Introduction
Breast cancer is a leading cause of cancer related
mortality among females worldwide and its incidence and mortality
rate have been increasing throughout the recent years (1). The treatment of breast cancer has
progressed over the past three decades with the development of the
combination of chemotherapy, endocrine therapies and human
epidermal growth factor receptor 2 (HER2)-targeted therapies
(2–5). However, triple-negative breast cancer
(TNBC), which is defined by the lack of the estrogen receptor (ER),
the progesterone receptor (PgR) and HER2 expression, has not fully
benefited from such advances in treatment. Therefore, patients with
TNBCs are currently categorized as a sub group with the worst
possible outcome. Although recent progress in gene sequencing
technology has revealed the genetic profile of breast cancer
including that of TNBC, the effort to understand breast cancer
based on the diverse view from a more inclusive perspective is
still needed (6–8).
Annexin A1 (ANXA1) is a 37-kDa calcium-dependent
phospholipid-linked protein belonging to the annexin superfamily
and it is related to anti-inflammatory effects, regulation of
cellular differentiation, proliferation and apoptosis (9–11).
However, through those functions, ANXA1 is involved in
tumorigenesis and the pivotal role of ANXA1 is not clearly
understood. One of the main reasons for this is due to the fact
that the functional role of ANXA1 in malignant tumors is quite
different depending on the cancer type, such as head-and-neck,
esophageal, gastric, colorectal, pancreatic, hepatic and prostate
cancer (12–19). While ANXA1 is highly expressed in
malignant tumors (20,21), some studies have demonstrated that
lower expression of ANXA1 is associated with poor prognosis
(22) and cancer progression
(23–25) in breast cancer.
Our recent studies, as well as others, have
highlighted the functional role of ANAX1 in cancer progression. One
study suggested that ANXA1 may increase metastatic potential
through the regulation of NF-κB in breast cancer (26). Another study reported that ANXA1 may
promote metastasis formation by enhancing the TGFβ/Smad signaling
pathway (27). On the other hand,
we previously reported that the expression of ANXA1 is upregulated
in gastric and colon cancer and it is involved in cancer invasion
as well as lymph node metastasis (16). Here, we investigated the ANXA1
expression in breast cancer and the relationship between ANXA1
expression and clinicopathological features. Furthermore, the
biological significance of the ANXA1 expression was examined to
clarify the function of ANXA1 in breast cancer.
Materials and methods
Clinical samples of the patients
Surgically resected 211 breast cancer tissues were
examined in the present study. All cases underwent surgical
resection at Fukushima Medical University between 2002 and 2005.
Information regarding age, TNM stage (the 7th classification), ER,
PgR and HER2, and pathological diagnosis including lymphatic and
venous invasion, were retrospectively collected. The patient median
age was 55 years. These cases included 85 stage I cases, 120 stage
II cases and 6 stage III cases. The present study was approved by
the Ethics Committee of Fukushima Medical University. Written
informed consent was obtained from all participants.
Immunohistochemical (IHC) staining and
evaluation
Formalin-fixed paraffin-embedded (FFPE) sections
were immunostained for ANXA1, ER, PgR and HER2, and evaluated for
staining intensity. Briefly, breast cancer specimens were fixed in
a 10% formalin and embedded in paraffin. They were cut into thin (4
μm) sections and stained with hematoxylin and eosin or other
antibodies. Analysis of ER, PgR and HER2 was performed by the
Ventana HX automatic system BenchMark (Ventana Medical Systems SA,
Illkirch Cedex, France). Antibodies used for IHC staining were as
follows: anti-ANXA1 (clone 29; BD Biosciences, San Jose, CA, USA),
anti-ER (SP1), anti-PgR (1E2), anti-HER2 (4B5) (all from Ventana
Medical Systems SA). The expression of ANXA1 protein was evaluated
by the ratio of the numbers of positively stained cells to those
negatively stained. Tissues were dichotomized as positive (≥5%
staining) or negative (<5% staining) for ANXA1. ER, PgR and HER2
expression levels were also dichotomized as positive or negative as
previously described (28).
Cell line culture
The breast cancer cell lines were originally
obtained from the American Type Culture Collection (Rockville, MD,
USA) and were cultured in the recommended media with 10% fetal
bovine serum. These monolayer cells were maintained in a 37°C
incubator with 5% CO2. Cells were checked regularly
under a light microscope and were subcultured when reaching 80 to
90% confluence. For hypoxia exposure, each cell type was cultured
for 24 h in a modulator incubator chamber (Billups-Rothenberg, Del
Mar, CA, USA) at 37°C with 1% O2, 5% CO2 and
94% N2. To mimic hypoxia, the cells were cultured for 24
h with 100 μM cobalt chloride (CoCl2) (Sigma-Aldrich,
St. Louis, MO, USA).
Western blotting
Cells were washed twice with ice-cold PBS and
immediately scraped. The cells were then centrifuged at 15,000 rpm
to pellet cellular debris and were stored overnight at −80°C. The
protein lysates were collected using ice-cold RIPA buffer
containing Halt Protease Inhibitor Single-Use Cocktail (Thermo
Scientific, San Jose, CA, USA). The protein concentration was
determined by the Bradford method (Bio Rad, Hercules, CA, USA).
