Introduction
Breast cancer is the first most commonly diagnosed
invasive malignant disease and the second leading cause of cancer
mortality in women. In 2014, the estimated new cases and deaths due
to breast cancer in the United States were 232,670 and 40,000,
respectively. The incidence of breast cancer accounts for up to 29%
of female malignant diseases, while the mortality accounts for up
to 15% (1). Breast cancer is
therefore considered a major public health issue worldwide. The
proto-oncogene HER-2 encodes a transmembrane tyrosine
kinase receptor with extensive homology to the epidermal growth
factor receptor. Overexpression of HER2 has been shown in 20–30% of
patients with breast cancer and is associated with metastasis and
poor prognosis (2). HER2-positive
tumors constitute a group of breast cancers with specific
biological features and therapeutic options (3).
The HER2 gene targeted therapy has been
applied in the clinical treatment of breast cancer. Trastuzumab
(Herceptin), a humanized monoclonal anti-ERBB2 antibody, is known
to significantly improve clinical outcome for early and advanced
HER2-positive breast cancer (4).
Trastuzumab inhibits HER2 dimerization and growth factor signaling
cascades downstream of HER2, including the phosphatidylinositol
3-kinase (PI3K)/AKT/mTOR pathway and RAS/RAF/MEK/MAP kinase (MAPK)
pathway. In addition, it has been demonstrated that Fc portion of
trastuzumab participates in antibody-dependent cellular
cytotoxicity (ADCC) function (5–7).
Despite its initial efficacy, acquired resistance to trastuzumab
develops in the majority of patients with metastatic breast cancer,
and a large subset never responds, demonstrating primary resistance
(5,8,9).
MicroRNAs (miRNAs) are small non-coding,
single-stranded RNAs that regulate crucial biological processes by
inhibiting gene expression at a post-transcriptional level. The
abnormal expression of miRNAs was observed in a various types of
human cancer. miRNAs may function as tumor suppressors or
oncogenes, depending on whether they specifically target oncogenes
or tumor-suppressor genes (10,11).
Recent findings have demonstrated that miRNA-542-3p is associated
with tumor progression via c-Src-related oncogenic pathways
(12). Furthermore, miRNA-542-3p
induces growth arrest and inhibits tumor angiogenesis by targeting
angiopoietin-2 (13,14).
In the present study, we detected the function of
miRNA-542-3p in breast cancer. The result showed that miRNA-542-3p
expression was induced by trastuzumab in SKBR3 and MCF7/Her2 cell
lines. Knockdown of miRNA-542-3p impaired trastuzumab-mediated
apoptosis and G1/S checkpoint blockage. Furthermore, miRNA-542-3p
depletion activated the PI3K-AKT pathway and LY294002 reversed the
effect of miRNA-542-3p knockdown. Collectively, our results
suggested that miRNA-542-3p is an important regulator of the
PI3K-AKT pathway and downregulation of miRNA-542-3p contributes to
trastuzumab response and resistance.
Materials and methods
Cell culture, antisense miRNA and
transfection
SKBR3 and MCF7/HER2 breast cancer cell lines were
obtained from the Type Culture Collection of Chinese Academy of
Sciences and were maintained in DMEM with 10% FBS in a 5%
CO2-humidified, 95% air incubator. Antisense
miRNA-542-3p was purchased from Ambion (Austin, TX, USA). Transient
transfection was performed using the Lipofectamine 2000 reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA).
Chemicals and antibodies
Trastuzumab was obtained from the Tianjin Medical
University Cancer Institute and Hospital. LY294002 was purchased
from Sigma-Aldrich (St. Louis, MO, USA). Anti-cyclin D1,
anti-p27kip, anti-AKT, anti-p-AKT, anti-GSK-3β,
anti-p-GSK-3β, anti-FOXO1a, anti-p-ERK and anti-β-actin antibody
were purchased from Cell Signaling Technologies Inc. (Danvers, MA,
USA).
Apoptosis and BrdU incorporation
Apoptotic rate and proliferation rate were measured
by a PE Annexin V Apoptosis Detection kit (BD Pharmingen, San
Diego, CA, USA) and a Cell Proliferation ELISA kit (Roche
Diagnostics, Mannheim, Germany), respectively. The measurements
were performed following the manufacturer’s instructions.
