Introduction
Nasopharyngeal carcinoma (NPC) is one of the most
common malignant tumors of the head and neck, and is endemic in
Southeast Asia, particularly in Southern China (1). Local recurrence and metastasis are the
main reasons that restrict the efficacy and prognosis after
radiotherapy. Some studies have shown that radioresistance is a
significant factor for local recurrence and metastasis of NPC
(2,3). Weakening radiation resistance to
increase the radiation sensitivity of tumor cells may be a
promising strategy by which to improve the 5-year survival of
patients with NPC.
Transcription factors play an important role in gene
expression. c-jun, an important component of activating protein-1
(AP-1) transcription factor, is closely related to cell
proliferation, apoptosis and malignant transformation (4). Clinically, c-jun overexpression has
been associated with oral squamous cell carcinomas, breast cancer,
non-small cell lung cancer and colorectal cancer (5–9). c-jun
was found to promote tumor growth and progression, and c-jun binds
the cyclin Dl promoter to promote its expression transcriptionally.
Overexpression of c-jun was found to lead to abnormal cell
proliferation and loss of apoptosis, thus it is considered to be a
positive regulator of the cell cycle (9). Previous studies have shown that the
expression of cyclin Dl is positively correlated with radiation
resistance (10,11). Our preliminary research indicated
that the expression of c-jun was significantly upregulated in
CNE-2R cells compaired to its expression in CNE-2 cells, and c-jun
may be associated with the radioresistance of NPC (12). However, the underlying mechanism of
such outcomes remains to be elaborated by subsequent
investigation.
In the present study, our data indicated that
knockdown of c-jun gene expression increased the sensitivity of
CNE-2R cells to radiation.
Materials and methods
Construction of the lentiviral
vectors
Small interfering RNA (siRNA) targeting the c-jun
sequence (CAAACCTCAGCAA CTTCAA) and a vector containing a scrambled
sequence (TTCTCCGAACGTGTCACGT) were transformed into short hairpin
RNA (shRNA) (stem-loop-stem structure) and cloned into
pLV-GV115-lentiviral vectors with AgeI/EcoRI sites
(Shanghai Genechem Biotechnology, Shanghai, China).
Cell culture and infection
CNE-2R, a radioresistant human NPC cell line, was
constructed and maintained at the Cancer Laboratory of Guangxi
Medical University. The cells were grown in RPMI-1640 culture
medium (Hyclone, Logan, UT, USA), supplemented with 10% fetal calf
serum (Gibco, Grand Island, NY, USA), in a humidified chamber at
37°C in 5% CO2. For the lentiviral infection, CNE-2R
cells were cultured in 6-well plates. Then, the
c-jun-shRNA-expressing lentivirus (c-jun-shRNA) or the scrambled
shRNA-expressing lentivirus (NC) was added, with a multiplicity of
infection (MOI) of 20 in the CNE-2R cells for 96 h. The
transduction efficiency was determined by inverted fluorescence
microscopy (Ix71; Olympus, Tokyo, Japan).
RNA extraction and RT-PCR analysis
Total RNA was extracted with TRIzol reagent
(Invitrogen, Carslbad, CA, USA) following the manufacturer’s
instructions. Single-strand cDNA templates were prepared from 1 μg
total RNA using the RT-PCR kit (Takara Biotechnology, Dalian,
China). Primers for c-jun and β-actin were designed (13) as follows: c-jun forward,
5′-TCCCCCAGCTATCTATATGCAAT-3′ and reverse,
5′-TCACAGCACATGCCACTTGA-3′; β-actin forward,
5′-ACCGAGCGCGGCTACAGC-3′ and reverse, 5′-CTCATTGCCAATGGTGAT-3′. PCR
amplification from cDNA was performed in a final volume of 20 μl.