Samples for SDS-PAGE were prepared by mixing aliquots of the
protein with Novex Tris-Glycine SDS sample buffer (Invitrogen,
Carlsbad, CA, USA) and heated at 100°C for 3 min. Protein samples
were run on 10% Bis-Tris gels at 100 V for 90 min with MES SDS
running buffer (Invitrogen). For western blotting, the gels were
electro-transferred to a nitrocellulose membrane using the iBlot
Dry Blotting System (Invitrogen). The proteins were blocked using
Starting Block (PBS) Blocking Buffer (Pierce, Rockford, IL, USA)
and were detected using anti-ANXA1, anti-hypoxia-inducible
factor-1α (HIF-1α) (BD Novus Bioscience Biologicals, Littleton, CO,
USA), anti-β-actin (Santa Cruz Biotechnology, Dallas, TX, USA) and
a goat anti-mouse secondary antibody phosphatase (Novagen,
Billerica, MA, USA). Western blottings were then incubated with
Super Signal West Pico detection system (Pierce) and detected using
LAS-4000 IR MultiColor (Fuji, Tokyo, Japan).
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
The total RNA was extracted from the cells using
TRIzol reagent (Invitrogen) according to the manufacturer’s
instructions. Complementary DNA (cDNA) was synthesized from 5 μg of
total RNA with a random hexamer using the SuperScript III
First-Strand Synthesis kit (Invitrogen). These cDNAs were used for
the measurement of gene expression with a 7500 Real-time PCR system
(Applied Biosystems, Foster City, CA, USA) using TaqMan probes, and
experiments were performed in triplicate to the blinded patient
information. β-actin was used as an internal control. Expression
assays were purchased from Applied Biosystems: ANXA1 (Hs00167549_
m1) and β-actin (Hs99999903_ m1). Normalized ANXA1 gene expression
was calculated using the 2−ΔΔCT method as previously
described (29).
Knockdown of ANXA1 expression using siRNA
technology
The siRNA oligonucleotides for ANXA1 (HSS100502 and
HSS100503) and the control were purchased from Invitrogen. ANXA1
siRNA sequences were designed as follows: siRNA-1,
5′-CAACCAUCAUUGACAUUCUAACUAA-3′ and siRNA-2,
5′-GCCUUGCAUAAGGCCAUAAUGGUUA-3′. One day prior to the siRNA
transfection, 5×105 cells were plated in 6 cm plates.
The cells were transfected with 40 nM of each siRNA using
Lipofectamine RNAiMAX (Invitrogen) following the manufacturer’s