Cell cycle analysis
Cells were collected and fixed in 75% ethanol at 4°C
overnight. After washing with PBS, the cells were stained with
PI/RNase staining buffer (BD Pharmingen) for 10 min at room
temperature. The DNA content of cells was measured by flow
cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA).
Proportions of cells in G1, S, and G2/M phases were analyzed using
ModFit Software (Verity Software House Inc., Topsham, ME, USA).
RNA extraction and RT-qPCR
RNA was extracted using the TRIzol RNA isolation kit
(Invitrogen Life Technologies). miRNAs were reverse-transcribed to
generate cDNA using stem-loop reverse transcriptase (RT) primers.
miRNA expression was calculated relative to the expression of RNU48
(P/N: 4373383, for human) (Applied Biosystems, Foster City, CA,
USA). miRNA-specific primers for miRNA-542-3p were obtained from
Applied Biosystems (P/N: 4378101).
MTT assay
Cells (8×103 cells/well) were placed in
96-well plates. At 24 h following treatment, the cells were
continually cultured for 24–72 h. At 24, 48 and 72 h, 10 μl of 0.5
μg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide
(MTT) was added to each well. The cells were incubated at 37°C for
another 2 h, the medium was removed and the precipitated formazan
was dissolved in 100 μl of DMSO. Following agitation for 20 min,
the absorbance was detected at 570 nm on a μQuant Universal
Microplate spectrophotometer (Bio-Tek Instruments, Winooski, VT,
USA).
Western blot analysis
Cell lysates were separated on 8% SDS denatured
polyacrylamide gel electrophoresis (PAGE) gels, transferred to
