Introduction
Hepatocellular carcinoma (HCC) is the most common
malignant tumor and the third leading cause of cancer mortality
worldwide (1). In China, HCC causes
~110,000 deaths annually (2).
Current chemotherapeutic drugs for treating HCC are restricted in
their clinical application because of toxicity and low efficacy
(3,4). Arsenic trioxide
(As2O3) (1–8 μM) arrests HCC (HepG2 and
SMMC-7721 cells) in the G2/M phase (5). However, applications there of are
controversial as arsenic compounds exhibit high toxicity.
Therefore, developing a novel high-efficacy therapeutic drug for
hepatoma is required (4). In 1935,
Raistrick and Smith (6) first
isolated (+)-terrein from Aspergillus terreus. Terreins with
distinct configurations have received considerable attention
because of their various substantial bioactivities, including
anticancer properties against human cells (7–10).
In antitumor therapy, numerous drugs affect
tumorigenesis and tumor growth; however, the key is determining
which drugs to exploit in the areas of signal transduction,
cell-cycle regulation, apoptosis, telomere biology, necrosis,
autophagy, cell senescence and angiogenesis (11–13).
Angiogenesis is a critical process for tumor growth, invasion, and
metastasis (14). Arakawa et
al (7) determined that
(−)-terrein inhibited angiogenin secretion in the tumor
angiogenesis of androgen-dependent prostate cancer cells. Apoptosis
is a form of cell death (12,15).
Furthermore, (+)-terrein suppresses the proliferation of breast
cancer cells (9), human cervical
carcinoma cells (10), and
pulmonary tumor cells (8) by
inducing an apoptotic mechanism. These results suggested that
terreins inhibit tumor cell growth through multiple strategies.
Findings of studies showed that the isolation and
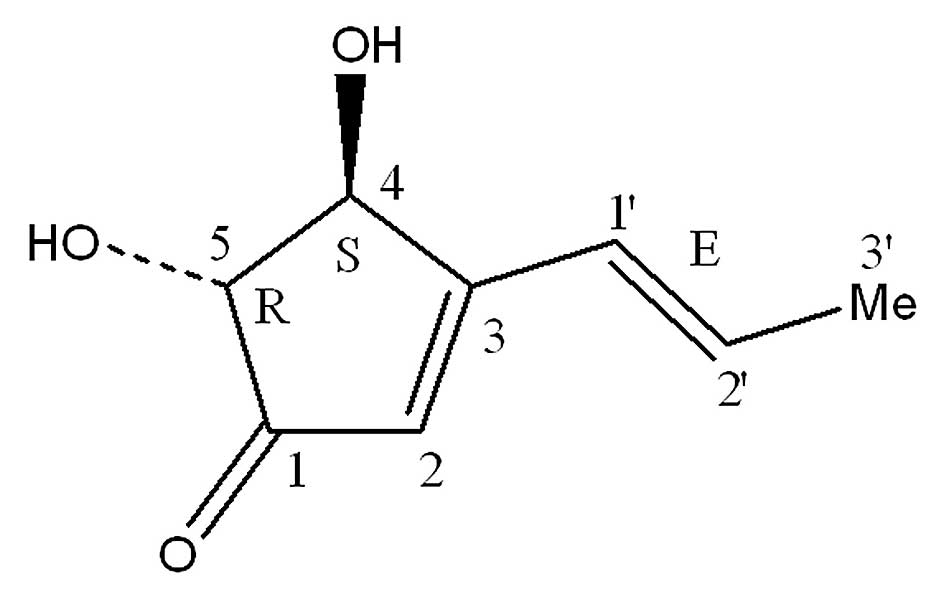
production of (+)-terrein (the molecular formula is
C8H10O3 and the molecular weight
is 154; Fig. 1) from the fungus
A. terreus PF-26 associated with marine sponges were improved
to ~9.07 g/l (16–19). However, the activity of (+)-terrein
against HCC and its mechanism remain unknown. In this study, the
anticancer activity and mechanism of (+)-terrein against HCC were
investigated using the Bel-7402 human hepatoma cell line. The
results showed that (+)-terrein suppressed Bel-7402 human hepatoma
cell growth and proliferation. The results indicated that 10 μM
(+)-terrein induced cell cycle arrest in the G2/M phase
and decreased the cell morphology gene expression of fibronectin,
N-cadherin, and vimentin. In addition, the high-throughput platform
with parallel detection of multiple mRNAs revealed that treating
Bel-7402 cells with (+)-terrein substantially altered the
expression of cell cycle-related genes. In addition, (+)-terrein
did not induce Bel-7402 cell apoptosis, indicating that (+)-terrein
inhibits cell proliferation through distinct mechanisms in
different cell strains.
Materials and methods
Reagents and cell lines
(+)-Terrein (Fig. 1)
was isolated from A. terreus PF-26, as described previously
(18). The isolated (+)-terrein was
dissolved in phosphate-buffered saline (PBS, pH 7.2) for subsequent
experiments. The human A549 lung adenocarcinoma epithelial cell
line was provided by Dr Wei Ma (Shanghai Jiao Tong University,
China), and the Bel-7402 human hepatoma cell line was obtained from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
The mentioned cells were cultured in Dulbecco’s modified Eagle’s
medium (DMEM) containing 10% fetal bovine serum and 100 U/ml of
penicillin and streptomycin. Unless otherwise mentioned, reagents
for cell cultures were purchased from Gibco/Invitrogen (New York,
Grand Island, USA) and biochemical reagents were obtained from
Sigma (New York, NY, USA) or Ameresco (Solon, OH, USA). The cells
were grown in a 5% CO2 atmosphere at 37°C.
Cell viability and proliferation
An MTT assay
[3-(4,5-dime-thylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was
performed with ~4×103 cells/well in 96-well plates. The
plates containing the cells were incubated at 37°C for 24 h, and
the cells were treated with (+)-terrein at 37°C for 48 h. The
protocol was performed using an MTT cell proliferation and
cytotoxicity detection kit (KeyGen, Nanjing, China) according to
the manufacturer’s instructions. Briefly, DMEM was supplemented
with 50 μl of MTT reagent to each well and incubated at 37°C for 4
h. Thereafter, the MTT solution was removed. Following the addition
of 150 μl of dimethyl sulfoxide (DMSO), the plates were incubated
at 37°C for 15 min to dissolve the formazan crystals. Absorbance of
DMSO extracts was detected at 550 nm by using an Enspire 2300
microplate reader (PerkinElmer, Foster City, CA, USA). A total of
~3×105 cells/well were inoculated in 6-well plates at
37°C for 24 h and treated with (+)-terrein at 37°C for 48 h. The
PBS-treated cells served as controls. These cells were used to
detect cell morphology, cell cycle, apoptosis, and RNA
extraction.
Light microscopy analysis
Cells (3×105) were cultured in 6-well
plates at 37°C for 24 h. The Bel-7402 and A549 cells were then
treated with 10 μM and 10 mM (+)-terrein at 37°C for 48 h,
respectively, and the PBS-treated cells served as controls. The
treated cells were used to observe cell morphology and apoptosis.
Light microscopy images of the cells were captured using a Nikon
Eclipse Ti-inverted microscope and a Nikon digital sight s-Qi1Mc
camera (both from Yokohama, Japan). For each surface, three
non-overlapping images were selected.