instructions and harvested after 48 h.
Invasion assay
The cell invasion assay was conducted using a
24-well BD Bio-Coat Tumor Invasion System (BD Biosciences). Cells
were harvested and seeded (5×103 cells) into the top
chamber and incubated at 37°C for 22 h. The cells were post-stained
with 4 μg/ml of Calcein-AM (Molecular Probes, Carlsbad, CA, USA) in
Hank’s buffered salt solution at 37°C in 5% CO2 for 1 h.
The labeled cells that invaded the BD Matrigel Matrix and passed
through the pores of the BD FluoroBlok membrane were detected by
Varioskan Flash Multimode Reader (Thermo Scientific).
Statistical data analysis
Statistical analysis was carried out with an
unpaired Student’s t-test using GraphPad Prism v5.0 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant result.
Results
ANXA1 protein expression in breast cancer
tissues
The ANXA1 protein expression in the breast cancer
tissues was examined by IHC staining using an anti-ANXA1 antibody.
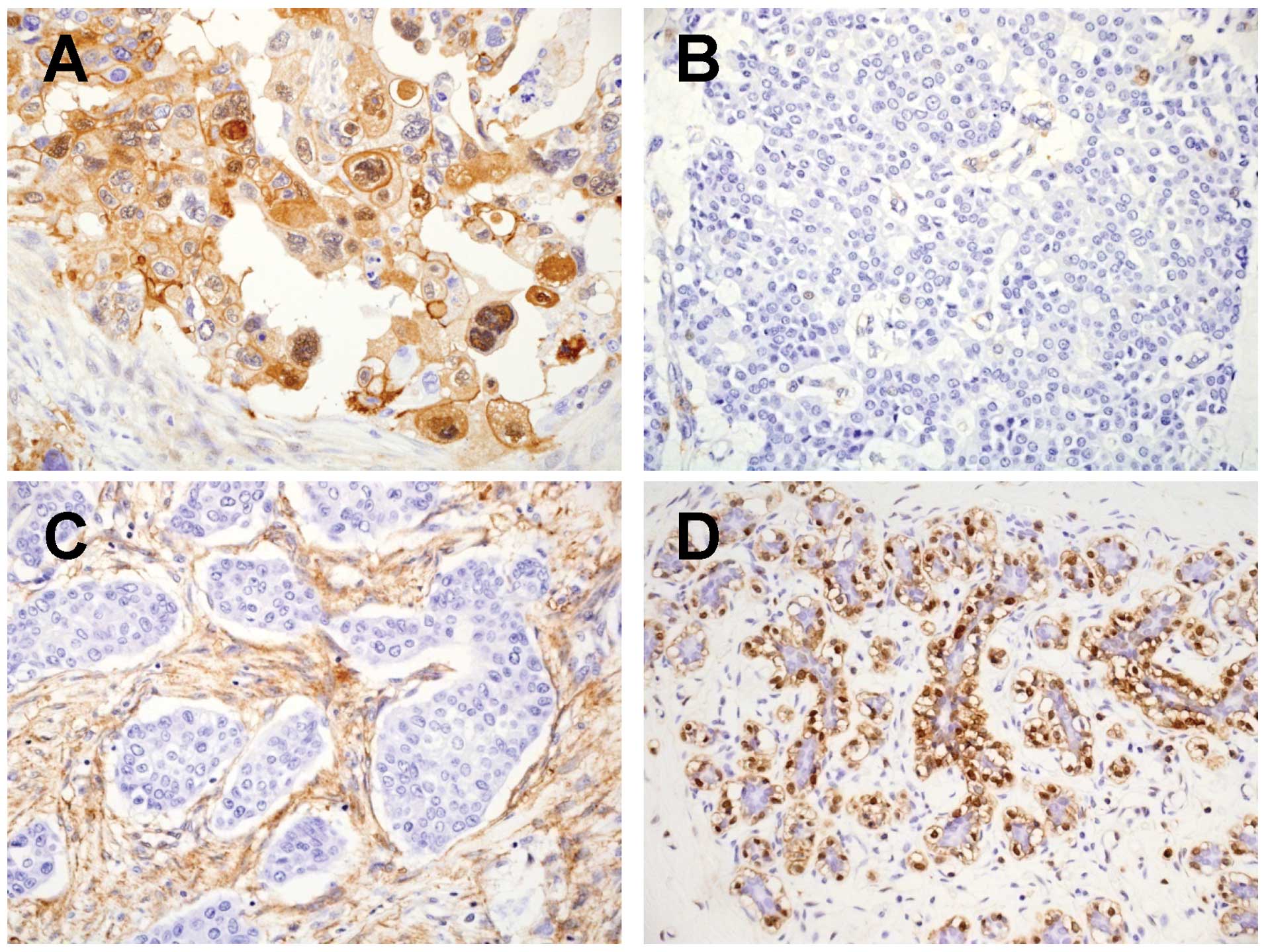
In the breast cancer tissues, the ANXA1 expression was mainly
detected in the cytoplasm of tumor cells, interstitial cells and
myoepithelial cells (Fig. 1). In
contrast, in the adjacent normal breast tissues, the ANXA1
expression was detected in myoepithelial cells, but not in normal
duct-epithelial cells.
Of the 211 cases, 31 (14.7%) showed positive ANXA1
staining in the breast cancer tissues. Such cases showed a positive
correlation with triple-negative (ER-negative, PgR-negative and
HER2-negative) expression (P=0.007) and venous invasion (P=0.028)
(Table I). However, such cases did
not show any correlation with age, TNM stage, lymph node
metastasis, histology, ER and PgR expression and recurrence. These
results suggest that positive expression of ANXA1 is associated
with tumor cell invasion in breast cancer, which was particularly
significant in the TNBC cases.
 | Table IClinicopathological factors and ANXA1
expression in 211 breast cancer cases. |
Table I
Clinicopathological factors and ANXA1
expression in 211 breast cancer cases.
| | ANXA1 | |
|---|
| |
| |
|---|
| Characteristics | Total (n=211) | Positive
(n=31)
n (%) | Negative
(n=180)
n (%) | P-value |
|---|
| Age (years) | | | | 1 |