nitrocellulose membranes and blocked in phosphate-buffered
saline/Tween-20 containing 5% non-fat milk. The membranes were
incubated with antibodies overnight at 4°C. The membranes were then
incubated with the HRP-labeled corresponding IgG for 1 h. The
protein expression level was assessed by enhanced chemiluminescence
and the membranes were exposed to film (Fujifilm, Tokyo, Japan). We
also performed western blot analysis to detect the expression of
cyclin D1 and p27kip.
Statistical analysis
Experimental results are presented as mean ±
standard deviation (SD). Statistically significant differences
between groups were indicated using a two-tailed unpaired Student’s
t-test. P<0.05 was considered significant.
Results
Trastuzumab induces miRNA-542-3p
expression in breast cancer cells
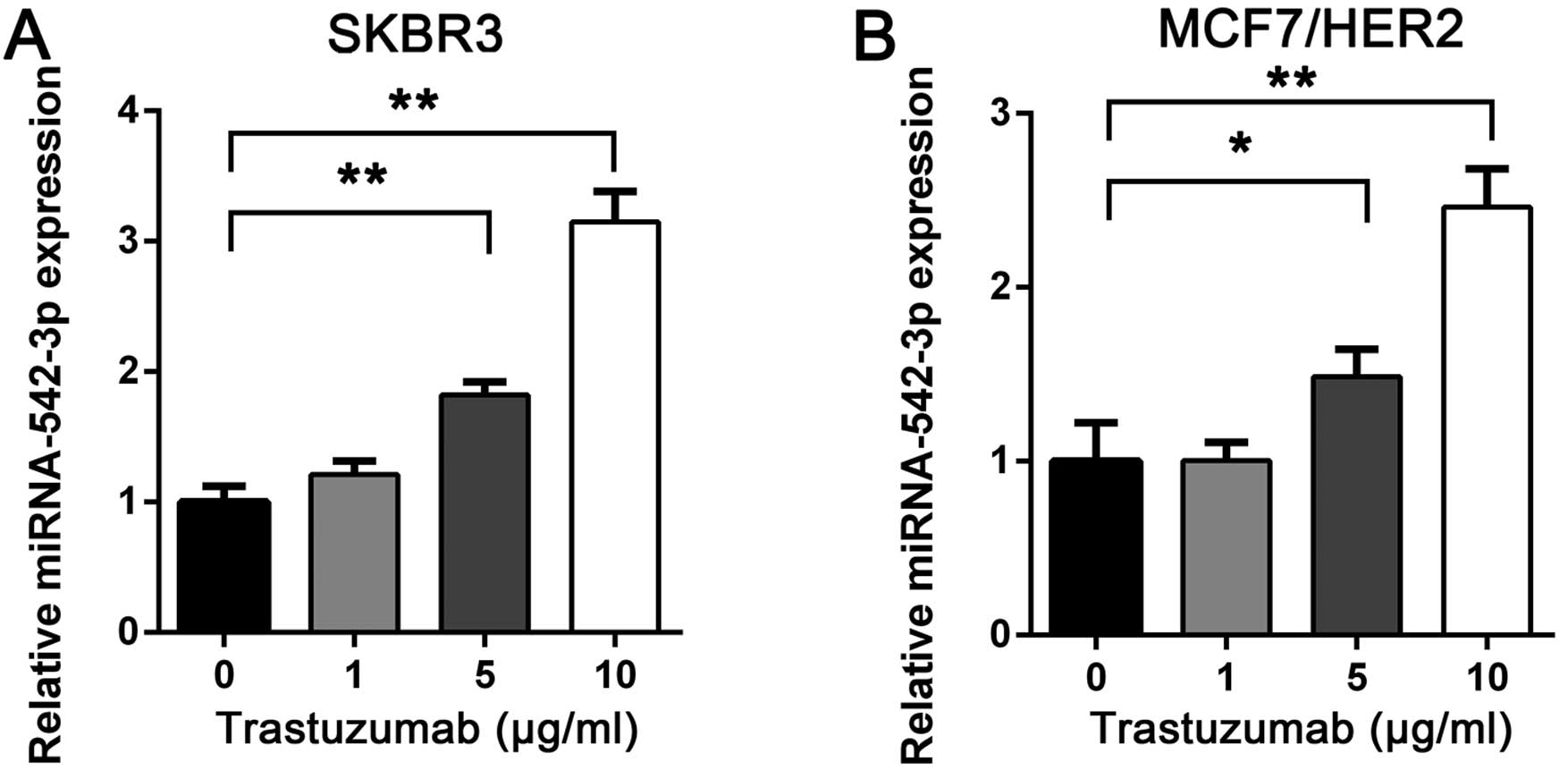
To investigate the potential role of miRNA-542-3p in
trastuzumab resistance, we treated the breast SKBR3 cancer cell
line with trastuzumab at concentrations of 1, 5 and 10 μg/ml or
vehicle. After 24 h, miRNA-542-3p expression was analyzed by
RT-qPCR. The miRNA-542-3p expression was upregulated after
trastuzumab treatment and correlated with trastuzumab concentration
(Fig. 1A). Furthermore, when we
treated the MCF7/HER2 cell line, a MCF7 breast cancer cell line
overexpressed with HER2, with trastuzumab, similar results were
obtained (Fig. 1B). The results
suggested that miRNA-542-3p may be induced by trastuzumab in breast
cancer cells and may be important in trastuzumab-mediated antitumor
effects.
miRNA-542-3p suppression restores