Cell analysis via flow cytometry
The cells were rinsed once in chilled PBS, digested
with 0.25% trypsin (Gibco), and then resuspended in DMEM and 10%
serum. The suspended cells were centrifuged at 2,000 × g at 4°C for
5 min and washed once in cold PBS. The cells were stained with
Alexa Fluor® 488 Annexin V and PI by using an Alexa
Fluor® 488 Annexin V/Dead cell apoptosis kit
(Invitrogen, New York, USA) according to the manufacturer’s
instructions. The stained cells were analyzed using flow cytometry
(FACSAria-II, BD Biosciences, San Jose, CA, USA).
Gene expression analysis of cell cycle
and cell morphology
The Bel-7402 cells treated with 10 μM (+)-terrein
were trypsinized and washed with a PBS buffer. Fifty microliters of
single cell suspension was examined using a cell cycle detection
kit (KeyGen). The stained cells were analyzed using flow cytometry
(FACSAria II).
Total RNA extraction
The Bel-7402 cells treated with (+)-terrein or PBS
were trypsinized and washed with a PBS buffer. The cells were
collected using centrifugation at 2,000 × g for 5 min. The cell
pellet was then resuspended in RL buffer and centrifuged at 13,000
× g for 5 min. Total RNA was extracted according to the
manufacturer’s instructions (CWBio, Beijing, China). The purity and
concentration of RNA was confirmed by the relative absorbance ratio
at 260/280 nm and 260 nm, respectively, by using NanoDrop 2000
(Thermo Fisher Scientific, Waltham, MA, USA).
Reverse transcription
Reverse transcription was performed according to the
manufacturer’s instructions (Thermo Fisher Scientific). Total RNA
(1 μg) was mixed with 4 μl of a 5X reaction buffer, 1 μl of oligo
(dT)18 primer, 1 μl of RiboLock RNase inhibitor, 2 μl of a 10 mM
dNTP mix, 1 μl of RevertAid reverse transcriptase (100 U/μl), and
then, ddH2O was added to increase the volume to 20 μl.
Reverse transcription was performed at 37°C for 60 min and then at
70°C for 5 min. The resulting cDNA was stored at −70°C until
use.
Polymerase chain reaction (PCR) array and
primer design
Table I shows that
73 potential genes involved in the cell cycle were used as the
target mRNAs. GAPDH, b2-MG, β-actin,
RPL27, HPRT1, and OAZ1 were used as
housekeeping genes for the control cells. The primers were designed
(CT Bioscience Co., Changzhou, China) to cover all of the
transcripts of each gene. Table I
shows the RefSeq accession IDs. All of the primers had a similar
melting temperature (Tm), and it was not located in the genomic
repetitive regions. The primers were selected based on criteria
such as a typical amplification curve and single peak from the
post-PCR melting curve. FN, gene ID 2335; N-cadherin, gene ID 1000;
and vimentin, gene ID 7431 which are involved in cell morphology
were also investigated.
 | Table IGene information detected by the
primers. |
Table I
Gene information detected by the
primers.
| Gene ID as in PCR
array | Symbol | Gene ID | Gene name |
|---|
| 1 | ANAPC2 | 29882 | Anaphase promoting
complex subunit 2 |
| 2 | ANAPC4 | 29945 | Anaphase promoting
complex subunit 4 |
| 3 | ANAPC5 | 51433 | Anaphase promoting
complex subunit 5 |
| 4 | BUB1 | 699 | Budding uninhibited
by benzimidazoles 1 homolog (yeast) |
| 5 | BUB1B | 701 | Budding uninhibited
by benzimidazoles 1 homolog β (yeast) |
| 6 | BUB3 | 9184 | Budding uninhibited
by benzimidazoles 3 homolog (yeast) |
| 7 | CCNE1 | 898 | Cyclin E1 |
| 8 | CCNE2 | 9134 | Cyclin E2 |
| 9 | CCND1 | 595 | Cyclin D1 |
| 10 | CCND2 | 894 | Cyclin D2 |
| 11 | CCND3 | 896 | Cyclin D3 |
| 12 | CCNH | 902 | Cyclin H |
| 13 | CDC16 | 8881 | Cell division cycle
16 homolog (S. cerevisiae) |
| 14 | CDC20 | 991 | Cell division cycle
20 homolog (S. cerevisiae) |
| 15 | CDC23 | 8697 | Cell division cycle
23 homolog (S. cerevisiae) |
| 16 | CDC25A | 993 | Cell division cycle
25 homolog A (S. pombe) |
| 17 | CDC25B | 994 | Cell division cycle
25 homolog B (S. pombe) |
| 18 | CDC25C | 995 | Cell division cycle
25 homolog C (S. pombe) |
| 19 | CDC26 | 246184 | Cell division cycle
26 homolog (S. cerevisiae) |
| 20 | CDC27 | 996 | Cell division cycle
27 homolog (S. cerevisiae) |
| 21 | CDC6 | 990 | Cell division cycle
6 homolog (S. cerevisiae) |
| 22 | CDC7 | 8317 | Cell division cycle
7 homolog (S. cerevisiae) |
| 23 | CDK4 | 1019 | Cyclin-dependent
kinase 4 |
| 24 | CDK6 | 1021 | Cyclin-dependent
kinase 6 |
| 25 | CDK7 | 1022 | Cyclin-dependent
kinase 7 |
| 26 | CDKN1B | 1027 | Cyclin-dependent
kinase inhibitor 1B (p27, Kip1) |
| 27 | CHEK1 | 1111 | Checkpoint kinase
1 |
| 28 | CHEK2 | 11200 | Checkpoint kinase
2 |
| 29 | E2F1 | 1869 | E2F transcription
factor 1 |
| 30 | E2F2 | 1870 | E2F transcription
factor 2 |
| 31 | E2F3 | 1871 | E2F transcription
factor 3 |
| 32 | HDAC1 | 3065 | Histone deacetylase
1 |
| 33 | MAD2L1 | 4085 | MAD2 mitotic arrest
deficient-like 1 (yeast) |
| 34 | MAX | 4149 | MYC-associated
factor X |
| 35 | MCM2 | 4171 | Minichromosome
maintenance complex component 2 |
| 36 | MCM3 | 4172 | Minichromosome
maintenance complex component 3 |
| 37 | MCM4 | 4173 | Minichromosome
maintenance complex component 4 |
| 38 | MCM5 | 4174 | Minichromosome
maintenance complex component 5 |
| 39 | MCM6 | 4175 | Minichromosome
maintenance complex component 6 |
| 40 | MCM7 | 4176 | Minichromosome
maintenance complex component 7 |
| 41 | ORC1L | 4998 | Origin recognition
complex, subunit 1 |
| 42 | ORC2L | 4999 | Origin recognition
complex, subunit 2 |
| 43 | ORC6L | 23594 | Origin recognition
complex, subunit 6 |
| 44 | PCNA | 5111 | Proliferating cell
nuclear antigen |
| 45 | PKMYT1 | 9088 | Protein kinase,
membrane-associated tyrosine/threonine 1 |
| 46 | RB1 | 5925 | Retinoblastoma
1 |
| 47 | RBL1 | 5933 | Retinoblastoma-like
1 (p107) |
| 48 | SKP2 | 6502 | S-phase
kinase-associated protein 2, E3 ubiquitin protein ligase |
| 49 | SMC1A | 8243 | Structural
maintenance of chromosomes 1A |
| 50 | TOP2A | 7153 | Topoisomerase (DNA)
II α 170 kDa |
| 51 | TP53 | 7157 | Tumor protein
p53 |
| 52 | TFDP1 | 7027 | Transcription
factor Dp-1 |
| 53 | WEE1 | 7465 | WEE1 homolog (S.