| <50 | 75 | 11 (35) | 64 (36) | |
| ≥50 | 136 | 20 (65) | 116 (64) | |
| pTMN stage | | | | 0.597 |
| I | 85 | 11 (35) | 74 (41) | |
| II | 120 | 20 (65) | 100 (56) | |
| III | 6 | 0 | 6 (3) | |
| Lymph node
metastasis | | | | 0.318 |
| Positive | 82 | 15 (48) | 67 (37) | |
| Negative | 129 | 16 (52) | 113 (63) | |
| Histology | | | | 1 |
| Invasive
ductal | 186 | 28 (90) | 158 (88) | |
| Invasive
lobular | 7 | 1 (3) | 6 (3) | |
| Mixed | 2 | 0 | 2 (1) | |
| Other | 16 | 2 (7) | 14 (8) | |
| Histological
grade | | | | 0.055 |
| 0–2 | 181 | 23 (74) | 158 (88) | |
| 3 | 30 | 8 (26) | 22 (12) | |
| Estrogen
receptor | | | | 0.09 |
| Positive | 150 | 18 (58) | 132 (73) | |
| Negative | 61 | 13 (42) | 48 (27) | |
| Progesterone
receptor | | | | 0.432 |
| Positive | 124 | 16 (52) | 108 (60) | |
| Negative | 87 | 15 (48) | 72 (40) | |
| HER2 | | | | 1 |
| Positive | 16 | 2 (7) | 14 (8) | |
| Negative | 195 | 29 (93) | 166 (92) | |
|
Triple-negative | | | | 0.007 |
| Presence | 35 | 11 (35) | 24 (13) | |
| Absence | 176 | 20 (65) | 156 (87) | |
| Lymphatic
invasion | | | | 0.329 |
| Positive | 116 | 20 (65) | 96 (53) | |
| Negative | 95 | 11 (35) | 84 (47 | |
| Venous
invasion | | | | 0.028 |
| Positive | 58 | 14 (45) | 44 (24) | |
| Negative | 153 | 17 (55) | 136 (76) | |
| Recurrence | | | | 0.088 |
| Presence | 20 | 6 (19) | 14 (8) | |
| Absence | 191 | 25 (81) | 166 (92) | |
ANXA1 expression in the breast cancer
cell lines
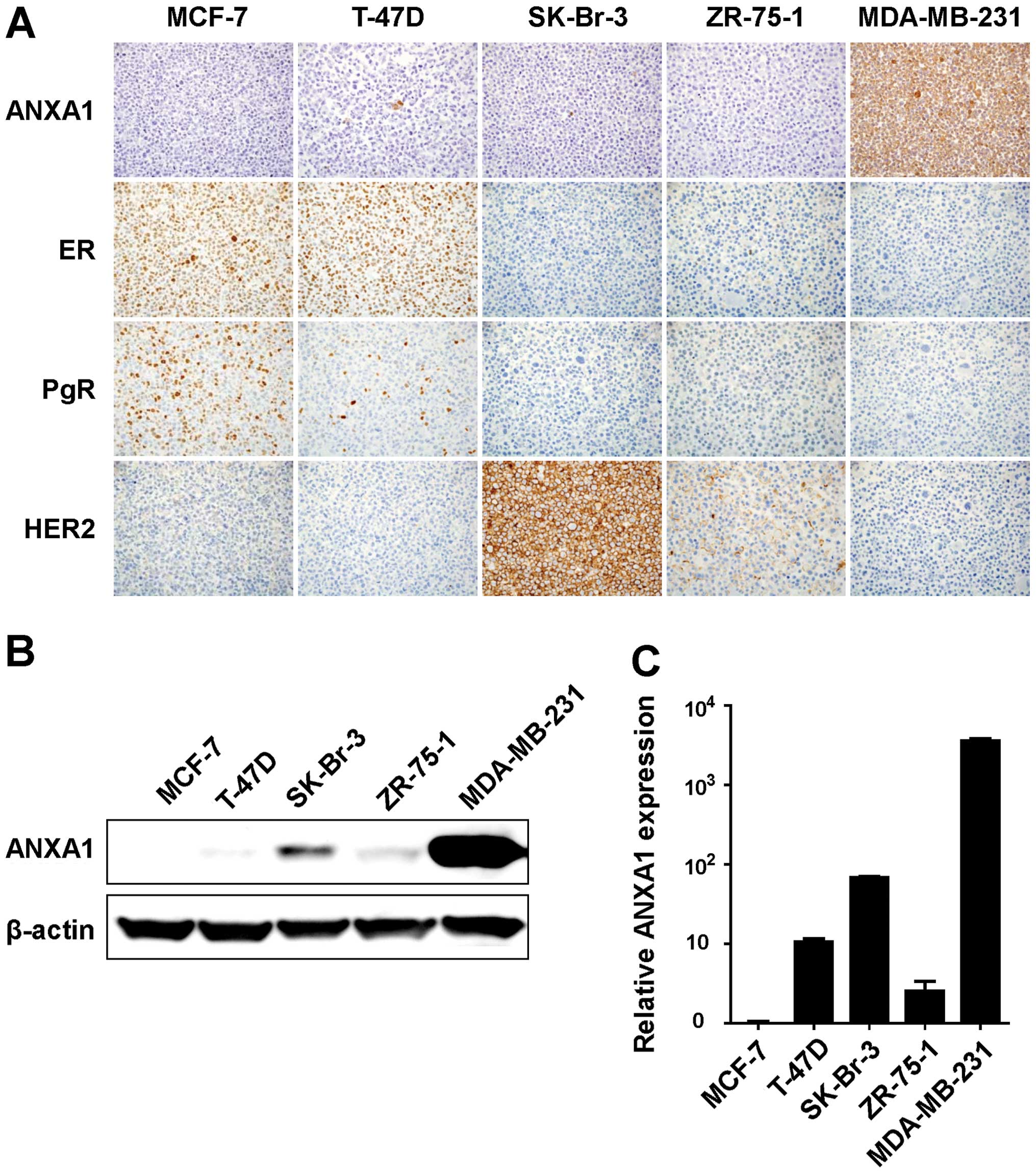
We next examined the ANXA1 expression in five human
breast cancer cell lines, MCF-7, T-47D, SK-Br-3, ZR-75-1 and
MDA-MB-231. IHC staining using the anti-ANXA1 antibody was
performed for those cell lines. Positive staining of ANXA1 was
detected in the cytoplasm of the MDA-MB-231 cells, while that of
ANXA1 was not detected in the other four cell lines (Fig. 2A). We also performed IHC staining
and evaluation for ER, PgR and HER2 in the same breast cancer cell
lines. Positive staining of both ER and PgR was observed in the
MCF-7 and T-47D cells. Strong positive staining of HER2 was
detected in the SK-Br-3 cells and weak positive staining of HER2
was detected in the ZR-75-1 cells. As a result, the MDA-MB-231 cell
line was found to be a TNBC with ANXA1-positive expression.
