Introduction
Colorectal cancer (CRC) is the third most common
type of cancer, and is a common gastrointestinal tumor. Malignant
colorectal cancer cells often possess the ability of rapid
progression and invasion, which contributes to tumor metastasis
(1,2). The mechanism of invasion is complex
and involves integrated biochemical processes requiring a
coordinated effort in managing a number of intracellular and
extracellular interactions. Tumor cells achieve this by excessive
production of several proteases and by modifying the extracellular
matrix (ECM). In addition, members of the Ras superfamily of
GTPases, most notably the Rho proteins, play a prominent role in
cell migration (3,4). Therefore, the development of more
effective treatments for the inhibition of invasion and metastasis
is required in patients with advanced CRC.
The miR-10b gene is located in the middle of the
Hoxd cluster on chromosome 2q31, near Hoxd4. This miRNA proceeds to
inhibit the translation of Hoxd10, resulting in the increased
expression of a well-characterized prometastatic gene, Rhoc. Hoxd10
belongs to the HOX gene family, and its expression is lost during
the malignant progression of breast cancer (5). Besides breast cancer, an aberrant
expression pattern of miR-10b has also been reported in
hepatocellular carcinoma, and higher-grade glioma, unlike that in
case of benign tumors (6,7). The silencing of miR-10b significantly
decreases miR-10b levels and increases the levels of Hoxd10, which
in turn inhibits metastasis (8,9). The
conserved 3′UTR element of Hoxd10-encoded mRNA is partially
complementary to miR-10b, by which miR-10b abrogates the function
of Hoxd10 by inhibiting protein translation but not mRNA
degradation (10). Rhoc was
reported to promote tumor metastasis in distinct carcinomas by
stimulating the activity of a series of kinases including protein
kinase B (AKT) and mitogen-activated protein kinase (MAPK)
(11,12). AKT mediates various basic cell
processes such as apoptosis and the cell cycle (13,14).
Abnormal AKT signaling has been found to be associated with tumor
metastasis.
The effect of miR-10b on the metastasis of
colorectal cancer and the related molecular mechanism remains
unclear. However, whether the overexpression of miR-10b is involved
in colorectal cancer remains to be investigated. Therefore, we
examined the expression level of miR-10b in 70 cases of colorectal
cancer and found that miR-10b was upregulated in all the cases,
unlike that in the case of the non-tumor tissue. Furthermore, we
found that miR-10b overexpression in colorectal cancer led to
decreased protein levels of Hoxd10, and increased Rhoc expression,
both of which were critical to miR-10b-induced invasion. In
addition, the overexpression of miR-10b was highly associated with
higher-grade colorectal cancer. From these findings, it is possible
to postulate that miR-10b plays a role in the invasion of
colorectal cancer, and may therefore be a highly attractive target
for novel molecular monotherapy or combination therapy for
colorectal cancer.
Materials and methods
Ethics and tumor specimen
acquisition
This study was performed in compliance with the
Helsinki Declaration and according to the protocol approved by the
Medical Ethics Committee of the Yangpu Hospital affiliated to
Shanghai Tongji University. All the subjects were informed of the
study and volunteered to participate there in. Written informed
consent was obtained from all patients and participants.
Study population
The study involved 70 patients with histologically
confirmed primary colorectal cancer, who had undergone surgical
resection at the Department of General Surgery at the Yangpu
Hospital affiliated to Shanghai Tongji University. Seventy healthy
individuals who had undergone physical examination in the same
hospital from 1, January 2013 to 1, January 2014 were also enrolled
in the study. The 70 colorectal cancer patients had an age range of
43–86 years, with a mean age of 62.8±11.6 years. There were no
other inclusion or exclusion criteria. The patients did not receive
any preoperative radiotherapy and/or chemotherapy, and the
diagnosis was confirmed by pathologic analysis. All the patients
were staged according to the tumor, node and metastasis (TNM)
staging system of the American Joint Committee on Cancer (AJCC) and
International Union Against Cancer (UICC) for colorectal cancer
(2010 seventh edition). The clinical and pathological profiles of
the patients are provided in Table
I. The 70 healthy individuals included 33 men and 37 women with
a mean age of 60.6±7.6 years (range, 38–76 years).
 | Table IPatient and tumor characteristics. |
Table I
Patient and tumor characteristics.
| Characteristic | No. of patients
(%) |
|---|
| Tumor site |
| Colon | 46 (65.7) |
| Rectum | 24 (34.3) |
| Liver
metastases | 1 (1.4) |
| Multifocal | 1 (1.4) |
| Age (years) |
| ≥50 | 62 (88.6) |
| <50 | 8 (11.4) |
| Gender |
| Male | 42 (60) |
| Female | 28 (40) |
| Tumor
classification |
| T1 | 4 (5.7) |
| T2 | 15 (21.4) |
| T3 | 46 (65.7) |
| T4 | 5 (7.2) |
| Lymph node
status |
| N0 | 38 (54.3) |
| N1 | 21 (30) |
| N2 | 11 (15.7) |
| Histological
differentiation |
| Well | 24 (34.3) |
| Moderate | 45 (64.3) |
| Poor | 1 (1.4) |
| Stage |
| I | 9 (12.9) |
| II | 21 (30) |
| III | 40 (57.1) |
| Hoxd10
staining |
| Strong
(>1+) | 20 (28.6) |
| Weak
(≤1+) | 50 (71.4) |
| Rhoc staining |
| Strong
(>1+) | 41 (58.6) |
| Weak
(≤1+) | 29 (41.4) |
Collection of tumor specimens, serum
samples, and preparation of total RNA
Colorectal cancer tissues were obtained from
therapeutic procedures performed as routine clinical management at
our institution. All the tissue samples were obtained at the time
of operation. Tissue samples were resected during surgery and
immediately frozen in liquid nitrogen for subsequent total RNA
extraction. Blood samples were collected in sterile tubes,
centrifuged at 2,000 × g for 10 min at room temperature, and the
supernatant serum was collected and preserved at −80°C until
further use.
RT-qPCR for miR-10b
Total RNA was isolated using TRIzol®
reagent (Invitrogen Life Technologies, Grand Island, NY, USA),
according to the manufacturer’s instructions. cDNA was synthesized
using the Reverse Transcriptase kit (Thermo Scientific, Shanghai,
China), as described by the manufacturer. Expression of miR-10b was
analyzed using the SYBR-Green PCR kit (Thermo Scientific),
according to the manufacturer’s instructions. Small nuclear 5S RNA
was used for normalization. The primers used for detecting miR-10b
were as follows: miR-10b reverse transcription primer: forward,
5′-TACCCTGTAGAACCGAATTTG-3′, and reverse,
5′-AACTGGTGTCGTGGAGTCGGC-3′ (Jrdun Biotechnogy, Shanghai, China).
These primers used for 5S RNA (QIR) were: forward,
5′-CCATACCACCCTGGAAACGC-3′ and reverse, 5′-TACTAACCGAGCCCGACCCT-3′.