pombe) |
| 54 | | | Tyrosine
3-monooxygenase/tryptophan 5-monooxygenase activation |
| YWHAE | 7531 | Protein, ɛ
polypeptide |
| 55 | CDKN2A | 1029 | Cyclin-dependent
kinase inhibitor 2A |
| 56 | CDKN2B | 1030 | Cyclin-dependent
kinase inhibitor 2B (p15, inhibits CDK4) |
| 57 | PSMD9 | 5715 | Proteasome
(prosome, macropain) 26S subunit, non-ATPase, 9 |
| 58 | CDKN1C | 1028 | Cyclin-dependent
kinase inhibitor 1C (p57, Kip2) |
| 59 | CDC2 | 983 | Cyclin-dependent
kinase 1 |
| 60 | CCNB2 | 9133 | Cyclin B2 |
| 61 | CCNB1 | 891 | Cyclin B1 |
| 62 | CDKN1A | 1026 | Cyclin-dependent
kinase inhibitor 1A (p21, Cip1) |
| 63 | CDK2 | 1017 | Cyclin-dependent
kinase 2 |
| 64 | CCNA1 | 8900 | Cyclin A1 |
| 65 | CCNA2 | 890 | Cyclin A2 |
| 66 | MYC | 4609 | V-myc
myelocytomatosis viral oncogene homolog (avian) |
| 67 | CDKN2C | 1031 | Cyclin-dependent
kinase inhibitor 2C (p18, inhibits CDK4) |
| 68 | GAPDH | 2597 |
Glyceraldehyde-3-phosphate
dehydrogenase |
| 69 | ACTB | 60 | Actin, β |
| 70 | B2M | 567 |
β-2-microglobulin |
| 71 | HPRT1 | 3251 | Hypoxanthine
phosphoribosyltransferase 1 |
| 72 | OAZ1 | 4946 | Ornithine
decarboxylase antizyme 1 |
| 73 | RPL27 | 6155 | Ribosomal protein
L27 |
Real-time PCR analysis
Each cDNA was diluted to 1 ml with ddH2O
and mixed with 1 ml of 2X SYBR Premix Ex Taq™ (Takara, Dalian,
China). Twenty microliters of this mixture was added to each well
of a 96-well PCR array, except for genomic DNA control (GDC). The
96-well PCR plates containing gene primers were prepared by the CT
Bioscience Company (Changzhou, China). The sealed PCR plate was
loaded in an Eppendorf Realplex 4S (Hamburg, German). The PCR was
performed under the following conditions: 95°C for 5 min, 40 cycles
at 95°C for 15 sec, 60°C for 15 sec, and 72°C for 20 sec. The
melting curve procedure (95°C for 15 sec, 60°C for 15 sec, and 95°C
for 15 sec) was implemented to analyze the PCR specificity.
Dissociation curves (DC) and melting temperatures (Tm) were
recorded. Relative changes in gene expression were calculated using
the threshold cycle (Ct) method (20). The formula used is presented as
follows: n-fold change = 2−ΔΔCt = (Ct target gene − Ct
internal control gene) treated sample − (Ct target gene − Ct
internal control gene) control sample.
Statistical and pathway analysis
Statistical product and service solutions (SPSS ver.
13.0) was used for the data analysis. All of the experiments were
conducted in duplicate. The results are presented as mean ± SD
(standard deviation) unless otherwise specified. The P-values were
two-tailed. P≤0.05 was considered to indicate a statistically
significant difference. The cell-cycle pathway is a highly
regulated process that incorporates three major checkpoints
including the participation of several genes (21). The functional pathways associated
with the set of differentially expressed genes were analyzed using
the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis
(http://www.kegg.jp/kegg/pathway.html). Differentially
expressed gene with >2-fold change were analyzed in the
cell-cycle pathway (22). The
pathway map was created using the Pathview™ package (23).
Results
(+)-Terrein reduces cell growth
The in vitro toxicity of (+)-terrein against
Bel-7402 cells and the A549 human lung adenocarcinoma epithelial
cell line was evaluated using the MTT method
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide] to
determine the potential inhibitory concentration. MTT analysis
revealed that(+)-terrein inhibited cell viability and proliferation
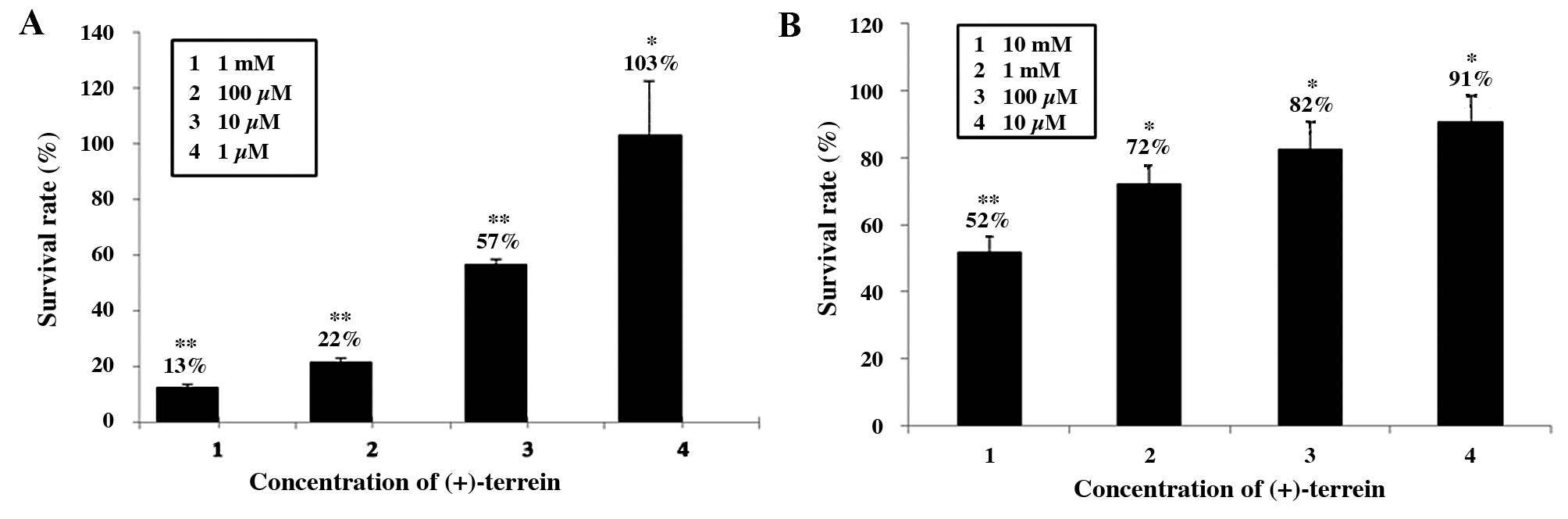
in a concentration-dependent manner. Fig. 2A shows the inhibition of Bel-7402
cells at various (+)-terrein concentrations. The IC50
(half maximal inhibitory concentration) value of Bel-7402 was
calculated as 11.63 μM ±0.02. The dose-dependent inhibition of
Bel-7402 indicated that 1 μM (+)-terrein was non-toxic and 10 μM
(+)-terrein was cytotoxic and associated with a survival rate of
57%. The survival rate of the Bel-7402 cells treated with 100 μM
and 1 mM (+)-terrein exhibited inhibitory activities of ~22 and
13%, respectively. A low dose of (+)-terrein did not substantially
affect the A549 cells. As indicated in Fig. 2B, (+)-terrein at various
concentrations induced inhibitory activity against the A549 cells.
(+)-Terrein at concentrations of 10 μM, 100 μM, and 1 mM exhibited
weak inhibitory activity, and the survival rate of the treated
cells was 91, 82, and 72%, respectively. Moreover, (+)-terrein at
10 mM was cytotoxic to A549 cells, and the survival rate of the
treated cells was 52%.