proliferation rate of trastuzumab-treated cells
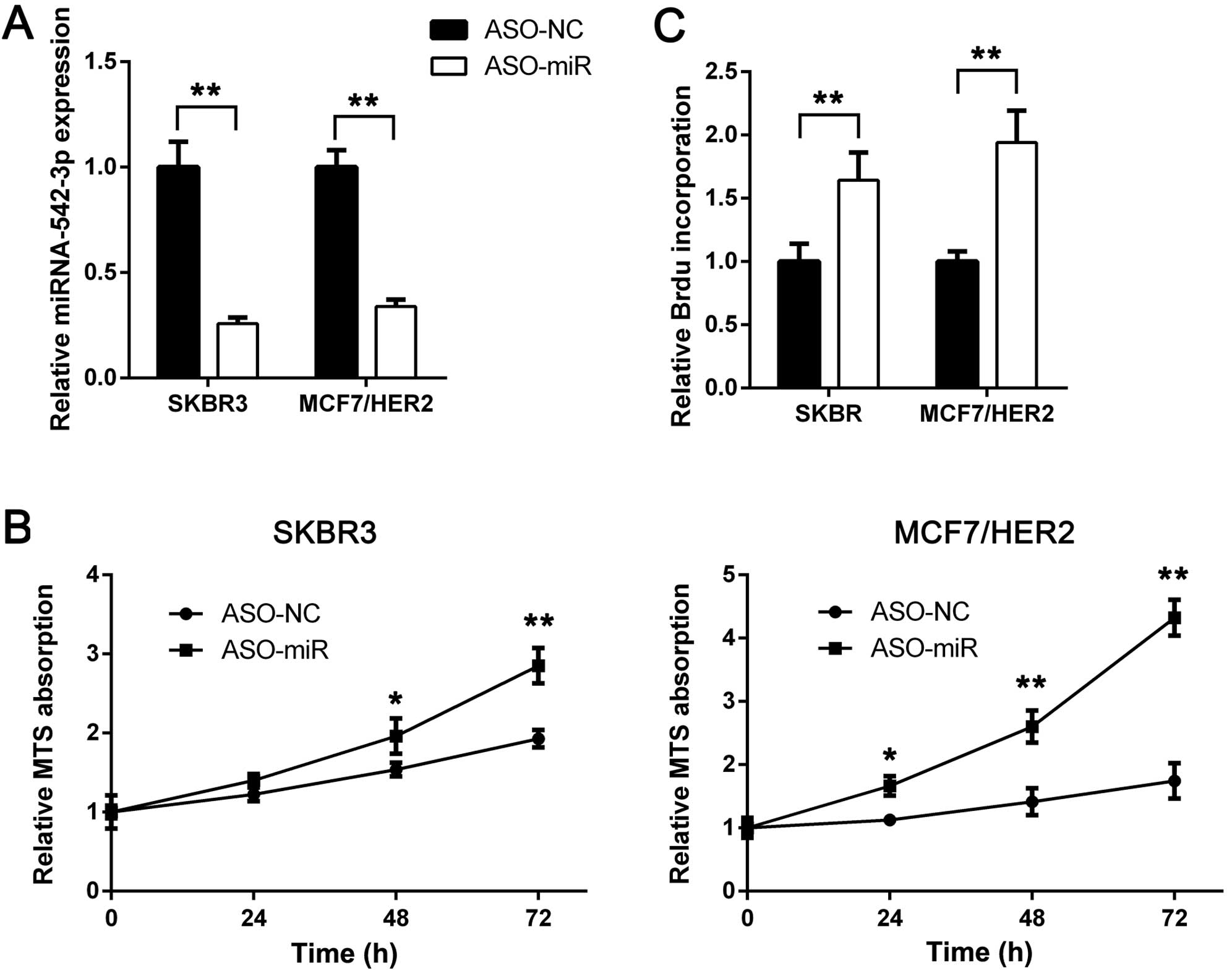
To investigate the role of miRNA-542-3p in
trastuzumab treatment, we suppressed miRNA-542-3p expression using
miRNA-542-3p antisense oligonucleotides (ASO-miR). ASO-NC was used
as thecontrol. SKBR3 and MCF7/HER2 cells were transfected with
ASO-miR and ASO-NC. Then miRNA-542-3p expression was analyzed using
RT-qPCR. As shown in Fig. 2A,
miRNA-542-3p expression was inhibited efficiently. After
miRNA-542-3p knockdown, trastuzumab was added. Compared with the
control, miRNA-542-3p depletion rescued trastuzumab-induced
proliferation suppression (Fig.
2B). To confirm these findings, we performed BrdU incorporation
assay. miRNA-542-3p depletion increased BrdU incorporation rate of
SKBR3 and MCF7/HER2 cells following trastuzumab treatment (Fig. 2C). Taken together, these results
indicated that miRNA-542-3p may participate in trastuzumab-induced
tumor growth suppression and downregulation of miRNA-542-3p may be
a cause of trastuzumab resistance.
miRNA-542-3p suppression rescues
trastuzumab-mediated cell cycle arrest
Cells treated with trastuzumab undergo arrest during
the G1 phase of the cell cycle, with a concomitant reduction in
proliferation (9). To investigate
whether miRNA-542-3p restored breast cancer cells proliferation by
rescuing cell cycle arrest, we determined alteration of cell cycle
profile. SKBR3 and MCF7/HER2 cells exhibited G1/S checkpoint arrest
following treatment with trastuzumab. However, when miRNA-542-3p
was depleted, trastuzumab-induced G1 arrest was rescued (Fig. 3A and B).