After 95°C for 30 sec, the experimental reaction was subjected to
40 cycles at 95°C for 5 sec, 60°C for 30 sec, followed by a final
elongation at 95°C for 30 sec, and 60°C for 1 min. The PCR products
were subjected to amplification curve analysis, and fluorescence
was analyzed using the Light Cycler 480 software release 1.5.0
(Roche Diagnostics, Switzerland). All samples were examined in
triplicate. c-jun expression data were normalized against β-actin
using the comparative threshold cycle ΔΔCt method.
Western blot analysis
Total protein was extracted from the CNE-2R cells,
and each sample protein concentration was determined by Bradford
assay (Beyotime, China). Proteins were separated on 10% SDS-PAGE
and blotted onto PVDF membranes (Millipore, Bedford, MA, USA).
After blocking in 5% non-fat dry milk in Tris-buffered saline with
Tween-20 for 1 h at room temperature, the membranes were incubated
with the anti-c-jun monoclonal antibody (1:1,000 dilution; Cell
Signaling Technology, Boston, MA, USA) and anti-GAPDH antibody
(1:3,000 dilution; Proteintech, Chicago, IL, USA) at 4°C overnight.
The membranes were washed and then incubated with a secondary
antibody (1:15,000 dilution; Cell Signaling Technology) for 1 h at
room temperature. Images of c-jun were obtained by an infrared
fluorescence imaging system (Odyssey; Li-Cor Co., Lincoln, NE,
USA). GAPDH served as a control.
CCK-8 assay
The effects on cell survival of the
c-jun-shRNA-transfected cells were examined by CCK-8 assay. Cells
were plated in 96-well plates at 5×103 cells/well and
allowed to attach overnight. The cells were then irradiated with 6
MV X-radiation at doses of 2, 4, 6 and 8 Gy. After a 24-h
culture,10 μl of 10 mg/ml CCK-8 solution was added to each well for
1 h at 37°C. The absorbance of each well was measured using a
microplate reader (Bio-Rad, Hercules, CA, USA) at 450 nm. All
experiments were performed in triplicate. Cell survival was
calculated according to the following formula: Survival fraction %
= OD treated/OD untreated × 100%, where OD is the mean
absorbance.
Colony formation assay for
radiosensitivity
For the colony assay, cells were plated onto 6-well
plates and allowed to attach overnight. The cells were exposed to
different doses of radiation (2, 4, 6 and 8 Gy), and then the cells
were cultured for 14 days in a 5% CO2 atmosphere at 37°C
until colonies appeared. The colonies were fixed with carbinol
(KeLong Chemical Reagent Factory, ChengDu, China) for 20 min and
then stained with 0.1% Giemsa (Solarbio Science, Beijing, China)
for 15 min. The numbers of single colonies containing >50 cells
were scored as survivors. All experiments were performed three
times. Graphad Prism 5.0 software was used to create a fit curve.
The dose responses were analyzed using multi-target single-hit
mode, SF = 1 − (1 − e−D/D0)N, where D is the
single radiation dose, D0 is the single dose of
radiation producing a 37% survival rate, SF is the survival
fraction at dose D and N is the radiobiological parameter.
Cell cycle analysis
Cells were harvested and resuspended using a
cell-cycle kit from Beckman Coulter (Brea, CA, USA) according to
the manufacturer’s instructions. The DNA content was analyzed using
the FC500 flow cytometry systems (Beckman Coulter).
Apoptosis analysis
Cells were collected and resuspended in PBS, and
stained using the Annexin V-PE apoptosis detection kit to detect
apoptosis cells and 7-ADD to monitor dead cells according to the
manufacturer’s instructions (eBioscience, San Diego, CA, USA).
Samples were analyzed on the FC500 flow cytometry systems.
Statistical analysis
Resulted are presented as the mean ± standard
deviation (SD) of three independent experiments. Significant
difference among groups were analyzed by one-way ANOVA. P<0.05
was considered to denote statistically significant differences. All
statistical analyses were carried out with SPSS 16.0 statistical
software.