(+)-Terrein induces cell morphology
change
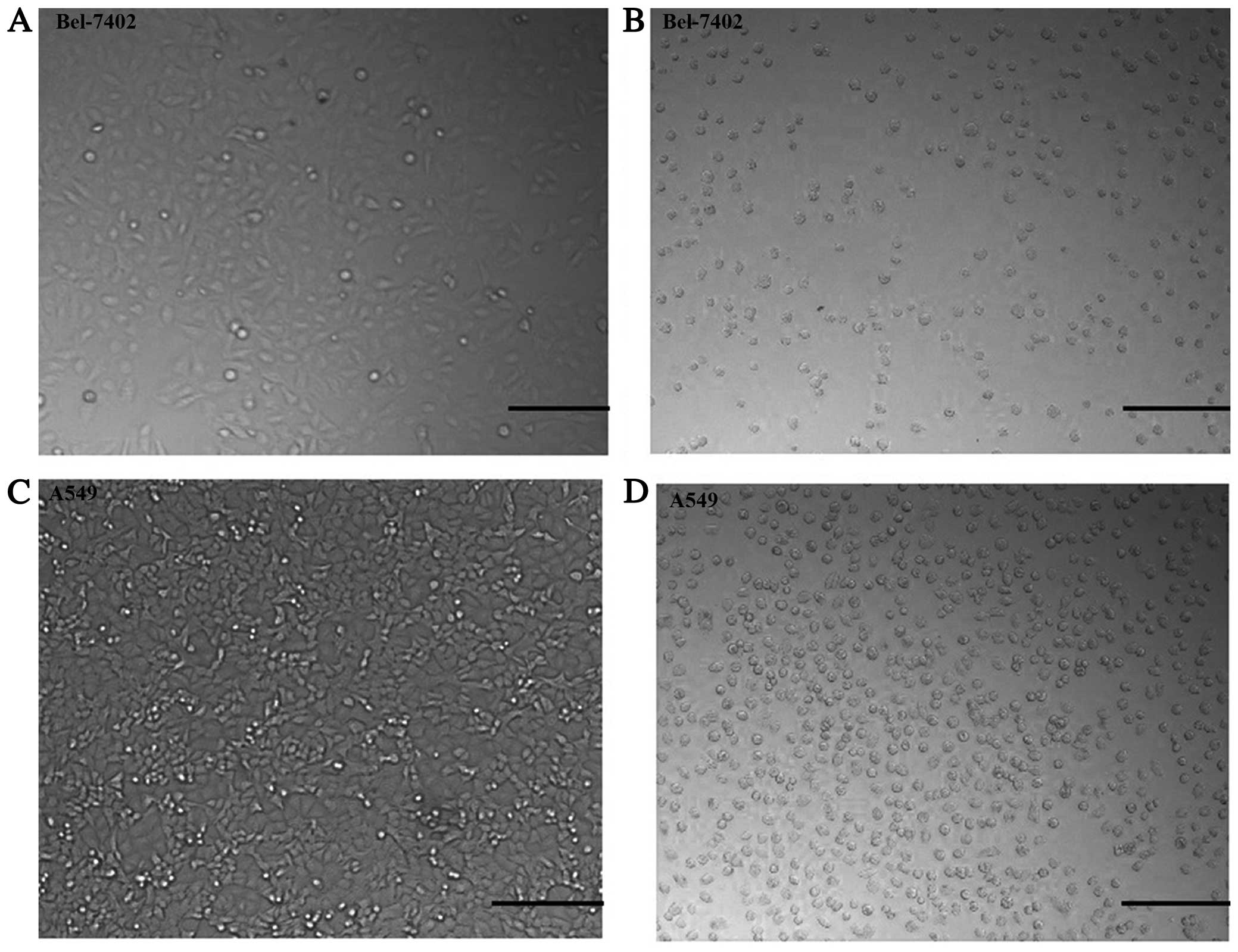
We observed a marked phenomenon regarding cell
morphology alterations when cells were treated with (+)-terrein.
Morphological changes in the Bel-7402 cells exposed to 10 μM
(+)-terrein and in the A549 cells exposed to 10 mM (+)-terrein for
48 h were examined using light microscopy (Fig. 3). The Bel-7402 cell morphology
alterations were from epithelial-like to spherical, when the cells
were treated with 10 μM (+)-terrein (Fig. 3A and B). The morphological changes
from epithelial-like to spherical of the A549 cells exposed to 10
mM (+)-terrein for 48 h were examined using light microscopy
(Fig. 3C and D). However, 10 μl of
(+)-terrein did not significantly affect the morphology of A549
cells (data not shown). The cells were adherent, and all of the
experiments were repeated at least five times.
The cell morphology gene (FN, N-cadherin, and
vimentin) expression of the Bel-7402 cells treated with 10 μM
(+)-terrein was investigated. The results showed that the gene
expression of FN, N-cadherin, and vimentin decreased −3.61-,
−2.39-, and −2.05-fold, respectively, compared with the control
(Table II).
 | Table IIDownregulated expression of cell
morphology genes in the Bel-7402 cells treated with
(+)-terrein. |
Table II
Downregulated expression of cell
morphology genes in the Bel-7402 cells treated with
(+)-terrein.
| Gene name | Gene ID | Primers | Expression
fold |
|---|
| FN
(fibronectin) | 2335 | F, FN1:
5′-AACCTCGGCTTCCTCCATAA-3′
R, FN1: 5′-AACAGTGGGAGCGGACCTA-3′ | −3.61 |
| VIM (vimentin) | 7431 | F, VIM:
5′-GCCAACCGGAACAATGAC-3′
R, VIM: 5′-GTGAGGGACTGCACCTGTCT-3′ | −2.05 |
| CDH2
(N-cadherin) | 1000 | F, CDH2:
5′-CTAACCCGTCGTTGCTGTTT-3′
R, CDH2: 5′-ACAGAATCAGTGGCGGAGAT-3′ | −2.39 |
(+)-Terrein inhibits cell apoptosis and
necrosis
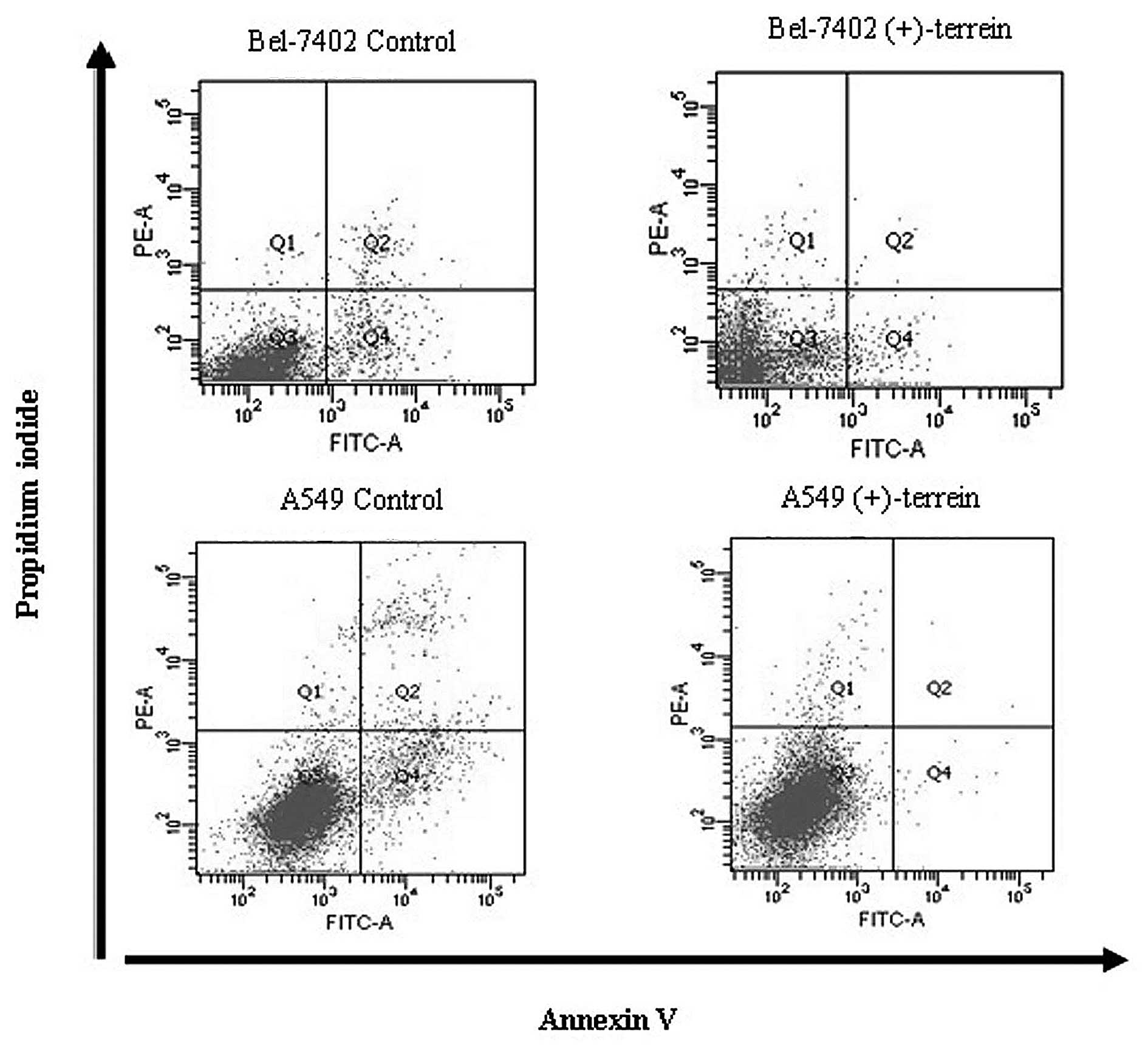
To determine whether this reduction in cell growth
induced by (+)-terrein was mediated by apoptosis, flow cytometric
analysis was performed using PI and Annexin-V staining. The results
indicated that the apoptotic levels of Bel-7402 and A549 cells
treated with (+)-terrein (10 μM and 10 mM) did not increase
(Fig. 4), but the number of cells
treated with (+)-terrein was less than that of the control cells,