We also performed western blot analysis to detect
the expression of cyclin D1 and p27kip. Trastuzumab may
reduce the expression of cyclin D1 and upregulate cyclin-dependent
kinase (cdk) inhibitor p27kip (9). As expected, trastuzumab downregulated
cyclin D1 expression and induced p27kip expression in
SKBR3 and MCF7/HER2 cells. However, when miRNA-542-3p was
suppressed, the expression of cyclin D1 and p27kip was
restored (Fig. 3C). Taken together,
these results indicated that miRNA-542-3p is important in
trastuzumab-induced G1 arrest.
miRNA-542-3p suppression impairs
trastuzumab enhancement on taxol-induced apoptosis
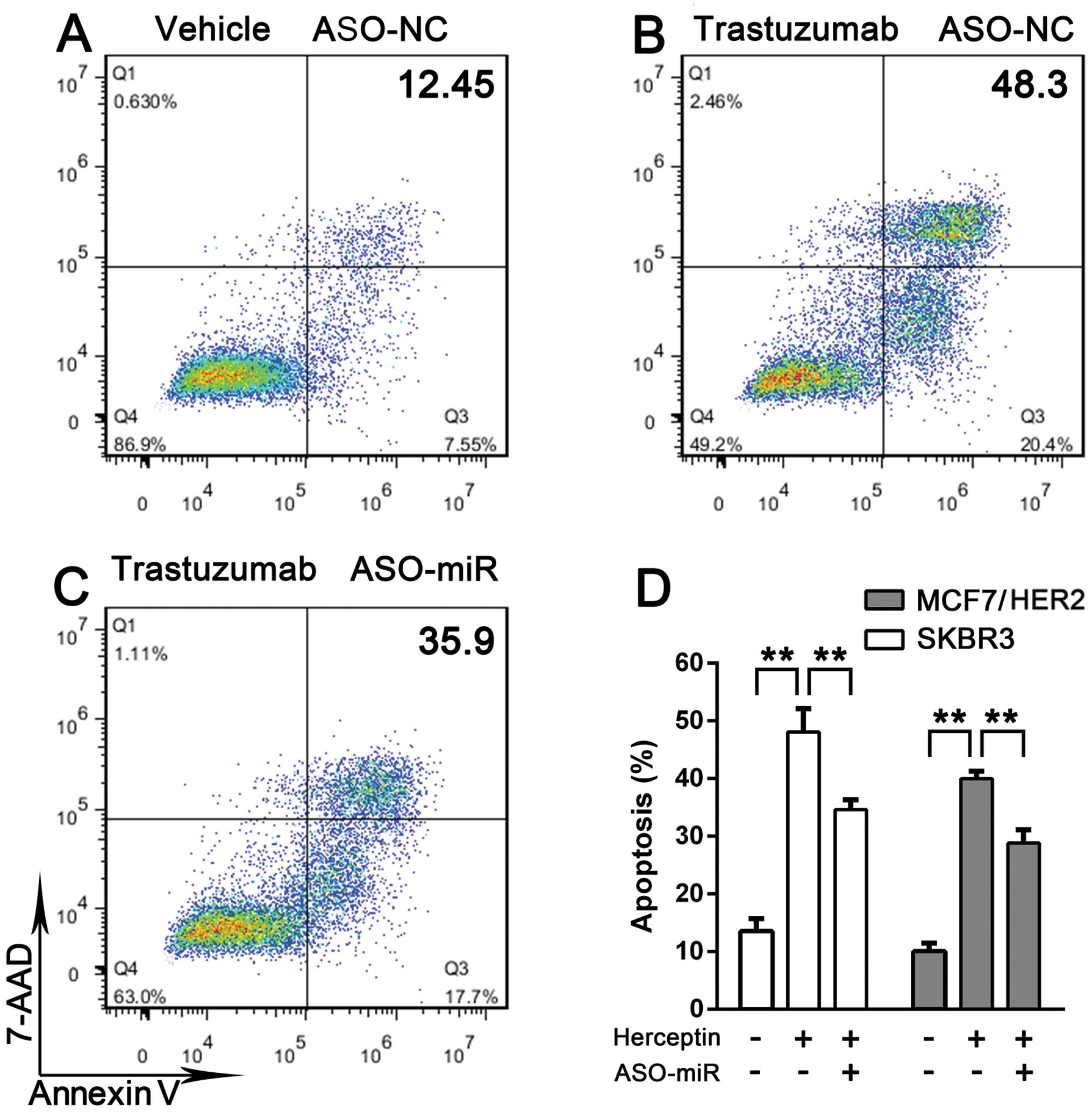
Trastuzumab pretreatment increases taxol-induced
apoptosis (15,16). We further investigated whether
miRNA-542-3p participates in breast cancer cell survival. SKBR3 and
MCF7/HER2 cells were or were not treated with taxol plus
trastuzumab. The two cell lines were more vulnerable to taxol when
pretreated with trastuzumab compared with treatment with taxol
alone. When miRNA-542-3p expression was suppressed, SKBR3 and
MCF7/HER2 cells exhibited resistance to trastuzumab-induced
apoptosis enhancement (Fig. 4).