Results
Effective shRNA-mediated knockdown of
c-jun in the CNE-2R cells
The lentiviral shRNA was successfully constructed
and transduced into the CNE-2R cells. The transduction rate was
~90% at 96 h (Fig. 1). The
efficiency of the c-jun gene silencing of these recombinants was
confirmed by both RT-PCR and western blotting. As shown in Fig. 2, both the mRNA and protein
expression levels of c-jun were significantly decreased in the
shRNA-transduced group compared to the control group
(P<0.05).
Cell proliferation following c-jun-shRNA
transduction was examined by CCK-8 assay
To further assess the role of c-jun in regulating
cell proliferation, CCK-8 assays were performed on CNE-2R cells
following lentiviral infection for 96 h. As shown in Fig. 3, there were no statistically
significant differences between the scrambled shRNA-transfected
cells (NC) and the non-infected cells (control), indicating that
the lenti-viral system itself had no cytotoxic effect on the cells,
whereas the inhibition of c-jun expression significantly reduced
the growth rate of the CNE-2R cells following 6 MV X-radiation
compared with the control group (P<0.05).
Cells infected with the c-jun-shRNA
lentivirus display enhanced radiosensitivity
Next, we investigated the survival curves of the
colony formation assay. The curve of the c-jun-shRNA group was
higher than that of the other groups. The main parameters presented
in Table I and Fig. 4 are the normalized results from the
clonogenic experiments. From the D0 (dose of radiation
producing a 37% survival rate), Dq (quasi-threshold dose required
for cell damage) and SF2 (survival fraction at 2 Gy) values, it can
be concluded that the shRNA-mediated c-jun silencing led to a
greater decrease in the surviving fractions when compared with the
control. When the data were analyzed by the multi-target single-hit
model, SF = 1 − (1 − e−D/D0)N, by calculating
the sensitization enhancement ratio (SER), we used the
D0 of the control cells divided by the D0 of
the c-jun silenced cells, the values of SERD0 = 1.41
>1, suggesting that silencing of c-jun in the CNE-2R cells
sensitized the cells to radiation.
 | Table ICorrelation parameters in the
multi-target single-hit model. |
Table I
Correlation parameters in the
multi-target single-hit model.
| Cell lines | SF2 | D0 | Dq
(lnN*D0) |
|---|
| Control | 0.88 | 3.32 | 2.18 |
| NC | 0.85 | 3.33 | 2.08 |
| c-jun-shRNA | 0.61 | 2.36 | 1.37 |
Inhibition of c-jun induces
G2/M cell cycle arrest
To investigate the mechanism involved in the
inhibition of cell proliferation mediated by c-jun-shRNA in the
CNE-2R cells, we further employed flow cytometry to study the
effect of the shRNA-mediated c-jun downregulation on cell cycle
progression. As shown in Fig. 5,
compared with the control group, an obvious increase in the
G2/M-phase cell cycle population was observed in the
c-jun-shRNA group in the CNE-2R cells (P<0.05). Our results
revealed that c-jun-shRNA might exert an inhibitory effect on
CNE-2R cell proliferation via G2/M cell-cycle arrest,
which was related to the enhanced radiosensitivity.