The PCR reaction was conducted at 95°C for 10 sec, followed by 40
cycles of 95°C for 15 sec and 60°C for 45 sec, annealing at 95°C
for 15 sec, 60°C for 1 min, 95°C for 15 sec and 60°C for 15 sec in
a 7500 Fast Real-Time PCR System (Power SYBR-Green PCR Master Mix).
A template-free reaction was used as a negative control. Each
sample was run in triplicate. Expression levels were calculated by
the ΔCt method using the Applied Biosystems 7500 Fast Real-Time PCR
System SDS software, version 1.2.
RT-qPCR for Rhoc
Total RNA was isolated using TRIzol®
reagent (Invitrogen Life Technologies), and reverse transcription
was performed using a Reverse Transcriptase kit (Thermo
Scientific), according to the manufacturer’s instructions.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Life Technologies)
was used as the endogenous control. Expression of Rhoc was analyzed
using the SYBR-Green Mix (Thermo Scientific). The primers used for
detecting Rhoc mRNA were: forward, 5′-GGAGGTCTACGTCCCTACTGT-3′ and
reverse, 5′-CGCAGTCGATCATAGTCTTCC-3′. The primers used for
detecting GAPDH were: forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and
reverse, 5′-GGCTGTTGTCATACTTCTCATGG-3′. Reaction mixtures were
incubated for an initial denaturation at 95°C for 10 min followed
by 40 cycles of 95°C for 15 sec and 60°C for 45 sec, annealing at
95°C for 15 sec, 60°C for 1 min, 95°C for 15 sec and 60°C for 15
sec. Each sample was run in triplicate. Fold-change (relative copy
number) was obtained and analyzed by the ΔCt method by using the
Applied Biosystems 7500 Fast Real-Time PCR System SDS software,
version 1.2.
Western blot analysis
Tissue samples were lysed in RIPA lysis buffer (150
mM NaCl, 10 mM Tris-HCl, pH 7.5, 1% NP-40, 1% deoxycholate, 0.1%
SDS, and protease inhibitor cocktail). Proteins from total tissue
sample lysates were analyzed using NuPAGE 4–12% Bis-Tris gradient
gels (Invitrogen) and transferred onto PVDF membranes, which were
blocked in Tris-buffered saline (TBS) plus 0.1% Tween-20 (TTBS) and
5% non-fat dry milk. The membranes were blotted using antibodies
for Rhoc and Hoxd10 (dilution, 1:200) (Abcam, Shanghai, China) and
β-actin (dilution, 1:10,000), and developed with secondary antibody
anti-goat IgG HRP (dilution, 1:10,000). BCA was used for protein
quantification. Signals were detected using an ECL Prime Western
Blotting Detection kit (GE Healthcare, Buckinghamshire, UK).
Immunoreactive signal intensities were analyzed using the Image J
software (NIH, Bethesda, MD, USA).
Immunohistochemistry
Sections (4 μm) were cut from formalin-fixed,
paraffin-embedded samples, and stained with hematoxylin and eosin.
These samples were reviewed by experienced pathologists. The
primary antibody used for Rhoc immunostaining was a rabbit
anti-Rhoc polyclonal antibody (Abcam), and for Hoxd10
immunostaining, a goat polyclonal anti-Hoxd10 antibody (Abcam). The
sections were incubated with HRP-conjugated anti-rabbit IgG
polyclonal secondary antibody (Abcam) for 45 min, and the reaction
products were visualized by immersing the sections in 0.03%
diaminobenzidine solution containing 2 mM hydrogen peroxide for 1–5
min. Nuclei were lightly stained with Mayer’s hematoxylin. For
control studies of the antibodies, serial sections were treated
with phosphate-buffered saline, and a normal goat IgG monoclonal
antibody (Abcam) was used instead of the primary antibodies. These
were confirmed to be unstained. Quantitative comparative analysis
of immunohistochemical staining was carried out in each case. Two
independent pathologists performed quantitative assessment of the
immunohistochemical staining. Staining in nuclei was graded as
follows: 0, no immunoreactive cells evident; 1, proportion of
immunoreactive cells <20%; 2, 20–70%; 3, >70%.
Immunohistochemical reactivity was evaluated and classified into
the groups: 1, negative (−); 2, weakly positive (+); 3, moderately
positive (++); 4, strongly positive (+++).
Statistical analysis
Data were expressed as mean ± SD. Measurement data
between groups were compared with the t-test (two-tailed), while
enumeration data were compared using the χ2 test. The
correlation between miR-10b and mRNA levels of Rhoc and Hoxd10 was
assessed using Spearman’s rank test. Differences between two groups
for immunostaining were analyzed using the Mann-Whitney U test.
Statistical analyses were performed using the SPSS®
statistical package, version 17.0 (SPSS Inc., Chicago, IL, USA) for
Windows®. P<0.05 was considered statistically
significant.