according to cell counting (data not shown). The mean percentage ±
SD (n=3) of early apoptosis for the Bel-7402 and A549 control cells
was 5.33±1.05 and 6.17±0.93, respectively, but that for the
Bel-7402 and A549 cells after treatment with (+)-terrein for 48 h
was 2.26±0.50 and 0.53±0.31, respectively (Table III). Fig. 4 shows that (+)-terrein at 10 μM and
10 mM inhibited early apoptosis. As indicated in Table III, the mean percentage ± SD (n=3)
of late apoptosis and necrosis for the Bel-7402 and A549 control
cells was 1.77±0.61 and 5.43±2.55, respectively, but that for the
Bel-7402 and A549 cells following treatment with (+)-terrein for 48
h was 0.83±0.23 and 1.93±1.07, respectively (Table III). Based on these results,
(+)-terrein inhibited late cell apoptosis and necrosis (Fig. 4). Standard deviation was larger than
the average deviation; however, for every independent experiment,
the percentage value (%) of apoptosis and necrosis in the treated
Bel-7402 and A549 cells was lower than that in the control
cells.
 | Table III(+)-Terrein induced cell apoptosis
and necrosis. |
Table III
(+)-Terrein induced cell apoptosis
and necrosis.
| Apoptosis (%) | Necrosis (%) |
|---|
|
|
|
|---|
| Cells | Control | Treated cells | Control | Treated cells |
|---|
| Bel-7402 | 5.33±1.05 | 2.26±0.50 | 1.77±0.61 | 0.83±0.23 |
| A4549 | 6.17±0.93 | 0.53±0.31 | 5.43±2.55 | 1.93±1.07 |
Effect of (+)-terrein on Bel-7402 cell
cycle
Cell proliferation depends on the specific
progression of the cell cycle (21). Thus, the cell cycle was analyzed to
investigate the anti-proliferative mechanism of (+)-terrein against
Bel-7402 cells. Provided the influence of (+)-terrein on A549
occurs at an exceedingly high concentration (>mmol), the
anti-proliferative mechanism of (+)-terrein against A549 was not
examined. When the Bel-7402 cells were treated with 10 μM
(+)-terrein for 48 h, the proportion of the cells in the
G0/G1 and S phases was reduced, whereas the
proportion of cells in the G2/M phase was increased
(Table IV). This result suggested
that the cell cycle was arrested by (+)-terrein. Thus, (+)-terrein
might decrease Bel-7402 cell growth by inducing cell cycle
arrest.
 | Table IVEffect of (+)-terrein on the cell
cycle of the Bel-7402 cells. |
Table IV
Effect of (+)-terrein on the cell
cycle of the Bel-7402 cells.