To confirm the ANXA1 expression by IHC evaluation in
the cancer cell lines, we performed western blotting and qRT-PCR
using the same breast cancer cell lines. Consistent with the IHC
results, the ANXA1 protein was highly expressed in the MDA-MB 231
cells (Fig. 2B). The mRNA of ANXA1
was also highly expressed in MDA-MB-231 cells, suggesting that the
ANXA1 expression was upregulated at the transcriptional level
(Fig. 2C).
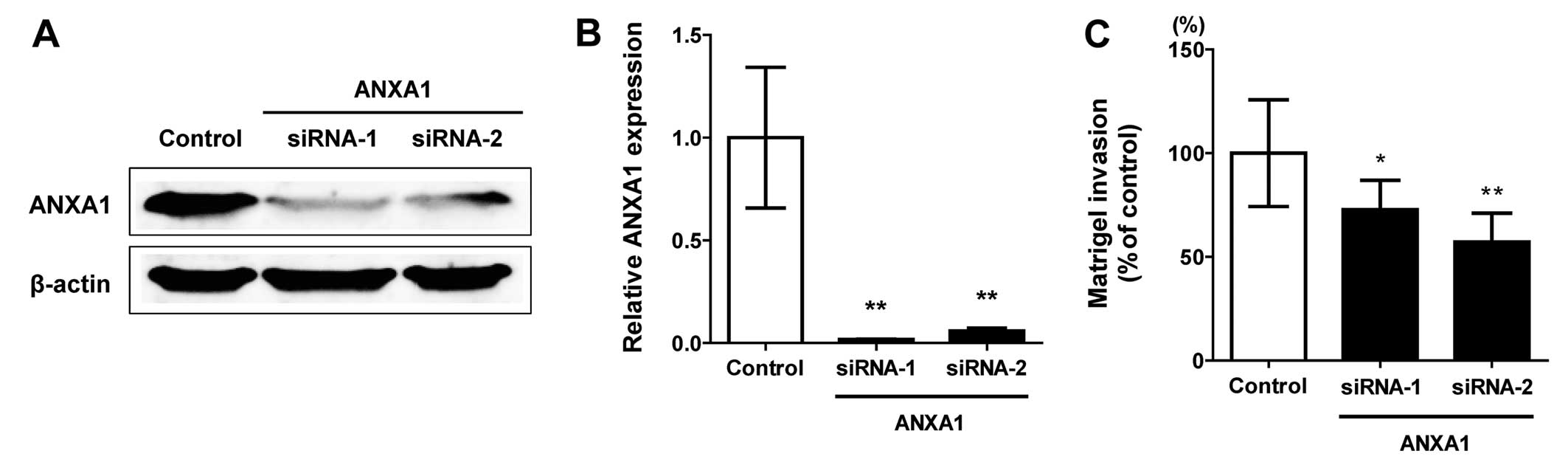
Knockdown of ANXA1 in the MDA-MB-231
cells
According to the comparisons of our IHC results and
the clinicopathological factors, we hypothesized that ANXA1 is
upregulated in TNBCs and is associated with cellular invasion. To
investigate this hypothesis, we used gene knockdown technology and
cell invasion assay. Both protein and mRNA expression of ANXA1 was
downregulated by two independent siRNA oligonucleotides (siRNA-1
and -2) in the MDA-MB-231 cells which originally exhibited
upregulated ANXA1 (Fig. 3A and B).
While no morphological changes were observed in the ANXA1 knockdown
cells, the ability of cellular invasion was attenuated in the ANXA1
downregulated cells. The invasion ratio was significantly decreased
in the cells treated by both ANXA1 siRNA-1 (P<0.05) and siRNA-2
(P<0.01) (Fig. 3C).