Results
Expression level of miR-10b in colorectal
cancer
Quantitative RT-PCR (RT-qPCR) successfully amplified
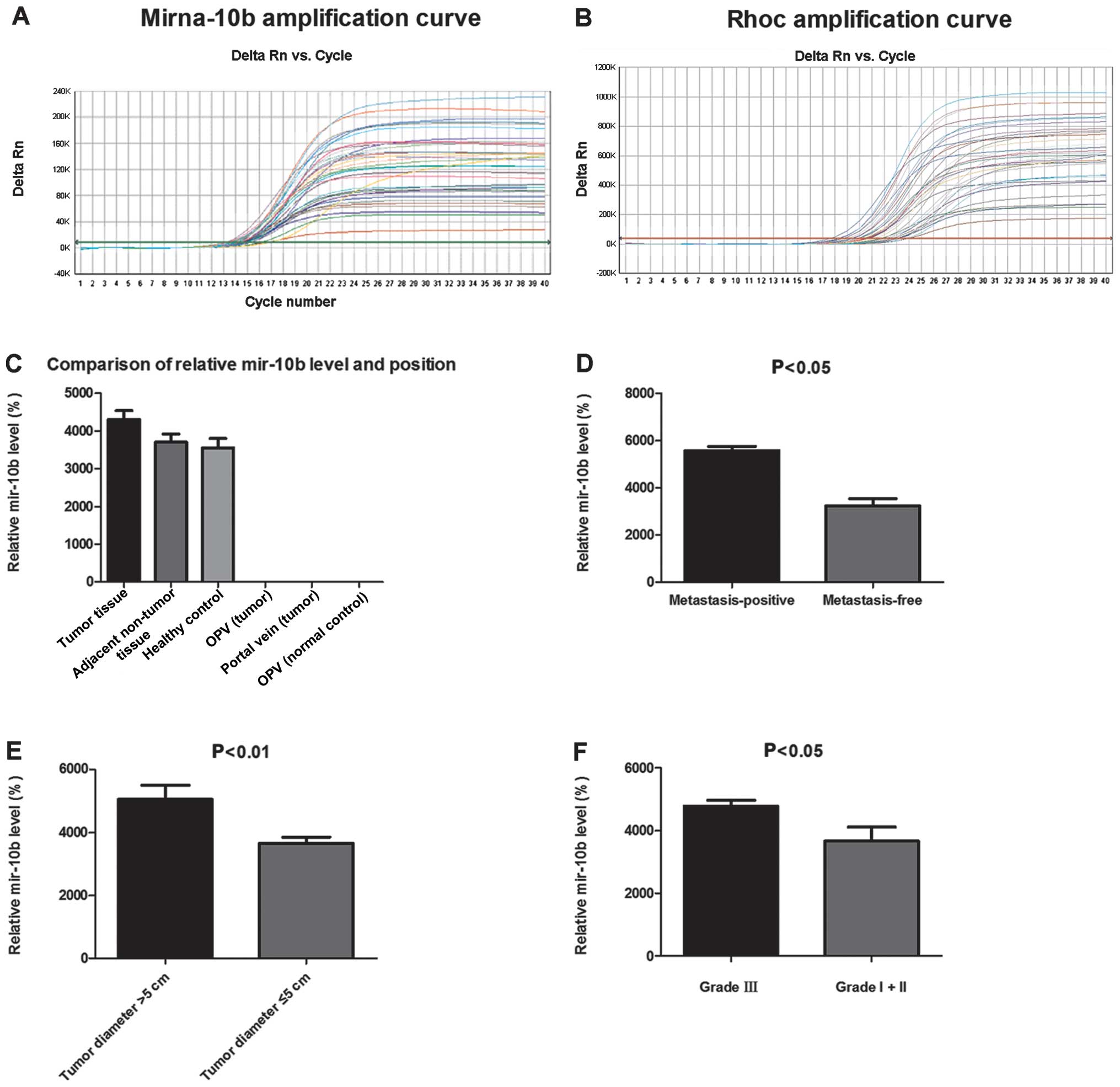
miR-10b in clinical specimens and serum samples (Fig. 1A). Quantitative miRNA expression
data were analyzed by the ΔCt method. The level of miR-10b in
colorectal cancer tissue was significantly higher compared with the
level of sera of patients and healthy controls (P=0.009, P=0.026,
respectively) (Fig. 1C). There were
significant differences in the mean miR-10b level between the
colorectal cancer tissue group and the adjacent non-tumor tissues
and healthy control tissues groups (P<0.05) (Table II). No statistical significance was
identified between the adjacent non-tumor tissue group and the
control tissue group (P>0.05). However, significant differences
in the mean miR-10b level between the healthy control outer
periphery vein group and the other two groups (P<0.05) was
observed, whereas no statistical significance was identified
between the outer periphery vein group and the portal vein group in
colorectal cancer (P>0.05). There was no significant difference
in miR-10b levels with respect to age (P>0.05) (Table III).
 | Table IIComparison of miR-10b expression
level in tissues from different sources (mean ± SD). |
Table II
Comparison of miR-10b expression
level in tissues from different sources (mean ± SD).
| Tissue source | miR-10b (AAM) |
|---|
| Tumor tissues | 43.05±18.85a,b |
| Adjacent non-tumor
tissues | 37.10±17.82a |
| Healthy control
tissues | 35.52±20.36 |
| Outer periphery
vein | 0.01±0.01a,c |
| Portal vein | 0.02±0.02a |
| Outer periphery
vein (healthy control) | 0.00±0.00 |
 | Table IIIVariation in miR-10b levels according
to clinicopathological factors analyzed using the t-test with
Bonferroni correction. |
Table III
Variation in miR-10b levels according
to clinicopathological factors analyzed using the t-test with
Bonferroni correction.
| Clincopathological
factors | Cases (n) | miR-10b level | F-value | P-value |
|---|
| Age (years) |
| ≥50 | 62 | 41.9 | 2.283 | 0.135 |
| <50 | 8 | 44.3 | | |
| Gender |
| Male | 42 | 41.6 | 0.243 | 0.624 |
| Female | 28 | 45.2 | | |
| Tumor diameter |
| ≤5 cm | 41 | 36.6 | 7.274 | 0.001 |
| >5 cm | 29 | 50.5 | | |
| Histological
grading |
| I + II | 30 | 36.6 | 6.712 | 0.012 |
| III | 40 | 47.9 | | |
| Lymph node
metastasis |
| Yes | 32 | 49.3 | 7.067 | 0.010 |
| No | 38 | 37.8 | | |
| Histological
differentiation |
|
Well-differentiated | 24 | 43.2 | 0.443 | 0.508 |
| Moderately
differentiateda | 45 | 41.9 | | |
Colorectal cancer patients were sub-classified for
clinicopathological characteristics according to tumor stage, size,
and histological differentiation for the calculation of miR-10b
levels. The expression level of miR-10b was markedly elevated in
lymph node metastasis-positive tumor tissue compared with lymph
node metastasis-free tumor tissue (Fig.
1D). The results showed that there were significant differences
in miR-10b levels between the colorectal cancer cases with regard
to tumor size (Fig. 1E and Table III). MiR-10b expression was also
significantly different between stage I+II and III tumors
(P<0.05) (Fig. 1F). There were
significant differences in miR-10b levels in cases of liver
metastases and multifocal with other tumor case (P<0.05)
(Table I).