| Sample |
G0/G1 (%) | S (%) | G2/M
(%) |
|---|
| Control | 59.91±2.8 | 35.29±2.1 | 4.8±0.53 |
| Treated cells (10
μM) | 56.88±3.5 | 35.26±2.8 | 7.36±0.27 |
Effect of (+)-terrein on cell-cycle
regulators
In this study, high-throughput gene expression
analysis of 73 genes was performed (Table I). Cell cycle-related gene
expression was performed by comparing the gene expression between
cDNA samples of (+)-terrein-treated Bel-7402 cells and control
cells. Melting curve analysis was used to assess the specificity of
the array. A single product peak observed from each reaction
without secondary products indicated a high specificity of PCR
assay (data not shown). A subset of differentially expressed genes
involved in the cell cycle was selected from all the microarray
data by performing initial filtration of the P-value (P≤0.05) and
expression level (>2-fold) of the 10 μM (+)-terrein-treated
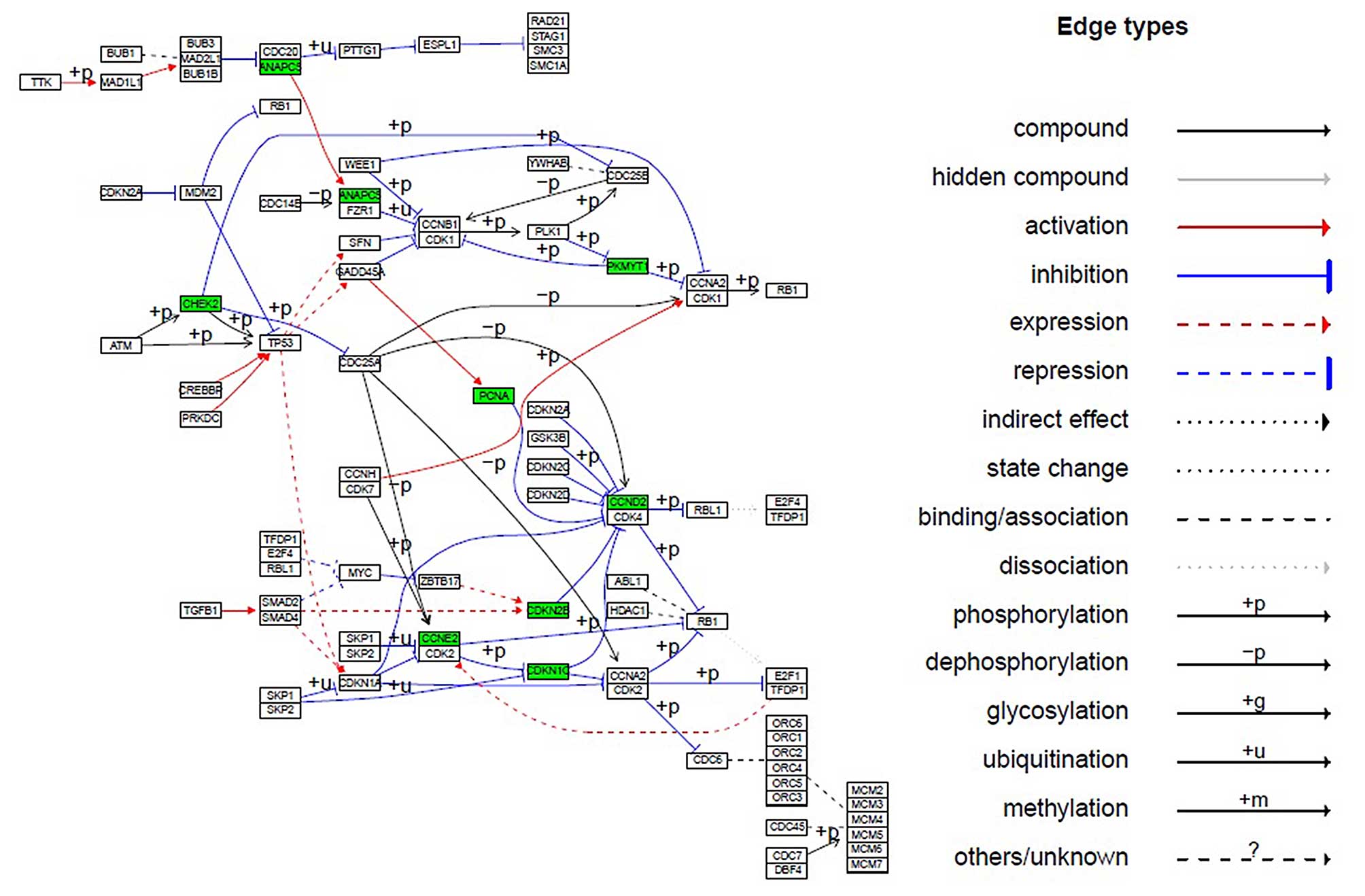
cells. The cell-cycle scheme from the KEGG database (http://www.kegg.jp/kegg/pathway.html)
was presented. Downregulated genes were labeled in green, while no
upregulated genes were overexpressed (Fig. 5). Compared with the control group,
the average expression values of CCND2, CCNE2,
CDKN1C, CDKN2B, ANAPC5, PKMYT1,
CHEK2, and PCNA genes in the 10 μM
(+)-terrein-treated group were significantly decreased by 5.52-,
3.30-, 5.32-, 2.24-, 2.52-, 2.52-, 2.31-, and 2.10-fold
(>2-fold; P≤0.05), respectively (Table V), and the expression level of the
remaining 65 genes was evidently unchanged (data not shown). Eight
obviously downregulated genes were visualized in the cell-cycle
pathway (Fig. 5). Moreover, the
results of flow cytometry (Table
IV) indicated that (+)-terrein arrested the cell cycle.
 | Table VDownregulated cell-cycle genes in the
Bel-7402 cells treated with (+)-terrein. |
Table V
Downregulated cell-cycle genes in the
Bel-7402 cells treated with (+)-terrein.
| Gene no. | Gene name | Function in cell
cycle | n-fold |
|---|
| 1 | CCND2 | G1 phase
and G1/S transition | −5.52 |
| 2 | CCNE2 | G1/S
checkpoint | −3.30 |
| 3 | CDKN1C | Causes arrest of
the cell cycle in G1 phase | −5.32 |
| 4 | CDKN2B | Cell-cycle
checkpoint and cell-cycle arrest | −2.24 |
| 5 | ANAPC5 | G2 phase
and G2/M transition | −2.52 |
| 6 | PKMYT1 | Protein kinase,
membrane-associated tyrosine/threonine 1 | −2.52 |
| 7 | CHEK2 | Checkpoint kinase
2 | −2.31 |
| 8 | PCNA | Proliferating cell
nuclear antigen | −2.10 |
Discussion
Numerous drugs affect tumorigenesis and tumor growth
through several mechanisms, including signal transduction,
cell-cycle regulation, apoptosis, telomere biology, angiogenesis
and cell senescence (11,13). Most available cancer drugs are
antimitotic and act by interfering with the basic mechanism of DNA
synthesis and cell division (20).
(+)-Terrein inhibited cell growth at various concentrations in
various human tumor cell strains (8–10). The
IC50 value of (+)-terrein against human breast cancer
MCF-7 cells was 1.1 nM (9). The
IC50 value was 0.29 mM for HeLa cells (10) and 0.3 mM for NCI-H292 (8). Strese et al (24) observed that the chemosensitivity of
various cell lines was differentially expressed, indicating that
distinct cell types with distinct genetic backgrounds exhibit
distinct responses to drug treatment (15,25).
Since the adhesion and distribution of
anchorage-dependent cells are prerequisites for cell viability and
proliferation, cell growth and survival also depend on cell
morphology (26). Changes in the
synthesis and structure of actin induce changes in cell morphology.
FN is a ubiquitous extracellular matrix glycoprotein assembled into
an FN matrix in all tissues and throughout all stages of life. Loss
of an FN matrix causes changes in cell morphology, cell signaling,
proliferation, and cell-cycle progression (27,28).
The aberrant expression of N-cadherin by cancer cells contributes
to invasiveness and metastasis by making the cells more motile
(29). Vimentin is responsible for
maintaining cell shape, adhesion and motility (30).
The inhibitory mechanism of (+)-terrein against the
Bel-7402 cell differed from the apoptosis of breast cancer and
pulmonary tumor cell lines (9,10).
Liao et al (9) and
Porameesanaporn et al (10)
reported that (+)-terrein suppressed the growth of breast cancer
and HeLa cancer cell lines by inducing apoptosis. Demasi et
al (8) determined that various
ranges of (+)-terrein induced pulmonary tumor cell apoptosis
through protease inhibitors.
Kim et al (31) determined that (±)-terrein inhibited
human epidermal keratinocyte proliferation through extracellular
signal-regulated protein kinase inactivation and G2/M
cell-cycle arrest. The fundamental task of the cell cycle is to
ensure that DNA is successfully replicated in the S phase and that
the identical chromosomal copies are equally distributed between
two daughter cells in the M phase (32,33).
Cell proliferation depends on the progression of the cell cycle
through the G0/G1 phase to the S phase
(34). In some biological systems,
cell-cycle delay and long-term arrest in the G2 phase
are well documented, but most variation in cell-cycle duration
among tissues is due to variability in the length of the
G1 phase (32,33). We determined that (+)-terrein
decreased the proportion of the Bel-7402 cells in the
G0/G1 and S phases (Table IV). The data suggested that
(+)-terrein delayed the progression of the cell cycle. The results
indicated that 10 μM (+)-terrein inhibited Bel-7402 cell growth
(Fig. 2) by arresting the cell
cycle in the G2/M phase (Table IV). The sensitivity of the
drug-induced DNA damage was, not only associated with the
interaction between drug and target, but also depended on the dose,
time (15) and cellular status
(34). The results of this study
confirmed that various strains (Bel-7402 and A549) exhibit a
distinct response to (+)-terrein (Fig.