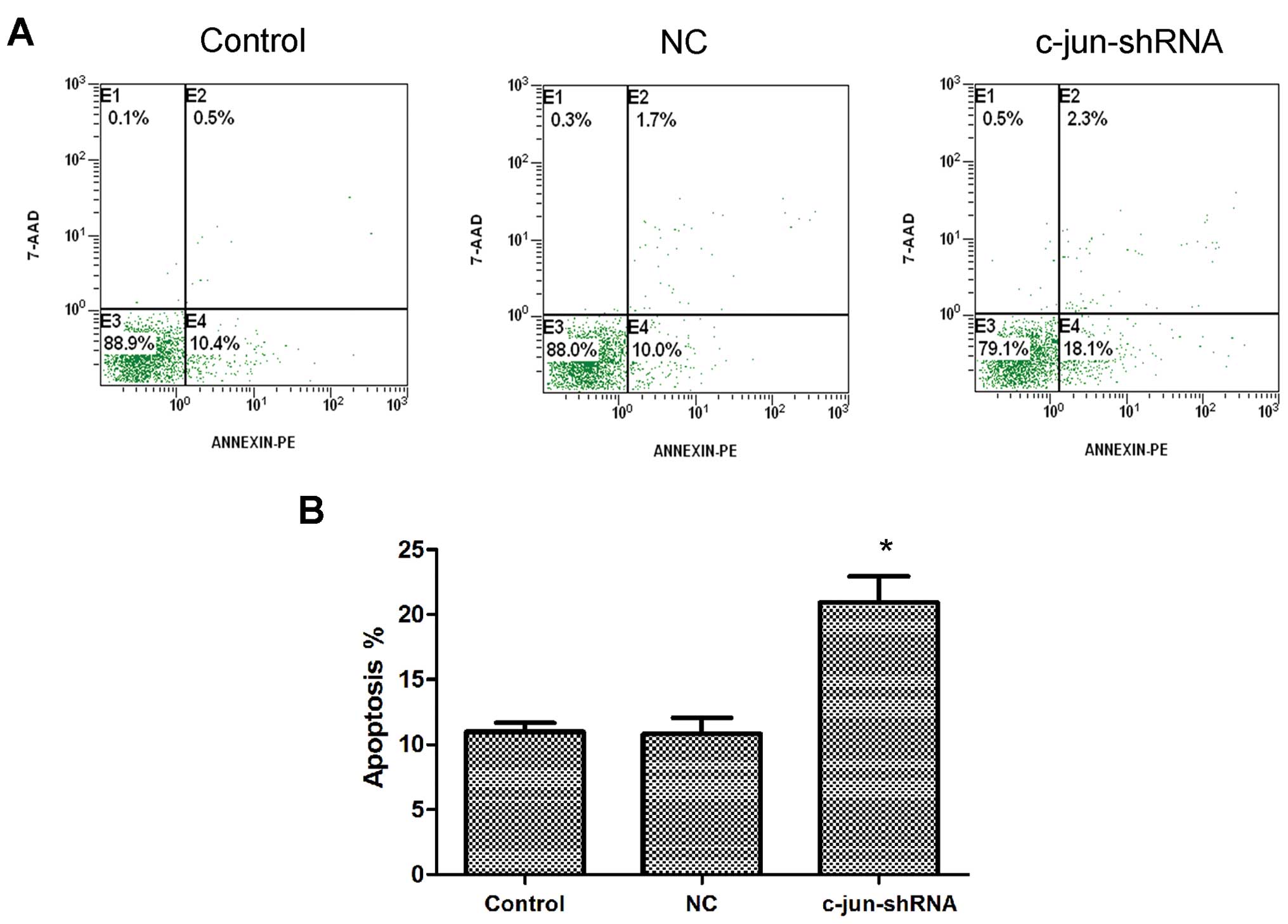
c-jun-shRNA promotes the apoptosis of
CNE-2R cells
FCM analysis was used to determine whether silencing
c-jun had an accelerated effect on the apoptosis of CNE-2R cells.
The results showed that the apoptosis rate of the CNE-2R cells
following c-jun-shRNA lentiviral transduction was obviously
increased, and the apoptosis rate of the control group, NC group
and c-jun-shRNA group was 10.97±0.7, 10.8±1.25 and 20.93±1.99%,
respectively (Fig. 6).
Discussion
NPC is a common type of cancer in Southern China.