Correlation between miR-10b with mRNA
expression of Rhoc
Sasayama et al (20) have shown that miR-10b inhibits the
translation of Hoxd10 mRNA. Two effectors of Hoxd10 are Rhoc and
uPAR, which are involved in invasion, migration, and metastasis
(15–17). Therefore, we examined the mRNA
expression of Rhoc in colorectal cancer tissue using RT-qPCR, which
was successfully amplified in samples from clinical specimens
(Fig. 1B). There were significant
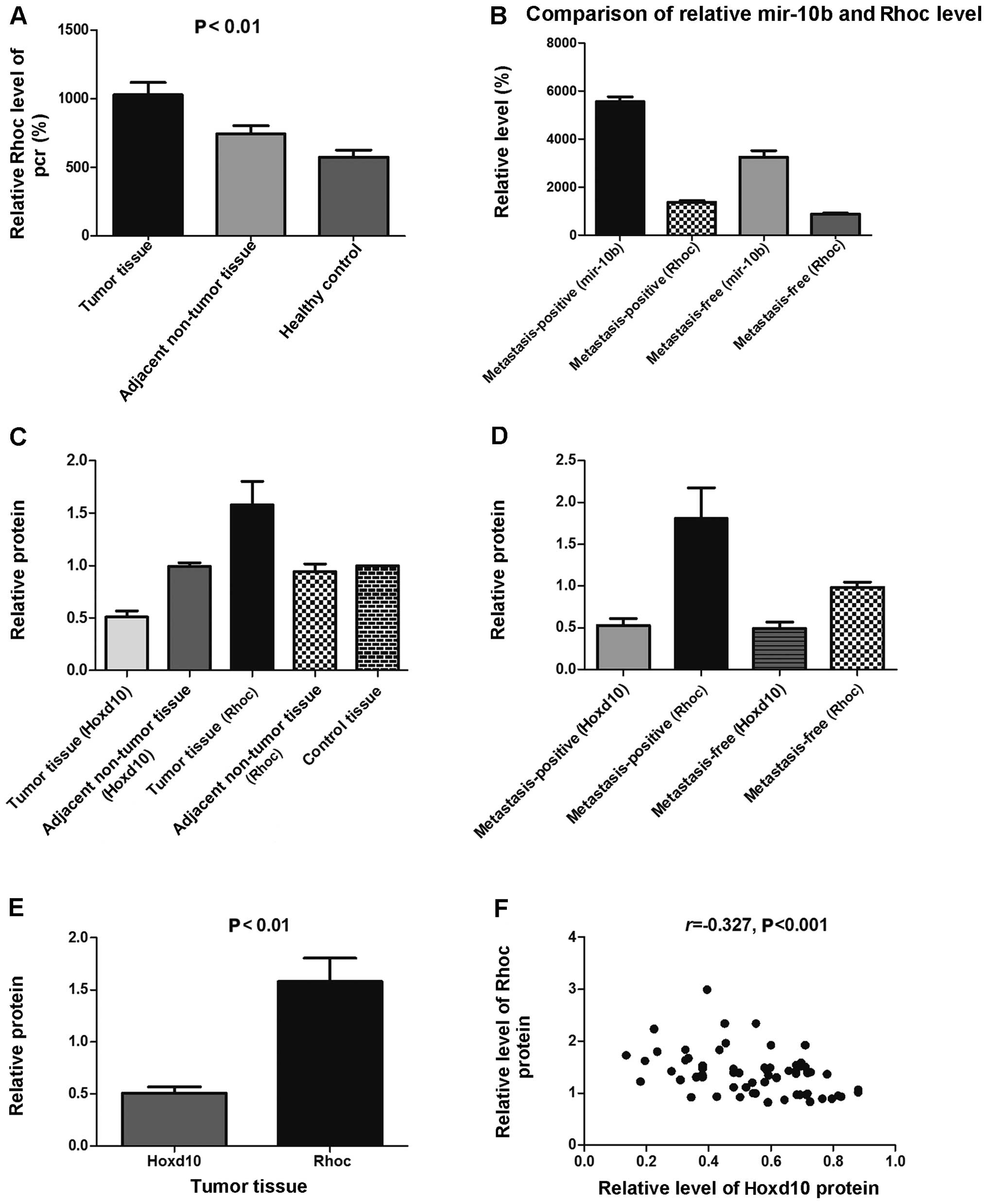
differences in the mean Rhoc mRNA level between the colorectal
cancer tissue group and the other two groups (P<0.01) (Fig. 2A). The difference was also
significant between the adjacent non-tumor tissue group and the
control tissue group (P=0.03). There were significant positive
correlations between miR-10b and mRNA expression of Rhoc (r=0.695,
P<0.001). The levels of miR-10b and Rhoc mRNA were markedly
elevated in lymph node metastasis-positive tumor tissue compared
with lymph node metastasis-free tumor tissue (Fig. 2B).
Western blot analysis of the protein
expression of Hoxd10 and Rhoc
Rhoc protein expression can enhance tumor cell
invasiveness and metastasis. We therefore determined whether
miR-10b inhibits the translation of Hoxd10 mRNA, thereby affecting
the expression of the upstream targets of Rhoc. Our results showed
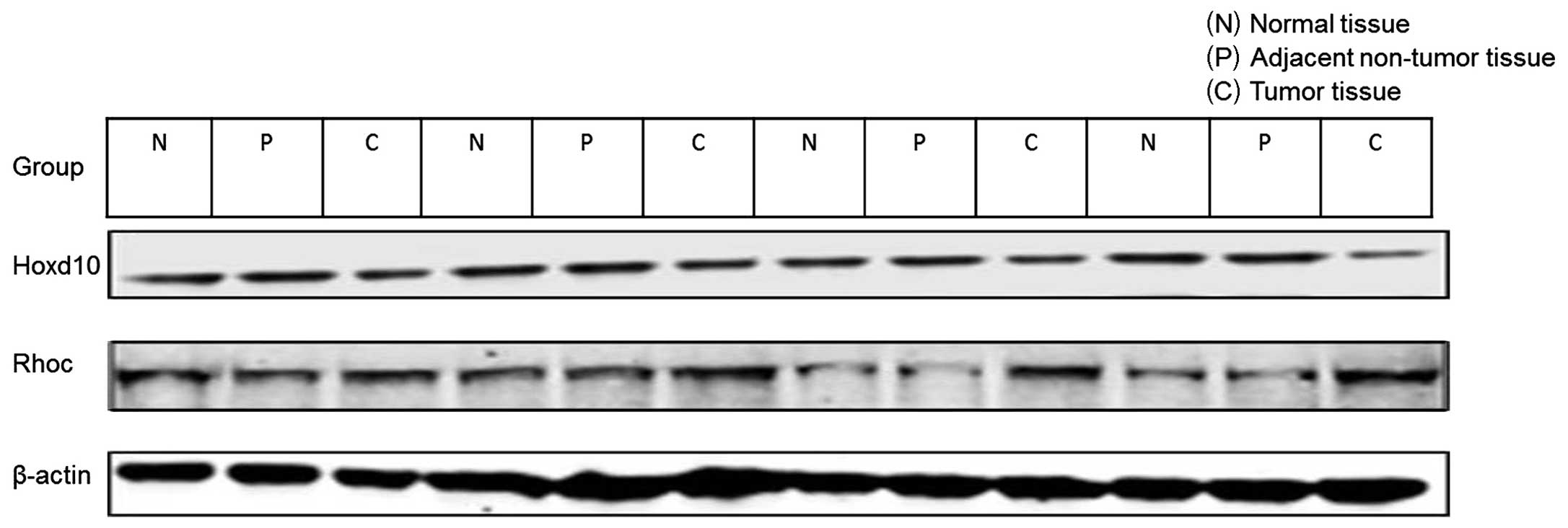
that miR-10b reduced Hoxd10 and increased Rhoc protein expression
(Figs. 2C and 3). The expression level of Hoxd10 and Rhoc
in colorectal cancer tissue was significantly different when
compared with that in the control tissue (P<0.01 and P=0.026,
respectively), while there was no statistical significance between
the adjacent non-tumor tissue group and the control tissue group
(P=0.863 and P=0.448, respectively). Rhoc protein levels were
markedly elevated in lymph node metastasis-positive tumor tissue as
compared to that in the lymph node metastasis-free tumor tissue
(P<0.01) (Figs. 2D and 4). The Spearman’s rank correlation test
showed that there was a high negative correlation between Rhoc
level and Hoxd10 protein level (−0.3<r<−0.5, P<0.001)
(Fig. 2E and F).