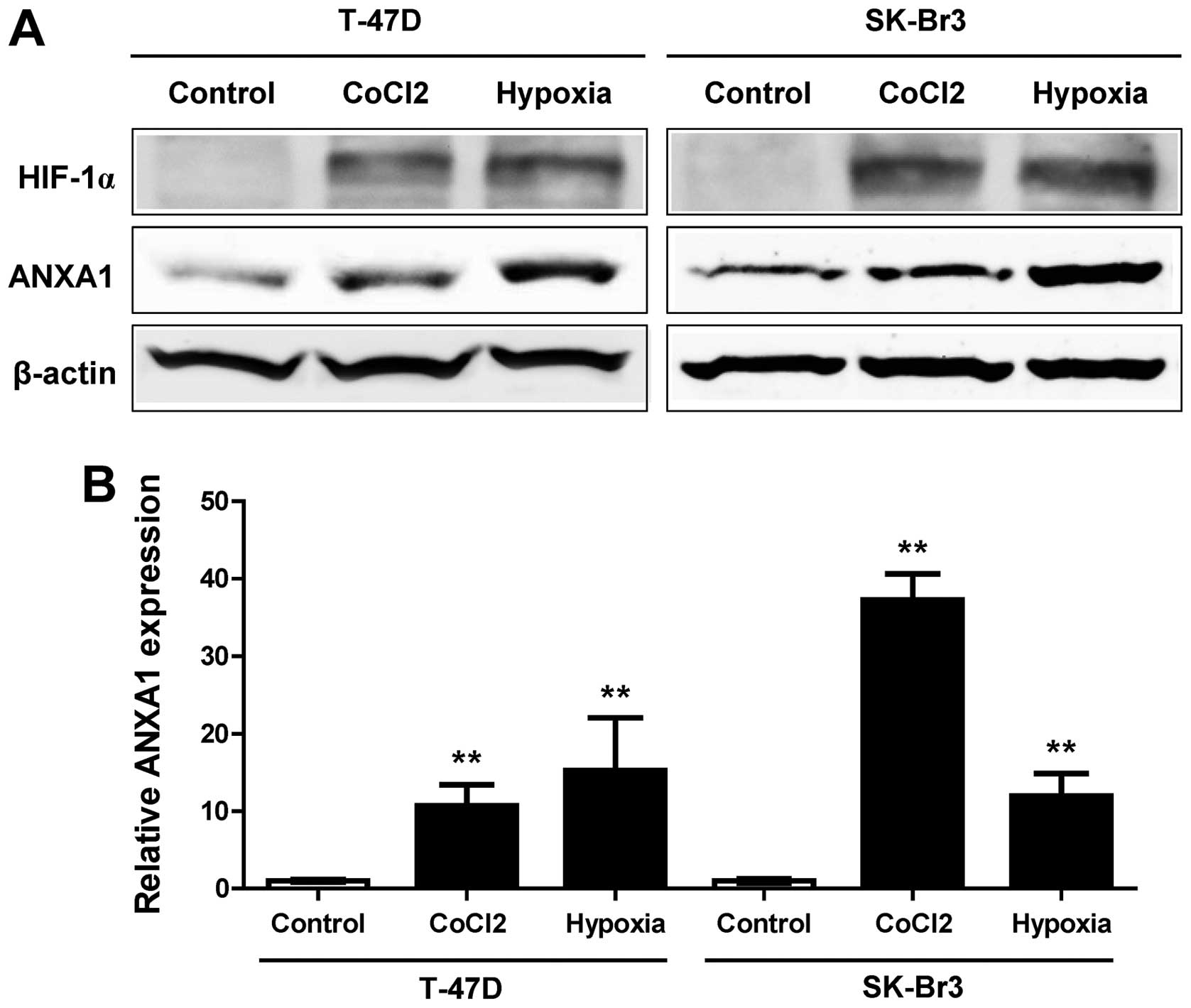
Induction of ANXA1 in hypoxia
To further assess the induction of ANXA1 during
cancer progression, we evaluated ANXA1 induction under hypoxic
conditions, which is one of the main characteristic features of a
tumor. T-47D and SK-Br3 breast cancer cell lines were treated with
hypoxia and the hypoxia mimic induced by CoCl2. HIF-1α
protein was induced in both cell lines (Fig. 4A). The ANXA1 protein was also
induced by hypoxia in both cell lines. This induction of ANXA1 was
also confirmed by examining mRNA expression (Fig. 4B). These results suggested that
hypoxia affects tumor invasion through induction of ANXA1 in
TNBC.
Discussion
Although it has been reported that ANXA1 plays an
important role in tumor development and progression, due to the
fact that the expression pattern of ANXA1 is different across tumor
types, the exact mechanisms of ANXA1 in cancer remain unknown.
While the ANXA1 expression on cancer tissues is downregulated in
head and neck, esophageal and prostate cancer, the expression is
upregulated in stomach, colorectal, pancreas and hepatic cancer
(12–19). In breast cancer, the expression and
functional roles of ANXA1 are controversial. Some reports describe
that cancer cells show positive ANXA1 expression, whereas
nonmalignant cells do not express ANXA1, except for myoepithelial
cells in normal tissues (20,21).
In vivo experiments using rats showed that ANXA1 was highly
expressed in lung metastatic tumors compared to parental cells
(20). However, other studies
demonstrated that low expression of ANXA1 was associated with poor
prognosis (22) and cancer
progression (23–25).
In the present study, we evaluated the ANXA1
expression in breast cancer tissues and detected positive
expression of ANXA1 in 31 (14.7%) of the 211 cases. Although the
positive ANXA1 cases appeared to have a relatively smaller ratio,
those cases had a significant correlation with TNBC and venous
invasion, suggesting that ANXA1 is associated with poor patient
outcome and malignant potential. We also evaluated the ANXA1
expression in several breast cancer cell lines. In the five breast
cancer cell lines, MDA-MB-231 cells highly expressed ANXA1 without
ER, PgR or HER2 expression. These cells are well known for their
invasive and metastatic potential (27,30–32).
Therefore, we investigated the biological significance of ANXA1 by
using knockdown methods in the MDA-MB-231 cells. As shown in
Fig. 3C, suppression of the ANXA1
expression resulted in a significant reduction of the cellular
invasion ability, showing the strong malignant potential of
ANXA1.