2). Therefore, the anti-proliferative mechanism of (+)-terrein
based on distinct drug doses, time and cellular status remains to
be investigated.
Cell-cycle progression in mammalian cells is
regulated by various proteins (35). Cell proliferation is closely
controlled by positive and negative regulators that determine cell
progress throughout the cell cycle (36). The cyclin/cyclin-dependent kinase
(Cdk) complexes and Cdk inhibitors (CDKIs) are crucial regulators
of cell-cycle progression (37).
Eight genes are related to the cell cycle (Fig. 5). The CCND2 (38), CCNE2 (39), CDKN1C (40) and CDKN2B (41) genes, positively regulated the
G1 and G1/S phases of the cell cycle, whereas
the APC gene negatively regulated the G2/M
transition in the cell cycle (42).
Distinct cyclins exhibited distinct expression and degradation
patterns, which contribute to the temporal coordination of each
mitotic event (38). Cyclin D
encoded by the CCND2 gene forms a complex regulatory subunit
of CDK4 or CDK6, the activity of which is required for cell-cycle
G1/S transition (38).
Cyclin E encoded by the CCNE2 gene controls the
G1- to S-phase transition in the cell cycle (39). Cyclin-dependent kinase inhibitor 1C
(p57, Kip2), also known as CDKN1C, causes cell cycle arrest in the
G1 phase (40).
Cyclin-dependent kinase 4 inhibitor B (ink4b) encoded by the
CDKN28B gene is a potential factor of cell cycle arrest in
the G1 phase (41). In
addition, anaphase-promoting complex subunit 5 (ANAPC5) consists of
at least eight protein subunits, including APC5, CDC27 (APC3; MIM
116946), CDC16 (APC6; MIM 603461), and CDC23 (APC8; MIM 603462).
The APC/C targets the mitotic cyclins for degradation, resulting in
the inactivation of mitotic cyclin-dependent kinase (M-CdK)
complexes, promoting exit from mitosis and cytokinesis (42). Although distinct processes are
responsible for this inhibition, a crucial process is the
activation of the APC/C by Cdh1. This continued activation prevents
the accumulation of cyclin, which triggers another round of mitosis
instead of exiting (42). The
results from the multiple mRNA analysis further proved that the
Bel-7402 cells were arrested in the G2/M phase by 10 μM
(+)-terrein.
Membrane-associated tyrosine- and threonine-specific
CDC2-inhibitory kinase encoded by the PKMYT1 gene negatively
regulates cell-cycle G2/M transition (43). A decrease in the expression of
PKMYT1 (Table V) and
ANAPC (Fig. 5 and Table V) genes was conducive to arresting
the cells in the G2/M phase of the cell cycle. The
CHEK2 gene provides instructions for producing checkpoint
kinase 2 (CHK2). CHEK2 activation in response to DNA damage
prevents the cell from entering mitosis (44). Proliferating cell nuclear antigen
(PCNA) is a nuclear protein that acts as a processivity factor for
DNA polymerase ɛ in eukaryotic cells (45). The expression levels of PCNA varied
throughout the cell-cycle progression, and the maximal expression
was observed in the G1 and S phases (46). Furthermore, the PCNA expression
level was downregulated as the cells exited the cell cycle and
differentiated (47). Based on the
PCNA expression level characteristics (46,47),
we obtained consistent results indicating that 10 μM (+)-terrein
increased the proportion of treated cells in the G2/M
phase (Table IV) and decreased the
PCNA expression 2.10-fold, compared with the control group
(Table V). Thus, (+)-terrein
induced cell cycle arrest by breaking down the balance between
multiple gene expressions of the cell cycle.
In conclusion, (+)-terrein exhibited cytotoxicity
against the Bel-7402 human hepatoma cell line, yielding an
IC50 value of 11.63 μM ±0.02. In addition, (+)-terrein
induced round-cell morphology of Bel-7402 and A549 cells but did
not induce cell apoptosis. Furthermore, (+)-terrein inhibited
Bel-7402 human hepatoma cell proliferation and arrested the cell
cycle in the G2/M phase by breaking down the expression
of the CCND2, CCNE2, CDKN1C, CDKN2B,
ANAPC5, PKMYT1, CHEK2 and PCNA cell
cycle-related genes. The results suggest that (+)-terrein inhibited
human tumor cell growth through various strategies. The potential
application of (+)-terrein remains to be investigated in future
studies.
Acknowledgements
We would like to thank Dr Qian Luo at the Instrument
Sharing and Technical Service Platform of SJTU for technical advice
on using flow cytometry. We would also like to thank Dr Valliappan
Karuppiah for providing assistance with the language. This study
was supported by the High-Tech Research and Development Program of
China (2011AA090702), the Medical and Engineering Cross Funds of
Shanghai Jiao Tong University (YG2011ms13), and the National
Natural Science Foundation of China (J1210047 and 31300104).
Abbreviations:
|
ANAPC5
|
anaphase-promoting complex subunit
5
|
|
Cdk
|
cyclin/cyclin-dependent kinase
|
|
CDKI
|
Cdk inhibitors
|
|
CDKN1C
|
cyclin-dependent kinase inhibitor
1C
|
|
Ct
|
threshold cycle
|
|
DC
|
dissociation curves
|
|
GDC
|
genomic DNA control
|
|
HCC
|
hepatocellular carcinoma
|
|
ink4b
|
cyclin-dependent kinase 4 ihibitor
B
|
|
M-CdK
|
mitotic cyclin-dependent kinase
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
SPSS
|
Statistical Product and Service
Solutions
|
|
Tm
|
melting temperature
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
References
|
1
|
Schütte K, Bornschein J and Malfertheiner
P: Hepatocellular carcinoma - epidemiological trends and risk
factors. Dig Dis. 27:80–92. 2009. View Article : Google Scholar
|
|
2
|
Xie SL, Zhu MG, Lv GY, Zhang Q and Wang
GY: The role of RhoC in the proliferation and apoptosis of
hepatocellular carcinoma cells. Med Oncol. 29:1802–1809. 2012.
View Article : Google Scholar
|
|
3
|
Cao H, Phan H and Yang LX: Improved
chemotherapy for hepatocellular carcinoma. Anticancer Res.
32:1379–1386. 2012.PubMed/NCBI
|
|
4
|
Nouso K: Current chemotherapies for
advanced hepatocellular carcinoma. Clin J Gastroenterol. 6:89–93.
2013. View Article : Google Scholar
|
|
5
|
Zhang X, Jia S, Yang S and Yang Y, Yang T
and Yang Y: Arsenic trioxide induces G2/M arrest in hepatocellular
carcinoma cells by increasing the tumor suppressor PTEN expression.
J Cell Biochem. 113:3528–3535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raistrick H and Smith G: Studies in the
biochemistry of micro-organisms: The metabolic products of
Aspergillus terreus Thom. A new mould metabolic product-terrein.
Biochem J. 29:606–611. 1935.PubMed/NCBI
|
|
7
|
Arakawa M, Someno T, Kawada M and Ikeda D:
A new terrein glucoside, a novel inhibitor of angiogenin secretion
in tumor angiogenesis. J Antibiot. 61:442–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Demasi M, Felicio AL, Pacheco AO, Leite
HG, Lima C and Andrade LH: Studies on terrein as a new class of
proteasome inhibitors. J Braz Chem Soc. 21:299–305. 2010.
