistance and EMT phenotype is linked with
activation of the endothelin A receptor pathway in ovarian
carcinoma cells. Clin Cancer Res. 17:2350–2360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qi S, Xin Y, Guo Y, Diao Y, Kou X, Luo L
and Yin Z: Ampelopsin reduces endotoxic inflammation via repressing
ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways. Int
Immunopharmacol. 12:278–287. 2012. View Article : Google Scholar
|
|
7
|
Xie XF, Wang JW, Zhang HP, Li QX and Chen
BY: Chemical composition, antimicrobial and antioxidant activities
of essential oil from Ampelopsis megalophylla. Natural Prod Res.
28:853–860. 2014. View Article : Google Scholar
|
|
8
|
Zhou Y, Shu F, Liang X, Chang H, Shi L,
Peng X, Zhu J and Mi M: Ampelopsin induces cell growth inhibition
and apoptosis in breast cancer cells through ROS generation and
endoplasmic reticulum stress pathway. PLoS One. 9:e890212014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ni F, Gong Y, Li L, Abdolmaleky HM and
Zhou JR: Flavonoid ampelopsin inhibits the growth and metastasis of
prostate cancer in vitro and in mice. PLoS One. 7:e388022012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo GQ, Zeng S and Liu DY: Inhibitory
effects of ampelopsin on angiogenesis. Zhong Yao Cai. 29:146–150.
2006.(In Chinese). PubMed/NCBI
|
|
11
|
Wilson AJ, Barham W, Saskowski J,
Tikhomirov O, Chen L, Lee HJ, Yull F and Khabele D: Tracking NF-κB
activity in tumor cells during ovarian cancer progression in a
syngeneic mouse model. J Ovarian Res. 6:632013. View Article : Google Scholar
|
|
12
|
Nishio H, Yaguchi T, Sugiyama J, Sumimoto
H, Umezawa K, Iwata T, Susumu N, Fujii T, Kawamura N, Kobayashi A,
Park J, Aoki D and Kawakami Y: Immunosuppression through
constitutively activated NF-κB signalling in human ovarian cancer
and its reversal by an NF-κB inhibitor. Br J Cancer. 110:2965–2974.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cianfrocca R, Tocci P, Semprucci E,
Spinella F, Di Castro V, Bagnato A and Rosano L: β-arrestin 1 is
required for endothelin-1-induced NF-κB activation in ovarian
cancer cells. Life Sci. Feb 12–2014.(Epub ahead of print).
View Article : Google Scholar
|
|
14
|
Maier HJ, Schmidt-Strassburger U, Huber
MA, Wiedemann EM, Beug H and Wirth T: NF-kappaB promotes
epithelial-mesenchymal transition, migration and invasion of
pancreatic carcinoma cells. Cancer Lett. 295:214–228. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu ZY, Dong R, Li D, Li WB, Xu FQ, Geng Y
and Zhang YS: SNAI1 overexpression induces stemness and promotes
ovarian cancer cell invasion and metastasis. Oncol Rep.
27:1587–1591. 2012.PubMed/NCBI
|
|
16
|
Parikh A, Lee C, Peronne J, Marchini S,
Baccarini A, Kolev V, Romualdi C, Fruscio R, Shah H, Wang F,
Mullokandov G, Fishman D, D’Incalci M, Rahaman J, Kalir T, Redline
RW, Brown BD, Narla G and DiFeo A: MicroRNA-181a has a critical
role in ovarian cancer progression through the regulation of the
epithelial-mesenchymal transition. Nat Commun. 5:29772014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adham SA, Al Harrasi I, Al Haddabi I, Al
Rashdi A, Al Sinawi S, Al Maniri A, Ba-Omar T and Coomber BL:
Immunohistological insight into the correlation between
neuropilin-1 and epithelial mesenchymal transition markers in
epithelial ovarian cancer. J Histochem Cytochem. 62:619–631. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim MK, Kim MA, Kim H, Kim YB and Song YS:
Expression profiles of epithelial-mesenchymal transition-associated
proteins in epithelial ovarian carcinoma. Biomed Res Int.
2014:4957542014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marchini S, Fruscio R, Clivio L, Beltrame
L, Porcu L, Fuso Nerini I, Cavalieri D, Chiorino G, Cattoretti G,
Mangioni C, Milani R, Torri V, Romualdi C, Zambelli A, Romano M,
Signorelli M, di Giandomenico S and D’Incalci M: Resistance to
platinum-based chemotherapy is associated with epithelial to
mesenchymal transition in epithelial ovarian cancer. Eur J Cancer.