In addition, MDA-MB-231 cells show a multidrug
resistance due to upregulated ANXA1 expression (33,34).
This cell line shows significant resistance to adriamycin,
melphalan and etoposide compared to MCF-7 cells, which lack ANXA1
expression. Drug resistance is also related to hypoxia of malignant
tumors (35). Hypoxia inhibits
tumor cell proliferation, induces cell cycle arrest and affects the
expression of various proteins, resulting in the reduction of
chemotherapeutic effects (36). For
example, HIF-1α, a transcription factor that contains a basic
helix-loop-helix motif as well as a PAS domain, is increased by
hypoxia in various types of cancers and precancerous lesions
(37–40). HIF-1α promotes tumor progression
through direct or indirect interactions with important oncogenes
and tumor suppressor genes, such as MYC and TP53 (41). Hypoxia and the hypoxia mimic induced
by CoCl2 also increased ANXA1 expression in both the
T-47D and SK-Br-3 breast cancer cell lines. As HIF inhibitors are
currently undergoing clinical evaluation as a useful treatment for
cancer, simultaneous inhibition of ANXA1 with HIF inhibitors may
increase the antitumor effects in breast cancer. Taken together,
our results indicate that the clinical significance of ANXA1
expression may be related to the tumor characteristics of TNBC,
which is of high importance for developing new treatments.
Our large-scale examination could make a major
contribution to the understanding of the role of ANXA1. Although
further investigation is necessary, our results are a first step
into the new ANXA1 aspect in breast cancer.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Early Breast Cancer Trialists’
Collaborative Group. Davies C, Godwin J, et al: Relevance of breast
cancer hormone receptors and other factors to the efficacy of
adjuvant tamoxifen: patient-level meta-analysis of randomised
trials. Lancet. 378:771–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Early Breast Cancer Trialists’
Collaborative Group. Peto R, Davies C, et al: Comparisons between
different polychemotherapy regimens for early breast cancer:
meta-analyses of long-term outcome among 100,000 women in 123
randomised trials. Lancet. 379:432–444. 2012. View Article : Google Scholar
|
|
4
|
Gianni L, Dafni U, Gelber RD, et al:
Treatment with trastuzumab for 1 year after adjuvant chemotherapy
in patients with HER2-positive early breast cancer: a 4-year
follow-up of a randomised controlled trial. Lancet Oncol.
12:236–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cortazar P, Zhang L, Untch M, et al:
Pathological complete response and long-term clinical benefit in
breast cancer: the CTNeoBC pooled analysis. Lancet. 384:164–172.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cancer Genome Atlas Network. Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Turner NC and Reis-Filho JS: Tackling the
diversity of triple-negative breast cancer. Clin Cancer Res.
19:6380–6388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cancer Genome Atlas Research Network.
Kandoth C, Schultz N, et al: Integrated genomic characterization of
endometrial carcinoma. Nature. 497:67–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rescher U and Gerke V: Annexins - unique
membrane binding proteins with diverse functions. J Cell Sci.
117:2631–2639. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parente L and Solito E: Annexin 1: more
than an anti-phospholipase protein. Inflamm Res. 53:125–132. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim LH and Pervaiz S: Annexin 1: the new
face of an old molecule. FASEB J. 21:968–975. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garcia Pedrero JM, Fernandez MP, Morgan
RO, et al: Annexin A1 down-regulation in head and neck cancer is
associated with epithelial differentiation status. Am J Pathol.
164:73–79. 2004. View Article : Google Scholar
|
|
13
|
Luo A, Kong J, Hu G, et al: Discovery of
Ca2+-relevant and differentiation-associated genes
downregulated in esophageal squamous cell carcinoma using cDNA
microarray. Oncogene. 23:1291–1299. 2004. View Article : Google Scholar
|
|
14
|
Sinha P, Hütter G, Köttgen E, Dietel M,
Schadendorf D and Lage H: Increased expression of annexin I and
thioredoxin detected by two-dimensional gel electrophoresis of drug
resistant human stomach cancer cells. J Biochem Biophys Methods.
37:105–116. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duncan R, Carpenter B, Main LC, Telfer C
and Murray GI: Characterisation and protein expression profiling of
annexins in colorectal cancer. Br J Cancer. 98:426–433. 2008.
View Article : Google Scholar
|
|
16
|
Sato Y, Kumamoto K, Saito K, et al:
Up-regulated Annexin A1 expression in gastrointestinal cancer is
associated with cancer invasion and lymph node metastasis. Exp Ther
Med. 2:239–243. 2011.PubMed/NCBI
|
|
17
|
Bai XF, Ni XG, Zhao P, et al:
Overexpression of annexin 1 in pancreatic cancer and its clinical
significance. World J Gastroenterol. 10:1466–1470. 2004.PubMed/NCBI
|
|
18
|
Masaki T, Tokuda M, Ohnishi M, et al:
Enhanced expression of the protein kinase substrate annexin in
human hepatocellular carcinoma. Hepatology. 24:72–81.