View Article : Google Scholar
|
|
9
|
Liao WY, Shen CN, Lin LH, Yang YL, Han HY,
Chen JW, Kuo SC, Wu SH and Liaw CC: Asperjinone, a nor-neolignan,
and terrein, a suppressor of ABCG2-expressing breast cancer cells,
from thermophilic Aspergillus terreus. J Nat Prod. 75:630–635.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Porameesanaporn Y,
Uthaisang-Tanechpongtamb W, Jarintanan F, Jongrungruangchok S and
Thanomsub Wongsatayanon B: Terrein induces apoptosis in HeLa human
cervical carcinoma cells through p53 and ERK regulation. Oncol Rep.
29:1600–1608. 2013.PubMed/NCBI
|
|
11
|
Anisimov VN: Biology of aging and cancer.
Cancer Control. 14:23–31. 2007.PubMed/NCBI
|
|
12
|
Edinger AL and Thompson CB: Death by
design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol.
16:663–669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gibbs JB: Mechanism-based target
identification and drug discovery in cancer research. Science.
287:1969–1973. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmitt CA and Lowe SW: Apoptosis and
therapy. J Pathol. 187:127–137. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao L, Yin Y, Sun W, Zhang F, Zhang F and
Li Z: Enhanced production of (+)-terrein by Aspergillus terreus
strain PF26 with epigenetic modifier suberoylanilide hydroxamic
acid. Proc Biochem. 48:1635–1639. 2013. View Article : Google Scholar
|
|
17
|
Xu B, Yin Y, Zhang F, Li Z and Wang L:
Operating conditions optimization for (+)-terrein production in a
stirred bioreactor by Aspergillus terreus strain PF-26 from marine
sponge Phakellia fusca. Bioprocess Biosyst Eng. 35:1651–1655. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin Y, Gao Q, Zhang F and Li Z: Medium
optimization for the high yield production of single (+)-terrein by
Aspergillus terreus strain PF-26 derived from marine sponge
Phakellia fusca. Process Biochem. 47:887–891. 2012. View Article : Google Scholar
|
|
19
|
Yin Y, Xu B, Li Z and Zhang B: Enhanced
production of (+)-terrein in fed-batch cultivation of Aspergillus
terreus strain PF-26 with sodium citrate. World J Microbiol
Biotechnol. 29:441–446. 2013. View Article : Google Scholar
|
|
20
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar
|
|
23
|
Luo W and Brouwer C: Pathview: an
R/Bioconductor package for pathway-based data integration and
visualization. Bioinformatics. 29:1830–1831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Strese S, Fryknäs M, Larsson R and Gullbo
J: Effects of hypoxia on human cancer cell line chemosensitivity.
BMC Cancer. 13:3312013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Evan G and Littlewood T: A matter of life
and cell death. Science. 281:1317–1322. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
French PW, Donnellan M and McKenzie DR:
Electromagnetic radiation at 835 MHz changes the morphology and
inhibits proliferation of a human astrocytoma cell line.
Bioelectrochem Bioenerg. 43:13–l8. 1997. View Article : Google Scholar
|
|
27
|
Bourdoulous S, Orend G, MacKenna DA,
Pasqualini R and Ruoslahti E: Fibronectin matrix regulates
activation of RHO and CDC42 GTPases and cell cycle progression. J
Cell Biol. 143:267–276. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yi M and Ruoslahti E: A fibronectin
fragment inhibits tumor growth, angiogenesis, and metastasis. Proc
Natl Acad Sci USA. 98:620–624. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramis-Conde I, Chaplain MAJ, Anderson ARA
and Drasdo D: Multi-scale modelling of cancer cell intravasation:
the role of cadherins in metastasis. Phys Biol. 6:0160082009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mendez MG, Kojima S and Goldman RD:
Vimentin induces changes in cell shape, motility, and adhesion
during the epithelial to mesenchymal transition. FASEB J.
24:1838–1851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim DS, Lee HK, Park SH, Lee S, Ryoo IJ,
Kim WG, Yoo ID, Na JI, Kwon SB and Park KC: Terrein inhibits
keratinocyte proliferation via ERK inactivation and G2/M cell cycle
arrest. Exp Dermatol. 17:312–317. 2008. View Article : Google Scholar
|
|
32
|
Heichman KA and Roberts JM: Rules to
replicate by. Cell. 79:557–562. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nilsson I and Hoffmann I: Cell cycle
regulation by the Cdc25 phosphatase family. Prog Cell Cycle Res.
4:107–114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gonzalez VM, Fuertes MA, Alonso C and
Perez JM: Is cisplatin-induced cell death always produced by
apoptosis? Mol Pharmacol. 59:657–663. 2001.PubMed/NCBI
|
|
35
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brooks G and La Thangue NB: The cell cycle
and drug discovery: the promise and the hope. Drug Discov Today.
4:455–464. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nurse P: A long twentieth century of the
cell cycle and beyond. Cell. 100:71–78. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mullany LK, White P, Hanse EA, Nelsen CJ,
Goggin MM, Mullany JE, Anttila CK, Greenbaum LE, Kaestner KH and
Albrecht JH: Distinct proliferative and transcriptional effects of
the D-type cyclins in vivo. Cell Cycle. 7:2215–2224. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lauper N, Beck AR, Cariou S, Richman L,
Hofmann K, Reith W, Slingerland JM and Amati B: Cyclin E2: a novel
CDK2 partner in the late G1 and S phases of the mammalian cell
cycle. Oncogene. 17:2637–2643. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matsuoka S, Edwards MC, Bai C, Parker S,
Zhang P, Baldini A, Harper JW and Elledge SJ: p57KIP2, a
structurally distinct member of the p21CIP1 Cdk inhibitor family,
is a candidate tumor suppressor gene. Genes Dev. 9:650–662. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hannon GJ and Beach D: pl5INK4B is a
potential effector of TGF-beta-induced cell cycle arrest. Nature.
371:257–261. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kraft C, Herzog F, Gieffers C, Mechtler K,
Hagting A, Pines J and Peters JM: Mitotic regulation of the human
anaphase-promoting complex by phosphorylation. EMBO J.
22:6598–6609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu F, Rothblum-Oviatt C, Ryan CE and
Piwnica-Worms H: Overproduction of human Myt1 kinase induces a G2
cell cycle delay by interfering with the intracellular trafficking
of Cdc2-cyclin B1 complexes. Mol Cell Biol. 19:5113–5123.
1999.PubMed/NCBI
|
|
44
|
Cybulski C, Górski B, Huzarski T, Masojć
B, Mierzejewski M, Debniak T, Teodorczyk U, Byrski T, Gronwald J,
Matyjasik J, Złowocka E, Lenner M, Nej K, Castaneda J, Medrek K,
Szymańska A, Szymańska J, Kurzawski G, Suchy J, Oszurek O, Witek A,
Narod SA and Lubinski J: CHEK2 is a multiorgan cancer
susceptibility gene. Am J Hum Genet. 75:1131–1135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kisielewska J, Lu P and Whitaker M:
GFP-PCNA as an S-phase marker in embryos during the first and
subsequent cell cycles. Biol Cell. 97:221–229. 2005. View Article : Google Scholar
|
|
46
|
Kumar D, Minocha N, Rajanala K and Saha S:
The distribution pattern of proliferating cell nuclear antigen in
the nuclei of Leishmania donovani. Microbiology. 155:3748–3757.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Barton KM and Levine EM: Expression
patterns and cell cycle profiles of PCNA, MCM6, cyclin D1, cyclin
A2, cyclin B1, and phosphorylated histone H3 in the developing
mouse retina. Dev Dynam. 237:672–682. 2008. View Article : Google Scholar
|