Taken together, these results indicated that miRNA-542-3p depletion
promotes breast cancer cell survival.
miRNA-542-3p suppression activates AKT
pathway
HER-2 activates multiple cell signaling pathways,
including the PI3K and MAPK cascades. Trastuzumab reduces signaling
from these pathways, promoting cell cycle arrest and apoptosis
(6). Since miRNA-542-3p knockdown
may reduce the tumor suppressive effect of trastuzumab, we
hypothesized that miRNA-542-3p may regulate the PI3K or MAPK
pathway. AKT is the core transducer and regulator of PI3K pathway
(17). Western blot analysis showed
that although total AKT expression was not affected, miRNA-542-3p
suppression upregulated phospho-AKT, an active form of the protein
(Fig. 5). GSK-3β is a downstream
effector of AKT that is involved in cell cycle regulation. Compared
with transfected ASO-NC, SKBR3 and MCF7/HER2 cells, ASO-miR
expressed a more active form than GSK-3β, and phospho-GSK-3β
(Fig. 5).
FOXO transcription factors are major substrates of
AKT kinase and have been suggested as a tumor suppressor (18). FOXO1a has been known to participate
in trastuzumab resistance through p27kip and cyclin D1
regulation (19). Since
miRNA-542-3p suppression regulates p27kip and cyclin D1,
we determined whether it also regulates FOXO1a. Western blot
analysis showed that trastuzumab increased FOXO1a expression in
SKBR3 and MCF7/HER2 cells. However, after miRNA-542-3p knockdown,
FOXO1a was obviously downregulated (Fig. 5).
We also examined whether miRNA-542-3p affects ERK
pathway activation. Although trastuzumab inhibited ERK
phosphorylation in the SKBR3 and MCF7/HER2 cell lines, miRNA-542-3p
knockdown had no impact on ERK phosphorylation (Fig. 5). Taken together, these results
showed that miRNA-542-3p is an essential PI3K-AKT pathway regulator
in breast cancer.
PI3K-AKT pathway activation is required
for miRNA-542-3p suppression-induced trastuzumab resistance
Since trastuzumab mediated PI3K-AKT, inhibition is
important in its antitumor effect (6,9). We
hypothesized that miRNA-542-3p suppression-induced trastuzumab
resistance is PI3K-AKT pathway-dependent. To determine this
possibility, we treated miRNA-542-3p knockdown cells with PI3K
inhibitor, LY294002. Phospho-AKT downregulation was confirmed by
western blot analysis (Fig. 6A).
Cell proliferation was examined using BrdU incorporation assay. As
expected, PI3K inhibition suppressed miRNA-542-3p-mediated BrdU
incorporation in SKBR3 and MCF7/HER2 cells (Fig. 6D). Furthermore, the cell cycle
arrest was examined. Compared with miRNA-542-3p knockdown alone,
the cell cycle arrest in SKBR3 and MFC7/HER2 cells was restored
after LY294002 treatment (Fig. 6C).
We also assessed LY294002 impact on miRNA-542-3p depletion-mediated
apoptosis resistance. In agreement with the above results, LY294002
restored trastuzumab enhancement on taxol-induced apoptosis
(Fig. 6B). Taken together, these
results indicated that miRNA-542-3p suppression-induced trastuzumab
resistance is, at least in part, PI3K-dependent.