Some patients can be cured by radiotherapy which is the main
therapeutic method (14), while
radioresistance of NPC affects the clinical efficiency. To date,
there are no effective biomarkers for predicting NPC
radioresistance.
c-jun is a major constituent of activating protein-1
(AP-1) transcription factor that transduces multiple mitogen growth
signals (15). Overexpression of
c-jun/AP-1 has been associated with tumor invasion, metastasis and
prognosis in many human cancers (5–9). In
our previous study (12), we used
fractionated radiation in vitro to construct the
radioresistant NPC (CNE-2R) cell line, and with cDNA microarray, we
found that the c-jun/AP-1 expression was upregulated in the
radioresistant CNE-2R cells when compared with the expression in
the parental CNE-2 cell line. Integrating microarray with gene
ontology and protein interaction networks showed that c-jun/AP-1
can positively regulate cell proliferation. Similarly, Kajanne
et al (16) suggested that
AP-1 could mediate EGFR and PI3K signaling in prostate cancer
cells, which is essential for cell proliferation, and confers
protection against radiation-induced cell death. This study
suggested that the transcription factor AP-1 promotes the growth
and radioresistance in prostate cancer cells.
The EGFR signaling pathway has been associated with
tumor radioresistance. Overexpression of EGFR is related with poor
response to radiotherapy in tumors (17). In head and neck cancer cells, a high
level of EGFR is correlated with radio-resistance. Anti-EGFR
monoclonal antibodies can improve radiosensitivity (18,19).
Among the downstream signaling pathways activated by EGFR, the
Ras/Raf/MEK/MAPK pathways have been well studied. Signals are
transferred to the nucleus by shc, grb2, c-jun and c-fos in order,
and then activate AP-1 affecting cell proliferation (20,21).
c-jun may play an essential role in radioresistance through the
EGFR pathway or AP-1. Therefore, specific downregulation of c-jun
may be a potential strategy to enhance radiosensitivity. However,
the effects of c-jun on radioresistance have not been reported in
NPC cells.
In the present study, we hypothesized that c-jun may
be correlated with radiation resistance in CNE-2R cells. Given the
prevalence and availability of RNA interference (RNAi) technology
in cancer research or cancer therapy (22), we used a lentivirus shRNA system
that effectively knocked down the expression of c-jun at both the
mRNA and protein levels. As shown in Fig. 2, RT-PCR and western blot analysis
showed effective silencing of c-jun, thus ensuring the reliability
of the subsequent assays. Our results demonstrated that inhibition
of c-jun by shRNA enhanced response to radiation (Fig. 4), which significantly reduced cell
proliferation (Fig. 3), altered the
cell cycle (Fig. 5), and promoted
cell apoptosis (Fig. 6).
The cell cycle phase determines the relative
radiosensitivity of cells. Cells are the most radiosensitive in the
G2/M phase, less sensitive in the G1 phase
and the least sensitive during the S phase (23). Our data revealed that shRNA-mediated
knockdown of c-jun increased G2/M arrest, and cells were
easily killed by radiation. In other words, inhibition of c-jun in
CNE-2R cells enhanced the radiosensitivity of the cells.
Apoptosis also known as programmed cell death,
removes injured or disabled cells to maintain the balance of the
micro-environment. The main mechanism of radiotherapy in tumors is
to induce the apoptosis of cells (24,25).
At present there has been some controversy regarding the effect of
c-jun/AP-1 on cell apoptosis (26,27).
Downregulation of c-jun expression by blocking JNK was found to
inhibit the activation of AP-1 and reduce apoptosis in certain
types of cells (28). However, some
studies also suggest that, in some types of stress, the expression
of c-jun was increased, but did not induce apoptosis. In contrast,
it may promote the proliferation and differentiation of cells
(29). In mice, deletion of the
c-jun gene or changes in JNK phos-phorylation sites were found to
shrink intestinal tumors, and increase the life span of mice
(30).