Correlation between miR-10b with the
protein expression of Hoxd10 and Rhoc
To examine the correlation between miR-10b and the
protein expression of Hoxd10 and Rhoc, immunohistochemical analysis
was performed. Immunohistochemical reactivity was evaluated and
classified as: negative (−), weak (+), moderate (++), and strong
(+++), (Fig. 5). All the samples
were further classified into two groups based on the levels of
miR-10b: samples showing >25-fold relative expression of miR-10b
were defined as the high miR-10b expression group. Of the 47 cases
in the high miR-10b expression group, moderate and strong
expressions of Rhoc protein were observed in 23 (48.9%) and 11
(23.4%) cases, respectively. On the other hand, in the low miR-10b
expression group, moderate and strong expressions of Rhoc protein
were observed in 7 (30.4%) and 0 (0%) of 23 cases, respectively.
Rhoc protein expression in the miR-10b high expression group was
significantly higher than that in the low expression group
(P<0.001, Mann-Whitney U test). In addition, of the 47 high
miR-10b expression samples, moderate and strong expressions of
Hoxd10 were found in 8 (17%) and 0 (0%) cases, respectively,
whereas, in the low miR-10b expression group, there were 10 (43.5%)
and 2 (8.7%) cases, respectively, (Table IV). There was a statistically
significant difference between high and low miR-10b expression
groups in Hoxd10 immunostaining (P<0.001, Mann-Whitney U test).
A significant inverse correlation between the immunoreactivity of
Hoxd10 and Rhoc was observed (Table