49:520–530. 2013. View Article : Google Scholar
|
|
20
|
Du F, Wu X, Liu Y, Wang T, Qi X, Mao Y,
Jiang L, Zhu Y, Chen Y, Zhu R, Han X, Jin J, Ma X and Hua D:
Acquisition of paclitaxel resistance via PI3K-dependent
epithelial-mesenchymal transition in A2780 human ovarian cancer
cells. Oncol Rep. 30:1113–1118. 2013.PubMed/NCBI
|
|
21
|
Uno M, Saitoh Y, Mochida K, Tsuruyama E,
Kiyono T, Imoto I, Inazawa J, Yuasa Y, Kubota T and Yamaoka S:
NF-κB inducing kinase, a central signaling component of the
non-canonical pathway of NF-κB, contributes to ovarian cancer
progression. PLoS One. 9:e883472014. View Article : Google Scholar
|
|
22
|
Block MS, Charbonneau B, Vierkant RA,
Fogarty Z, Bamlet WR, Pharoah PD, Rossing MA, Cramer D, Pearce CL,
Schildkraut J, Menon U, Kjaer SK, Levine DA, Gronwald J, Culver HA,
Whittemore AS, Karlan BY, Lambrechts D, Wentzensen N, Kupryjanczyk
J, Chang-Claude J, Bandera EV, Hogdall E, Heitz F, Kaye SB,
Fasching PA, Campbell I, Goodman MT, Pejovic T, Bean YT, Hays LE,
Lurie G, Eccles D, Hein A, Beckmann MW, Ekici AB, Paul J, Brown R,
Flanagan JM, Harter P, du Bois A, Schwaab I, Hogdall CK, Lundvall
L, Olson SH, Orlow I, Paddock LE, Rudolph A, Eilber U,
Dansonka-Mieszkowska A, Rzepecka IK, Ziolkowska-Seta I, Brinton LA,
Yang H, Garcia-Closas M, Despierre E, Lambrechts S, Vergote I,
Walsh CS, Lester J, Sieh W, McGuire V, Rothstein JH, Ziogas A,
Lubinski J, Cybulski C, Menkiszak J, Jensen A, Gayther SA, Ramus
SJ, Gentry-Maharaj A, Berchuck A, Wu AH, Pike MC, Van Den Berg D,
Terry KL, Vitonis AF, Ramirez SM, Rider DN, Knutson KL, Sellers TA,
Phelan CM, Doherty JA, Johnatty SE, deFazio A, Song H, Tyrer J,
Kalli KR, Fridley BL, Cunningham JM and Goode EL: Variation in
NF-κB signaling pathways and survival in invasive epithelial
ovarian cancer. Cancer Epidemiol Biomarkers Prev. 23:1421–1427.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Annunziata CM, Stavnes HT, Kleinberg L,
Berner A, Hernandez LF, Birrer MJ, Steinberg SM, Davidson B and
Kohn EC: Nuclear factor kappaB transcription factors are
coexpressed and convey a poor outcome in ovarian cancer. Cancer.
116:3276–3284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cichon MA and Radisky DC: ROS-induced
epithelial-mesenchymal transition in mammary epithelial cells is
mediated by NF-κB-dependent activation of Snail. Oncotarget.
5:2827–2838. 2014.PubMed/NCBI
|
|
25
|
Liu L, Salnikov AV, Bauer N,
Aleksandrowicz E, Labsch S, Nwaeburu C, Mattern J, Gladkich J,
Schemmer P, Werner J and Herr I: Triptolide reverses
hypoxia-induced epithelial-mesenchymal transition and stem-like
features in pancreatic cancer by NF-κB downregulation. Int J
Cancer. 134:2489–2503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu L, Mu Y, Sa N, Wang H and Xu W: Tumor
necrosis factor α induces epithelial-mesenchymal transition and
promotes metastasis via NF-κB signaling pathway-mediated twist
expression in hypopharyngeal cancer. Oncol Rep. 31:321–327.
2014.
|
|
27
|
Wang Y, Lin Z, Sun L, Fan S, Huang Z,
Zhang D, Yang Z, Li J and Chen W: Akt/Ezrin Tyr353/NF-κB pathway
regulates EGF-induced EMT and metastasis in tongue squamous cell
carcinoma. Br J Cancer. 110:695–705. 2014. View Article : Google Scholar
|
|
28
|
Deep G, Jain AK, Ramteke A, Ting H,
Vijendra KC, Gangar SC, Agarwal C and Agarwal R: SNAI1 is critical
for the aggressiveness of prostate cancer cells with low
E-cadherin. Mol Cancer. 13:372014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cano A, Perez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tuhkanen H, Soini Y, Kosma VM, Anttila M,
Sironen R, Hamalainen K, Kukkonen L, Virtanen I and Mannermaa A:
Nuclear expression of Snail1 in borderline and malignant epithelial
ovarian tumours is associated with tumour progression. BMC Cancer.
9:2892009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen KC, Chen CY, Lin CR, Yang TY, Chen
TH, Wu LC and Wu CC: Luteolin attenuates TGF-β1-induced
epithelial-mesenchymal transition of lung cancer cells by
interfering in the PI3K/Akt-NF-κB-Snail pathway. Life Sci.
93:924–933. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen Z, Liu M, Liu X, Huang S, Li L, Song
B, Li H, Ren Q, Hu Z, Zhou Y and Qiao L: COX-2 regulates E-cadherin
expression through the NF-κB/Snail signaling pathway in gastric
cancer. Int J Mol Med. 32:93–100. 2013.PubMed/NCBI
|