Our data also showed that the apoptosis rate of the
c-jun-shRNA group was higher than this rate in the control groups
(P<0.05). However, whether the mechanism involved in the
promotion of apoptosis by decreased c-jun expression in CNE-2R
cells is the same by inhibiting the activity of AP-1,
downregulating cyclin D1 (31),
arresting the cell cycle, inhibiting cancer cell proliferation or
other manners needs to be further studied.
In conclusion, the levels of c-jun appear to be
highly important for the radioresistance of CNE-2R cells.
shRNA-mediated knockdown of c-jun in the radioresistant CNE-2R
cells enhanced radiosensitivity, induced cell cycle arrest and
apop-tosis. Our findings imply that the overexpression of c-jun may
serve as a potential target to enhance the radiation sensitivity
for NPC therapy.
Acknowledgements
This study was supported by the National Natural
Science Foundation (grant no. 30860329), the Guangxi Natural
Science Foundation (grant no. 0832229) and Major Research Projects
of Guangxi Universities (grant no. 201101ZD004).
References
|
1
|
Jia WH, Huang QH, Liao J, et al: Trends in
incidence and mortality of nasopharyngeal carcinoma over a 20–25
year period (1978/1983–2002) in Sihui and Cangwu counties in
southern China. BMC Cancer. 6:1782006. View Article : Google Scholar
|
|
2
|
Lee AW, Poon YF, Foo W, et al:
Retrospective analysis of 5037 patients with nasopharyngeal
carcinoma treated during 1976–1985: overall survival and patterns
of failure. Int J Radiat Oncol Biol Phys. 23:261–270. 1992.
View Article : Google Scholar
|
|
3
|
Leung SF, Teo PM, Shiu WW, Tsao SY and
Leung TW: Clinical features and management of distant metastases of
nasopharyngeal carcinoma. J Otolaryngol. 20:27–29. 1991.PubMed/NCBI
|
|
4
|
Shaulian E: AP-1 - The Jun proteins:
Oncogenes or tumor suppressors in disguise? Cell Signal.
22:894–899. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao L, Huang S, Ren WH, et al: Jun
activation domain-binding protein 1 expression in oral squamous
cell carcinomas inversely correlates with the cell cycle inhibitor
p27. Med Oncol. 29:2499–2504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song X, Tao YG, Deng XY, et al:
Heterodimer formation between c-Jun and Jun B proteins mediated by
Epstein-Barr virus encoded latent membrane protein 1. Cell Signal.
16:1153–1162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gonzalez-Villasana V, Gutierrez-Puente Y
and Tari AM: Cyclooxygenase-2 utilizes Jun N-terminal kinases to
induce invasion, but not tamoxifen resistance, in MCF-7 breast
cancer cells. Oncol Rep. 30:1506–1510. 2013.PubMed/NCBI
|
|
8
|
Wang CY, Chen CL, Tseng YL, et al: Annexin
A2 silencing induces G2 arrest of non-small cell lung cancer cells
through p53-dependent and -independent mechanisms. J Biol Chem.
287:32512–32524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qing H, Gong W, Che Y, et al:
PAK1-dependent MAPK pathway activation is required for colorectal
cancer cell proliferation. Tumour Biol. 33:985–994. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimura T, Kakuda S, Ochiai Y, et al:
Acquired radioresistance of human tumor cells by
DNA-PK/AKT/GSK3beta-mediated cyclin D1 overexpression. Oncogene.
29:4826–4837. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimura T: Acquired radioresistance of
cancer and the AKT/GSK3β/cyclin D1 overexpression cycle. J Radiat
Res. 52:539–544. 2011. View Article : Google Scholar
|
|
12
|
Guo Y, Zhu XD, Qu S, et al: Identification
of genes involved in radioresistance of nasopharyngeal carcinoma by
integrating gene ontology and protein-protein interaction networks.