IV). Tumor samples were collected and sorted into two groups:
those that were lymph node metastasis-free and those that were
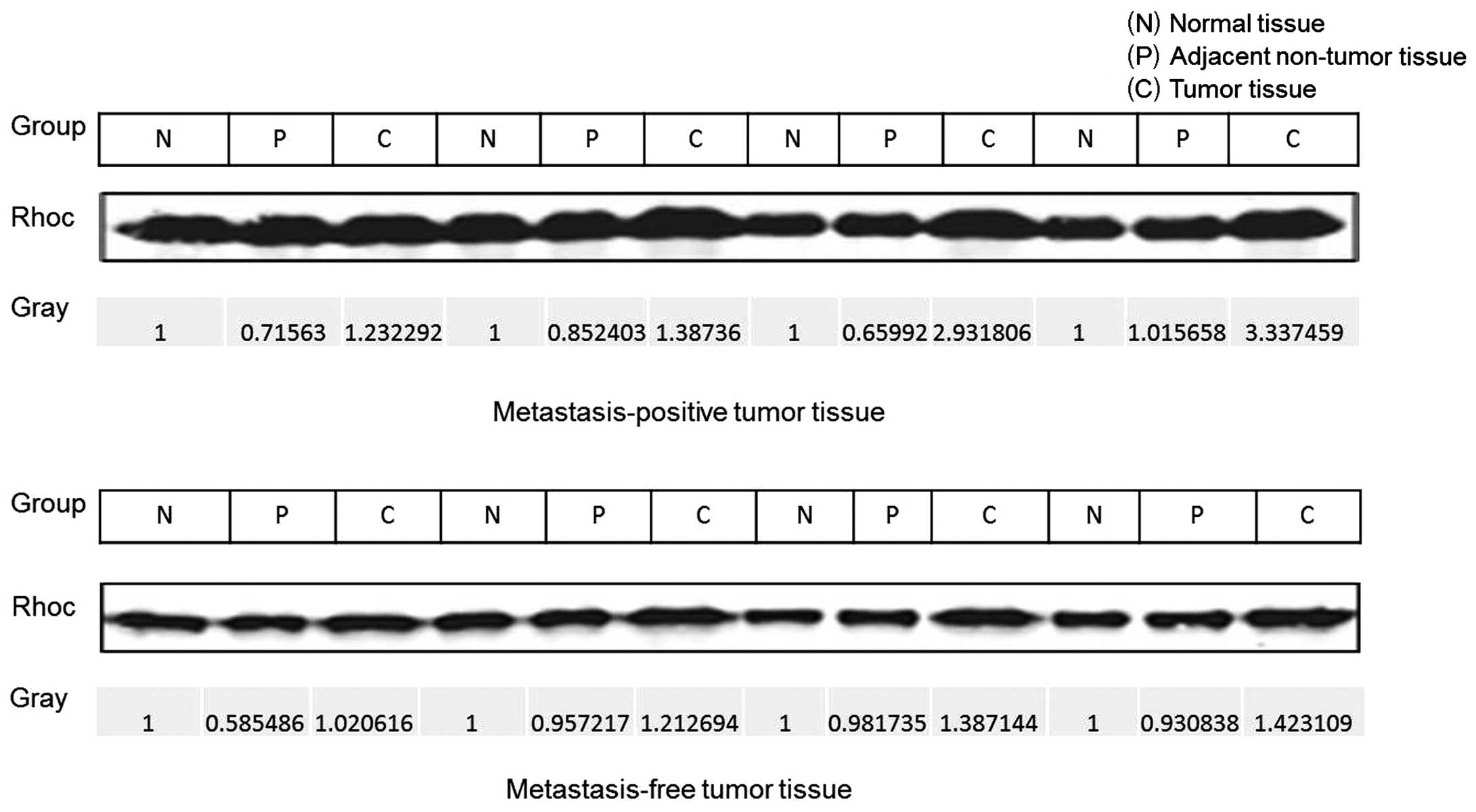
lymph node metastasis-positive. Results of the western blot
analysis indicated that Rhoc levels in tumor tissue from the
metastasis-positive group were 1-to 2-fold higher than those of the
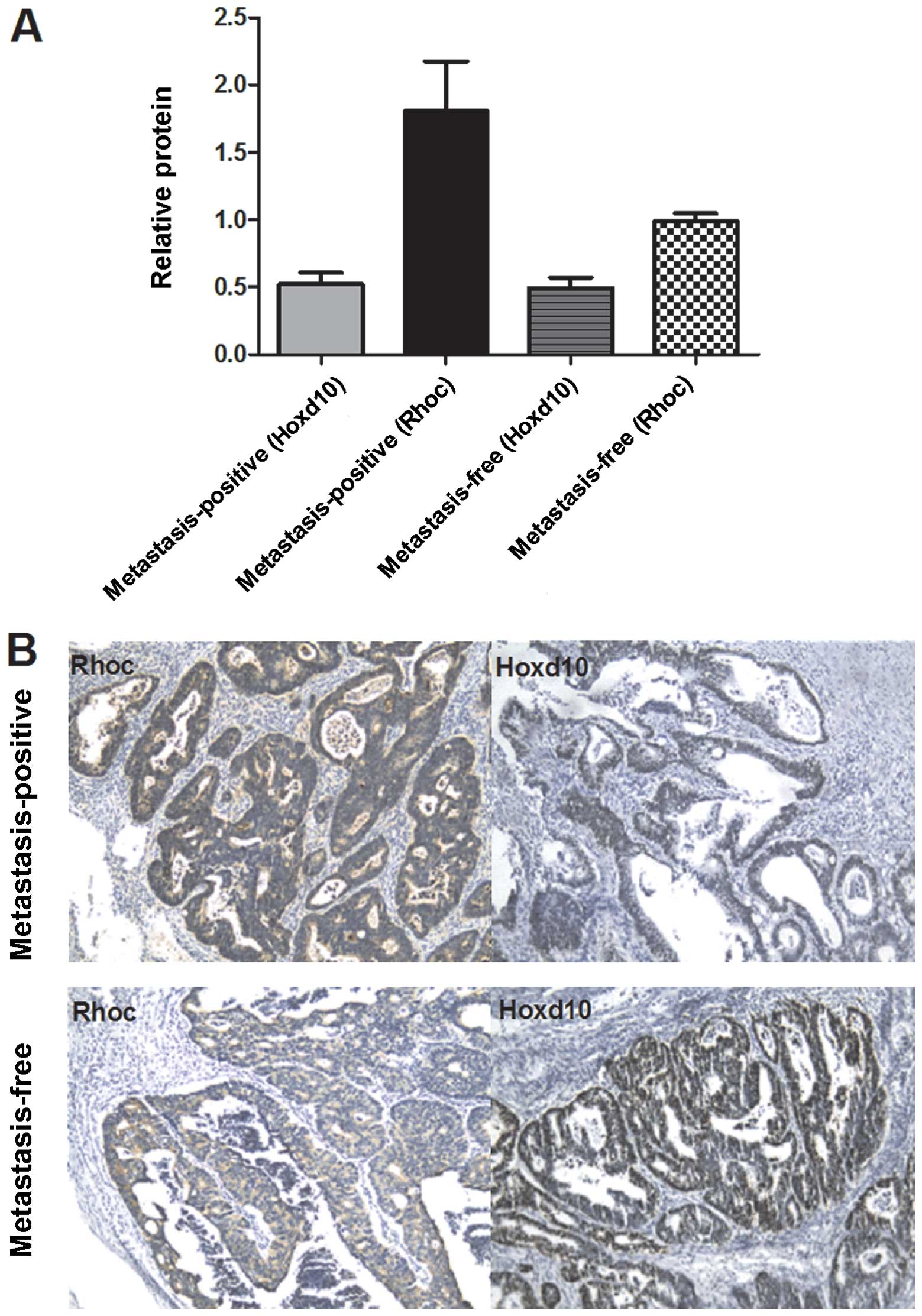
metastasis-free tissue (Fig. 6A).
To confirm the western blot analysis data, we performed
immunohistochemistry to detect the levels of Rhoc. We found that
Rhoc immunoexpression was enhanced in metastasis-positive tissue,
unlike that in the case of the metastasis-free tissue. A
significant inverse immunoreactivity of Hoxd10 was observed
(Fig. 6B). These data revealed that
Rhoc may be involved in the process of colorectal cancer
metastasis.
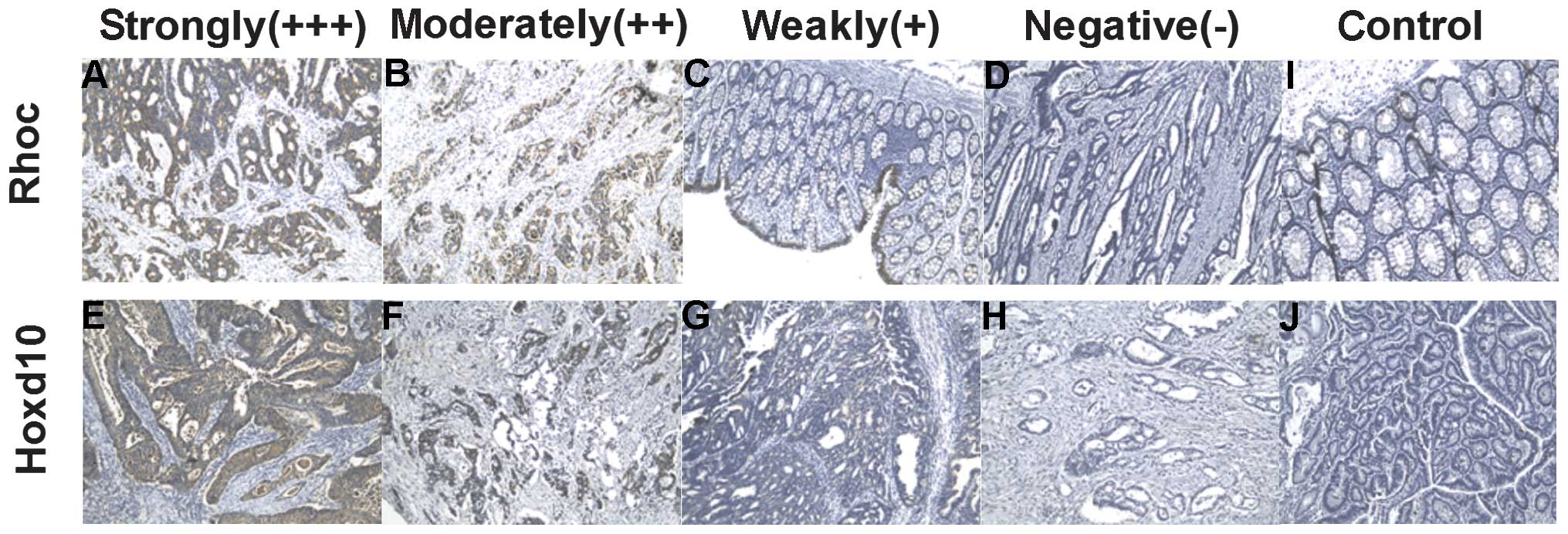 | Figure 5Expression of Rhoc and Hoxd10 protein
in colorectal cancer samples. Immunohistochemical reactivity was
evaluated and classified into 5 groups: negative (−), weak (+),
moderate (++), strong (+++) and control. Upper panels, colorectal
cancer samples showing Rhoc (A) strong, (B) moderate, (C) weak, (D)
negative and (I) control tissue specimens. Lower panels, colorectal
cancer samples showing Hoxd10 (E) strong, (F) moderate, (G) weak,
(H) negative and (J) control tissue specimens. Original
magnification, ×200. |
 | Table IVCorrelation between miR-10b level and
immunostaining of Rhoc and Hoxd10. |
Table IV
Correlation between miR-10b level and
immunostaining of Rhoc and Hoxd10.