1996.PubMed/NCBI
|
|
19
|
Kang JS, Calvo BF, Maygarden SJ, Caskey
LS, Mohler JL and Ornstein DK: Dysregulation of annexin I protein
expression in high-grade prostatic intraepithelial neoplasia and
prostate cancer. Clin Cancer Res. 8:117–123. 2002.PubMed/NCBI
|
|
20
|
Pencil SD and Toth M: Elevated levels of
annexin I protein in vitro and in vivo in rat and human mammary
adenocarcinoma. Clin Exp Metastasis. 16:113–121. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahn SH, Sawada H, Ro JY and Nicolson GL:
Differential expression of annexin I in human mammary ductal
epithelial cells in normal and benign and malignant breast tissues.
Clin Exp Metastasis. 15:151–156. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang LP, Bi J, Yao C, et al: Annexin A1
expression and its prognostic significance in human breast cancer.
Neoplasma. 57:253–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen D, Chang HR, Chen Z, et al: Loss of
annexin A1 expression in human breast cancer detected by multiple
high-throughput analyses. Biochem Biophys Res Commun. 326:218–227.
2005. View Article : Google Scholar
|
|
24
|
Shen D, Nooraie F, Elshimali Y, et al:
Decreased expression of annexin A1 is correlated with breast cancer
development and progression as determined by a tissue microarray
analysis. Hum Pathol. 37:1583–1591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao Y, Li Y, Edelweiss M, et al: Loss of
annexin A1 expression in breast cancer progression. Appl
Immunohistochem Mol Morphol. 16:530–534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bist P, Leow SC, Phua QH, et al: Annexin-1
interacts with NEMO and RIP1 to constitutively activate IKK complex
and NF-κB: implication in breast cancer metastasis. Oncogene.
30:3174–3185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Graauw M, van Miltenburg MH, Schmidt
MK, et al: Annexin A1 regulates TGF-beta signaling and promotes
metastasis formation of basal-like breast cancer cells. Proc Natl
Acad Sci USA. 107:6340–6345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saito M, Matsuzaki M, Sakuma T, et al:
Clinicopathological study of non-palpable familial breast cancer
detected by screening mammography and diagnosed as DCIS. Breast
Cancer. 21:140–145. 2014. View Article : Google Scholar
|
|
29
|
Saito M, Shiraishi K, Matsumoto K, et al:
A three-microRNA signature predicts responses to platinum-based
doublet chemotherapy in patients with lung adenocarcinoma. Clin
Cancer Res. 20:4784–4793. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mastro AM and Vogler EA: A
three-dimensional osteogenic tissue model for the study of
metastatic tumor cell interactions with bone. Cancer Res.
69:4097–4100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nicolson GL, Nawa A, Toh Y, Taniguchi S,
Nishimori K and Moustafa A: Tumor metastasis-associated human MTA1
gene and its MTA1 protein product: role in epithelial cancer cell
invasion, proliferation and nuclear regulation. Clin Exp
Metastasis. 20:19–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen WT, Lee CC, Goldstein L, et al:
Membrane proteases as potential diagnostic and therapeutic targets
for breast malignancy. Breast Cancer Res Treat. 31:217–226. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Serfass L, Roy MO, Wong J, Bonneau
AM and Georges E: Annexin-I expression modulates drug resistance in
tumor cells. Biochem Biophys Res Commun. 314:565–570. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chuthapisith S, Bean BE, Cowley G, et al:
Annexins in human breast cancer: Possible predictors of
pathological response to neoadjuvant chemotherapy. Eur J Cancer.
45:1274–1281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gottesman MM: Mechanisms of cancer drug
resistance. Annu Rev Med. 53:615–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gardner LB, Li Q, Park MS, Flanagan WM,
Semenza GL and Dang CV: Hypoxia inhibits G1/S transition through
regulation of p27 expression. J Biol Chem. 276:7919–7926. 2001.
View Article : Google Scholar
|
|
37
|
Giatromanolaki A, Koukourakis MI, Sivridis
E, et al: Relation of hypoxia inducible factor 1 alpha and 2 alpha
in operable non-small cell lung cancer to angiogenic/molecular
profile of tumours and survival. Br J Cancer. 85:881–890. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu L, Ning X, Sun L, et al:
Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced
chemoresistance in gastric cancer. Cancer Sci. 99:121–128.
2008.
|
|
39
|
Schindl M, Schoppmann SF, Samonigg H, et
al: Overexpression of hypoxia-inducible factor 1alpha is associated
with an unfavorable prognosis in lymph node-positive breast cancer.
Clin Cancer Res. 8:1831–1837. 2002.PubMed/NCBI
|
|
40
|
Welsh SJ, Bellamy WT, Briehl MM and Powis
G: The redox protein thioredoxin-1 (Trx-1) increases
hypoxia-inducible factor 1alpha protein expression: Trx-1
overexpression results in increased vascular endothelial growth
factor production and enhanced tumor angiogenesis. Cancer Res.
62:5089–5095. 2002.PubMed/NCBI
|
|
41
|
Keith B, Johnson RS and Simon MC: HIF1α
and HIF2α: sibling rivalry in hypoxic tumour growth and
progression. Nat Rev Cancer. 12:9–22. 2012.
|