Int J Oncol. 40:85–92. 2012.
|
|
13
|
Zhang JS, Li DM, Ma Y, et al:
γ-Tocotrienol induces paraptosis-like cell death in human colon
carcinoma SW620 cells. PLoS One. 8:e577792013. View Article : Google Scholar
|
|
14
|
Perri F, Bosso D, Buonerba C, Lorenzo GD
and Scarpati GD: Locally advanced nasopharyngeal carcinoma: Current
and emerging treatment strategies. World J Clin Oncol. 2:377–383.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kikuchi J, Kinoshita I, Shimizu Y, et al:
Simultaneous blockade of AP-1 and phosphatidylinositol 3-kinase
pathway in non-small cell lung cancer cells. Br J Cancer.
99:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kajanne R, Miettinen P, Tenhunen M and
Leppä S: Transcription factor AP-1 promotes growth and
radioresistance in prostate cancer cells. Int J Oncol.
35:1175–1182. 2009.PubMed/NCBI
|
|
17
|
Golding SE, Morgan RN, Adams BR, Hawkins
AJ, Povirk LF and Valerie K: Pro-survival AKT and ERK signaling
from EGFR and mutant EGFRvIII enhances DNA double-strand break
repair in human glioma cells. Cancer Biol Ther. 8:730–738. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hatanpaa KJ, Burma S, Zhao D and Habib AA:
Epidermal growth factor receptor in glioma: signal transduction,
neuropathology, imaging, and radioresistance. Neoplasia.
12:675–684. 2010.PubMed/NCBI
|
|
19
|
Grana TM, Sartor CI and Cox AD: Epidermal
growth factor receptor autocrine signaling in RIE-1 cells
transformed by the Ras oncogene enhances radiation resistance.
Cancer Res. 63:7807–7814. 2003.PubMed/NCBI
|
|
20
|
Bergström JD, Westermark B and Heldin NE:
Epidermal growth factor receptor signaling activates met in human
anaplastic thyroid carcinoma cells. Exp Cell Res. 259:293–299.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Denning MF, Dlugosz AA, Cheng C, et al:
Cross-talk between epidermal growth factor receptor and protein
kinase C during calcium-induced differentiation of keratinocytes.
Exp Dermatol. 9:192–199. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Izquierdo M: Short interfering RNAs as a
tool for cancer gene therapy. Cancer Gene Ther. 12:217–227. 2005.
View Article : Google Scholar
|
|
23
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shinomiya N: New concepts in
radiation-induced apoptosis: ‘premitotic apoptosis’ and
‘postmitotic apoptosis’. J Cell Mol Med. 5:240–253. 2001.
View Article : Google Scholar
|
|
25
|
Shi W, Bastianutto C, Li A, et al:
Multiple dysregulated pathways in nasopharyngeal carcinoma revealed
by gene expression profiling. Int J Cancer. 119:2467–2475. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eferl R and Wagner EF: AP-1: a
double-edged sword in tumorigenesis. Nat Rev Cancer. 3:859–868.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shaulian E and Karin M: AP-1 as a
regulator of cell life and death. Nat Cell Biol. 4:E131–E136. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kutuk O, Poli G and Basaga H: Resveratrol
protects against 4-hydroxynonenal-induced apoptosis by blocking JNK
and c-JUN/AP-1 signaling. Toxicol Sci. 90:120–132. 2006. View Article : Google Scholar
|
|
29
|
Hu Z, Tao YG, Yang LF, et al: Effect of
JIP on the proliferation and apoptosis of nasopharyngeal carcinoma
cells. Ai Zheng. 21:1182–1186. 2002.(In Chinese).
|
|
30
|
Nateri AS, Spencer-Dene B and Behrens A:
Interaction of phosphorylated c-Jun with TCF4 regulates intestinal
cancer development. Nature. 437:281–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su ZZ, Lee SG, Emdad L, et al: Cloning and
characterization of SARI (suppressor of AP-1, regulated by IFN).
Proc Natl Acad Sci USA. 105:20906–20911. 2008. View Article : Google Scholar : PubMed/NCBI
|