| miR-10b
expression | |
|---|
|
| |
|---|
| Immunostaining | High (10b>25)
(n=47) (%) | Low (10b<25)
(n=23) (%) | P-value |
|---|
| Rhoc | | | <0.001a |
| − | 1 (2.1) | 1 (4.4) | |
| + | 12 (25.6) | 15 (65.2) | |
| ++ | 23 (48.9) | 7 (30.4) | |
| +++ | 11 (23.4) | 0 (0) | |
| Hoxd10 | | | <0.001a |
| − | 6 (12.8) | 2 (8.7) | |
| + | 33 (70.2) | 9 (39.1) | |
| ++ | 8 (17) | 10 (43.5) | |
| +++ | 0 (0) | 2 (8.7) | |
Discussion
We found increased serum concentrations of miR-10b
in patients with colorectal cancer, unlike that in the case of the
healthy controls. The outer periphery and portal vein serum miR-10b
concentration levels were not significantly different in colorectal
cancer (t=1.620, P=0.110). There was a significant difference in
the mean level of miR-10b in tumor tissue compared with the control
tissue (t=−2.133, P=0.038), and with adjacent non-tumor tissues
(t=−2.179, P=0.033). However, miR-10b concentration levels were not
significantly different between the adjacent non-tumor tissues and
the control tissues (t=0.496, P=0.621) (Table II and Fig. 1C). Moreover, there was a significant
difference in the mean level of miR-10b in tissues in serum of
colorectal cancer (P<0.01). The results also showed that there
were significant differences in miR-10b levels between colorectal
cancer cases of different stages, tumor sizes, node status, but
that the differences concerning age, gender, and differentiations
were not significantly different (Table III). MiR-10b levels were markedly
elevated in lymph node metastasis-positive tumor tissue compared
with lymph node metastasis-free tumor tissue (Fig. 1D). In addition, there was a
statistically significant difference between the multifocal and
single cases (P=0.04) (Table I).
Serum miR-10b concentrations were significantly higher in
colorectal cancer patients than in healthy control subjects in the
present study (P<0.01). This finding is in accordance with
studies that have reported elevated miR-10b concentrations in
breast cancer. The results of the present study suggest that
miR-10b is worthy of further evaluation as a prognostic marker in
colorectal cancer, although its molecular mechanism requires
further investigation.
As one of the gene targets of miR-10b, Hoxd10 is
considered the main effector that negatively regulates tumor
metastasis (18). In the present
study, miR-10b was highly expressed, accompanied by increased Rhoc
levels in colorectal cancer (Fig.
1B, Fig. 2A and B). The
expression level of Hoxd10 and Rhoc in colorectal cancer tissue was
significantly different compared with that of the control tissue
(P<0.01, P=0.026, respectively), and with the adjacent non-tumor
tissue group (P<0.01 and P=0.018, respectively; Fig. 2C). However, there was no
statistically significant difference between the adjacent non-tumor
tissue group and the control tissue group (P=0.863 and P=0.448,
respectively). Rhoc protein levels were markedly elevated in lymph
node metastasis-positive tumor tissue compared with lymph node
metastasis-free tumor tissue (P<0.01) (Figs. 2D and 4). The Spearman’s rank correlation test
showed that there was a high negative correlation between Rhoc and
Hoxd10 protein levels (Fig. 2F and
3). Consistent with previous
findings that miR-10b was able to drive tumor metastasis (10), high levels of miR-10b were detected
in colorectal cancer, with a low degree of differentiation that
possesses a strong ability of metastasis. Hoxd10 has been known to
repress the expression of genes involved in tumor metastasis
including Rhoc. There was a statistically significant difference
between high and low miR-10b expression groups in Rhoc and Hoxd10
immunostaining (P<0.001, Mann-Whitney U test). A significant
inverse correlation between the immunoreactivity of Hoxd10 and that
of Rhoc was observed (Table IV and
Fig. 5). MiR-10b overexpression led
to the elevation of Rhoc, which indicates that Rhoc contributes to
miR-10b-induced invasiveness with the target gene of Hoxd10. Rhoc
expression was found to be attenuated in lowly differentiated and
lymph node metastasis-positive colorectal cancer compared with
those in well-differentiated and metastasis-free lymph nodes
(Figs. 2D, 4 and 6).
These results suggest that miR-10b reduces the threshold of
metastasis in colorectal cancer as the positive regulator, which is
inversely related to Hoxd10.
Significantly higher concentrations of tissue and
serum miR-10b were identified in patients with colorectal cancer
than those in the adjacent non-tumor tissues and healthy control
subjects. The concentrations of miR-10b increased as the clinical
stage progressed. These results suggest miR-10b is involved in the
development of colorectal cancer, and may be a new therapeutic
target. In the present study, we found that miR-10b has a critical
role in colorectal cancer cell invasion. Overexpression of miR-10b
led to increased Rhoc by targeting Hoxd10, which facilitates cell
invasion in colorectal cancer. Furthermore, miR-10b expression in
high-grade colorectal cancer with multifocal lesions was
statistically significant and higher than that in cancer with
single lesions. These findings indicate that miR-10b may play a
role in the invasion and migration of colorectal cancer. Ma et
al (19) identified miR-10b as
a highly expressed miRNA in metastatic breast tumors that promotes
cell migration and invasion. It has also been shown to inhibit the
translation of mRNA encoding Hoxd10, which modulates many genes,
including uPAR (20), Rhoc
(21), α3 (22), integrin (23), β integrin (24) and MMP-14 (25,26),
which promote invasion, migration, ECM remodeling, and tumor
progression (27). In addition,
TWIST, a well-known transcriptional factor related to EMT,
activates the transcription of miR-10b by binding directly to an
E-box sequence proximal to its putative promoter (28). Although miR-10b does not trigger EMT
by itself, it may be required for TWIST-induced cell motility and
invasiveness in cancer cells (29).
Rhoc has been identified as an especially important
player in metastasis, and its expression correlates with the
metastatic spread of various types of carcinomas (30–32).
In our study, the expression of Rhoc correlated with the expression
levels of miR-10b, and a significant inverse correlation between
the immunoreactivity of Hoxd10 and Rhoc was observed.
In conclusion, our study suggests that high levels
of miR-10b are associated with the degree of metastasis in
colorectal cancer patients. A high expression of Rhoc and
downregulation of Hoxd10 correlated with the expression levels of
miR-10b. These results indicate that miR-10b may play a role in
invasion in colorectal cancer. In addition, miR-10b overexpression
promoted the invasiveness of colorectal cancer by targeting Hoxd10,
which partially passed through Rhoc-AKT signaling. These
observations shed new light on the mechanisms underlying the
invasion of colorectal cancer and provide novel therapeutic targets
in inhibiting metastasis. Moreover, studies using clinical samples
are necessary to clarify whether the quantification of miR-10b in
peripheral blood may be applied as a biomarker of colorectal
cancer.
Acknowledgements
This study was supported by grants from youth issues
of the Shanghai Municipal Health Bureau of China (20124y155).