Discussion
Trastuzumab, a humanized anti-HER2 monoclonal IgG1
antibody, gained FDA approval in September 1998 for the treatment
of HER2-overexpressing breast cancer in adjuvant and metastatic
settings. However, the clinical benefit from trastuzumab therapy
may be limited due to trastuzumab resistance. The most intensively
studied general mechanisms of trastuzumab resistance are: i)
obstacles for trastuzumab binding to HER2; ii) upregulation of HER2
downstream signaling pathways; iii) signaling through alternate
pathways; and iv) failure to trigger immune-mediated mechanisms to
destroy tumor cells (5).
The results describe a new mechanism by which breast
cancer acquires trastuzumab resistance. We found that miRNA-542-3p
was upregulated in breast cancer cell lines when treated with
trastuzumab. Of note, when miRNA-542-3p was silenced breast cancer
cells showed resistance to trastuzumab. Trastuzumab-induced
proliferation and cell cycle arrest were rescued. The apoptotic
rate was also downregulated when miRNA-542-3p was silenced.
Furthermore, miRNA-542-3p silencing activated PI3K-AKT pathway in
breast cancer cells. The inhibition of this pathway restored
trastuzumab sensitivity. Taken together, these findings demonstrate
that miRNA-542-3p plays an important role in trastuzumab anticancer
function.
Recent studies focused on PTEN or PI3K
mutation-mediated trastuzumab resistance (20–22).
However, few studies have examined the role of miRNA in trastuzumab
resistance. In the present study, we found that trastuzumab induced
miRNA-542-3p expression. Suppression of miRNA-542-3p in breast
cancer cell lines caused trastuzumab resistance. These results
confirm the existence of miRNA regulation of trastuzumab
resistance.
FOXO transcription factors belong to the forkhead
family of transcription factors which are characterized by a
distinct forkhead domain. Emerging evidence suggests that FOXO
factors play a tumor suppressor role in various types of cancer and
are coupled with lifespan extension (18,23).
Recent evidence revealed that the FOXO protein family members,
FOXO1 and FOXO3 promote autophagy (24,25).
Furthermore, it has been suggested that FOXO factors participate in
trastuzumab resistance (19,26).
In the present study, we confirmed the importance of the role of
FOXO1a in trastuzumab antitumor effects. We also found that
miRNA-542-3p controlled FOXO1a expression. These results suggest
that there may exist a microRNA-dependent mechanism that regulates
FOXO1a expression.
The PI3K pathway regulates various cell processes,
such as proliferation, growth, apoptosis and cytoskeletal
rearrangement (17). There are
ample genetic and laboratory studies that suggest the PI3K-AKT
pathway is vital to the growth and survival of cancer cells
(27). In the present study, we
confirmed AKT activation in trastuzumab resistance. We also found
that miRNA-542-3p is a negative PI3K-AKT pathway regulator in
breast cancer. Furthermore, in the present study, we applied
LY294002, an AKT inhibitor, to treat trastuzumab-resistant cancer
cells. The results showed marked antitumor effects suggesting
targeting of PI3K-AKT pathway. Several therapies target the
PI3K-AKT pathway in clinical development for the treatment of
cancer. These include dual PI3K-mTOR, PI3K, AKT and mTOR complex
catalytic site inhibitors (27,28).
However, whether these PI3K-AKT pathway inhibitors also have
similar effects remains to be investigated.
In summary, we found a new trastuzumab resistance
regulatory mechanism in breast cancer. miRNA-542-3p acts as an
AKT-negative regulator to maintain breast cancer cells sensitivity
to trastuzumab. However, the exact miRNA-542-3p targets and its
application in tumor therapy remain to be investigated.
Acknowledgements
The present study was supported by the National
Science and Technology Support Program (no. 2013BAI09B08) and
Tianjin Municipal Major Scientific and Technological Special
Project for Significant Anticancer Development (no.
12ZCDZSY15700).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hynes NE and MacDonald G: ErbB receptors
and signaling pathways in cancer. Curr Opin Cell Biol. 21:177–184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baselga J and Swain SM: Novel anticancer
targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer.
9:463–475. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sliwkowski MX, Lofgren JA, Lewis GD,
Hotaling TE, Fendly BM and Fox JA: Nonclinical studies addressing
the mechanism of action of trastuzumab (Herceptin). Semin Oncol.
26:60–70. 1999.PubMed/NCBI
|
|
5
|
Pohlmann PR, Mayer IA and Mernaugh R:
Resistance to trastuzumab in breast cancer. Clin Cancer Res.
15:7479–7491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nahta R and Esteva FJ: Herceptin:
mechanisms of action and resistance. Cancer Lett. 232:123–138.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hudis CA: Trastuzumab - mechanism of
action and use in clinical practice. N Engl J Med. 357:39–51. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nahta R and Esteva FJ: Trastuzumab:
triumphs and tribulations. Oncogene. 26:3637–3643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valabrega G, Montemurro F and Aglietta M:
Trastuzumab: mechanism of action, resistance and future
perspectives in HER2-overexpressing breast cancer. Ann Oncol.
18:977–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He L and Hannon GJ: MicroRNAs: small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oneyama C, Morii E, Okuzaki D, et al:
MicroRNA-mediated upregulation of integrin-linked kinase promotes
Src-induced tumor progression. Oncogene. 31:1623–1635. 2012.
View Article : Google Scholar
|
|
13
|
Yoon S, Choi YC, Lee S, Jeong Y, Yoon J
and Baek K: Induction of growth arrest by miR-542-3p that targets
survivin. FEBS Lett. 584:4048–4052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He T, Qi F, Jia L, et al: MicroRNA-542-3p
inhibits tumour angiogenesis by targeting angiopoietin-2. J Pathol.
232:499–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee S, Yang W, Lan KH, et al: Enhanced
sensitization to taxol-induced apoptosis by herceptin pretreatment
in ErbB2- overexpressing breast cancer cells. Cancer Res.
62:5703–5710. 2002.PubMed/NCBI
|
|
16
|
Baselga J, Norton L, Albanell J, Kim YM
and Mendelsohn J: Recombinant humanized anti-HER2 antibody
(Herceptin) enhances the antitumor activity of paclitaxel and
doxorubicin against HER2/neu overexpressing human breast cancer
xenografts. Cancer Res. 58:2825–2831. 1998.PubMed/NCBI
|
|
17
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Greer EL and Brunet A: FOXO transcription
factors at the interface between longevity and tumor suppression.
Oncogene. 24:7410–7425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y, Shang X, Sarkissyan M, Slamon D and
Vadgama JV: FOXO1A is a target for HER2-overexpressing breast
tumors. Cancer Res. 70:5475–5485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sumikawa T, Shigeoka Y, Igishi T, et al:
Dexamethasone interferes with trastuzumab-induced cell growth
inhibition through restoration of AKT activity in BT-474 breast
cancer cells. Int J Oncol. 32:683–688. 2008.PubMed/NCBI
|
|
21
|
Grell P, Fabian P, Khoylou M, et al: Akt
expression and compartmentalization in prediction of clinical
outcome in HER2-positive metastatic breast cancer patients treated
with trastuzumab. Int J Oncol. 41:1204–1212. 2012.PubMed/NCBI
|
|
22
|
Chung SS, Giehl N, Wu Y and Vadgama JV:
STAT3 activation in HER2-overexpressing breast cancer promotes
epithelial-mesenchymal transition and cancer stem cell traits. Int
J Oncol. 44:403–411. 2014.
|
|
23
|
Davis R, Singh KP, Kurzrock R and Shankar
S: Sulforaphane inhibits angiogenesis through activation of FOXO
transcription factors. Oncol Rep. 22:1473–1478. 2009.PubMed/NCBI
|
|
24
|
Han J, Pan XY, Xu Y, et al: Curcumin
induces autophagy to protect vascular endothelial cell survival
from oxidative stress damage. Autophagy. 8:812–825. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou J, Liao W, Yang J, et al: FOXO3
induces FOXO1-dependent autophagy by activating the AKT1 signaling
pathway. Autophagy. 8:1712–1723. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chakrabarty A, Bhola NE, Sutton C, et al:
Trastuzumab-resistant cells rely on a HER2-PI3K-FoxO-survivin axis
and are sensitive to PI3K inhibitors. Cancer Res. 73:1190–1200.
2013. View Article : Google Scholar :
|
|
27
|
Engelman JA: Targeting PI3K signalling in
cancer: opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fruman DA and Rommel C: PI3K and cancer:
lessons, challenges and opportunities. Nat Rev Drug Discov.
13:140–156. 2014. View
Article : Google Scholar : PubMed/NCBI
|