References
|
1
|
Walker AS, Zwintscher NP, Johnson EK, et
al: Future directions for monitoring treatment response in
colorectal cancer. J Cancer. 5:44–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manser CN and Bauerfeind P: Impact of
socioeconomic status on incidence, mortality, and survival of
colorectal cancer patients: a systematic review. Gastrointest
Endosc. 80:42–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chi X, Wang S, Huang Y, et al: Roles of
rho GTPases in intra-cellular transport and cellular
transformation. Int J Mo Sci. 28:7089–7108. 2013. View Article : Google Scholar
|
|
4
|
Chu E: Personalized medicine and anti-EGFR
antibody therapy in the treatment of metastatic colorectal cancer:
KRAS and beyond. Oncology. 28:962014.PubMed/NCBI
|
|
5
|
Yu X, Li Z, Shen J, et al: MicroRNA-10b
promotes nucleus pulposus cell proliferation through RhoC-Akt
pathway by targeting HOXD10 in intervetebral disc degeneration.
PLoS One. 8:e830802013. View Article : Google Scholar :
|
|
6
|
Lang MF, Yang S, Zhao C, et al:
Genome-wide profiling identified a set of miRNAs that are
differentially expressed in glioblastoma stem cells and normal
neural stem cells. PLoS One. 7:e362482012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Preis M, Gardner TB, Gordon SR, et al:
MicroRNA-10b expression correlates with response to neoadjuvant
therapy and survival in pancreatic ductal adenocarcinoma. Clin
Cancer Res. 17:5812–5821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao H, Li H, Yu G, et al: MicroRNA-10b
promotes migration and invasion through KLF4 and HOXD10 in human
bladder cancer. Oncol Rep. 31:1832–1838. 2014.PubMed/NCBI
|
|
9
|
Schepeler T, Reinert JT, Ostenfeld MS, et
al: Diagnostic and prognostic microRNAs in stage II colon cancer.
Cancer Res. 68:6416–6424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Z, Zhu J, Cao H, et al: MiR-10b
promotes cell invasion through RhoC-AKT signaling pathway by
targeting HOXD10 in gastric cancer. Int J Oncol. 40:1553–1560.
2012.PubMed/NCBI
|
|
11
|
Malissein E, Meunier E, Lajoie-Mazenc I,
et al: RhoA and RhoC differentially modulate estrogen receptor α
recruitment, transcriptional activities, and expression in breast
cancer cells (MCF-7). J Cancer Res Clin Oncol. 139:2079–2088. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Y, Zheng HC, Chen S, et al: The role
of RhoC in ovarian epithelial carcinoma: a marker for
carcinogenesis, progression, prognosis, and target therapy. Gynecol
Oncol. 130:570–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goundiam O, Nagel MD and Vayssade M: Akt
and RhoA inhibition promotes anoikis of aggregated B16F10 melanoma
cells. Cell Biol Int. 36:311–319. 2012. View Article : Google Scholar
|
|
14
|
Gao W, Dong X, Xie N, et al:
Dehydroabietic acid isolated from Commiphora opobalsamum causes
endothelium-dependent relaxation of pulmonary artery via
PI3K/Akt-eNOS signaling pathway. Molecules. 19:8503–8517. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han X, Yan S, Weijie Z, et al: Critical
role of miR-10b in transforming growth factor-β1-induced
epithelial-mesenchymal transition in breast cancer. Cancer Gene
Ther. 21:60–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Parrella P, Barbano R, Pasculli B, et al:
Evaluation of microRNA-10b prognostic significance in a prospective
cohort of breast cancer patients. Mol Cancer. 13:1422014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Severino P, Brüggemann H, Andreghetto FM,
et al: MicroRNA expression profile in head and neck cancer:
HOX-cluster embedded microRNA-196a and microRNA-10b dysregulation
implicated in cell proliferation. BMC Cancer. 13:5332013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu Y, Li F, Zhao DY, et al: Interaction
between Tbx1 and Hoxd10 and connection with TGFβ-BMP signal pathway
during kidney development. Gene. 536:197–202. 2014. View Article : Google Scholar
|
|
19
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sasayama T, Nishihara M, Kondoh T, et al:
MicroRNA-10b is over-expressed in malignant glioma and associated
with tumor invasive factors, uPAR and RhoC. Int J Cancer.
125:1407–1413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakayama I, Shibazaki M, Yashima-Abo A, et
al: Loss of HOXD10 expression induced by upregulation of miR-10b
accelerates the migration and invasion activities of ovarian cancer
cells. Int J Oncol. 43:63–71. 2013.PubMed/NCBI
|
|
22
|
Carrio M, Arderiu G, Myers C and Boudreau
NJ: Homeobox D10 induces phenotypic reversion of breast tumor cells
in a three-dimensional culture model. Cancer Res. 65:7177–7185.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu B, Geerts D, Bu Z, et al: Regulation of
endometrial receptivity by the highly expressed HOXA9, HOXA11 and
HOXD10 HOX-class homeobox genes. Hum Reprod. 29:781–790. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park H, Choi HJ, Kim J, et al: Homeobox D1
regulates angiogenic functions of endothelial cells via integrin β1
expression. Biochem Biophys Res Commun. 408:186–192. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun L, Yan W, Wang Y, et al: MicroRNA-10b
induces glioma cell invasion by modulating MMP-14 and uPAR
expression via HOXD10. Brain Res. 1389:9–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong CG, Wu WK, Feng SY, et al:
Co-inhibition of microRNA-10b and microRNA-21 exerts synergistic
inhibition on the proliferation and invasion of human glioma cells.
Int J Oncol. 41:1005–1012. 2012.PubMed/NCBI
|
|
27
|
Biagioni F, Bossel Ben-Moshe N, Fontemaggi
G, et al: MiR-10b*, a master inhibitor of the cell
cycle, is down-regulated in human breast tumours. EMBO Mol Med.
4:1214–1229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gabriely G, Teplyuk NM and Krichevsky AM:
Context effect: microRNA-10b in cancer cell proliferation, spread
and death. Autophagy. 7:1384–1386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vimalraj S, Miranda PJ, Ramyakrishna B and
Selvamurugan N: Regulation of breast cancer and bone metastasis by
microRNAs. Dis Markers. 35:369–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gavert N, Vivanti A, Hazin J, et al:
L1-mediated colon cancer cell metastasis does not require changes
in EMT and cancer stem cell markers. Mol Cancer Res. 9:14–24. 2011.
View Article : Google Scholar
|
|
31
|
Pizzini S, Bisognin A, Mandruzzato S, et
al: Impact of microRNAs on regulatory networks and pathways in
human colorectal carcinogenesis and development of metastasis. BMC
Genomics. 14:5892013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang FJ, Ding Y, Mao YY, et al:
Associations between hsa-miR-603 polymorphism, lifestyle-related
factors and colorectal cancer risk. Cancer Biomark. 14:225–231.
2014.PubMed/NCBI
|