Introduction
The p53 tumor-suppressor plays a crucially role in
preventing neoplasia and tumor progression. p53 directly activates
a large number of genes, which mediate numerous cellular functions
that contribute to tumor suppression (1,2).
Furthermore, p53 may mediate the specific repression of many genes.
However, the mechanisms of gene repression by p53 are not well
understood and may be indirect to some extent (3).
In recent years, miRNAs have been identified as
important components of the signaling cascades that mediate and
regulate tumor suppression exerted by p53 (4–10). In
2007, the miR-34 families, miR-34a and miR-34b/c, were reported to
be directly regulated by p53 by a number of laboratories using
diverse approaches (11–16). miR-1246 has been identified as a
novel p53 target miRNA (17).
Notably, this miRNA regulates the expression of DYRK1A, a Down
syndrome-associated kinase, which inactivates a nuclear
transcriptional factor called NFAT1C by phosphorylating it and
preventing it from nuclear import (18).
In the present study, we found that p53 induces the
expression of miR-1246 in hepatocellular carcinoma (HCC) cell
lines. Alteration of miR-1246 modulated cell proliferation, colony
formation ability and apoptosis. These functions were, at least in
part, exerted through the direct targeting of nuclear factor I/B
(NFIB). In conclusion, we propose a new p53 pathway involving
miR-1246-NFIB potentially critical for cancer prevention.
Materials and methods
Human liver tumor samples
Fresh frozen human HCC tissue samples and matched
normal hepatocellular tissue samples were obtained from the Fourth
Affiliated Hospital of Hebei Medical University. The types of all
the tumors were confirmed by pathologic analysis. All the human
materials were used in accordance with the policies of the
Institutional Review Board of Hebei Medical University.
Cell culture and transfection
Seven human HCC cell lines (HepG2, Hep3B, Huh7, C3A,
PLC, LO2 and SUN387) were maintained in MEMα or RPMI-1640 medium
(Gibco), respectively, and supplemented with 10% fetal bovine serum
(FBS), 100 IU/ml of penicillin and 100 μg/ml of streptomycin. Cells
were incubated at 37°C in a humidified chamber supplemented with 5%
CO2. Transfection was performed with Lipofectamine 2000
reagent (Invitrogen, Carlsbad, CA, USA) following the
manufacturer’s protocol.
Fluorescent report assay
To confirm the direct interaction between miR-1246
and NFIB mRNA, HCC cells were simultaneously transfected with
miR-1246 or miR-control and the reporter vectors in 48-well plates.
The cells were lysed with radioimmunoprecipitation assay (RIPA)
buffer (150 mM NaCl, 50 mM Tris-HCl pH 7.2, 1% Triton X-100, 0.1%
SDS) 72 h later, and the proteins were harvested. The intensities
of luciferase were detected with a fluorescence spectrophotometer
F-4500 (Hitachi, Tokyo, Japan).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cell proliferation was determined using the MTT cell
proliferation kit (Solarbio, Beijing, China). HCC cells
(5×103) were seeded in each 96-well plate and allowed to
adhere overnight. The cells were then infected with the adenovirus
vector at an MOI of 10 for the indicated times prior to being used
for the MTT assay as per the manufacturer’s instructions (Bio-Rad
Laboratories Inc., Irvine, CA, USA).
Colony formation assay
After transfection, cells were counted and seeded in
12-well plates in triplicate at 100 cells/well. Fresh culture
medium was replaced every 3 days. The colony was counted only if it
contained >50 cells, and the number of colonies was counted from
day 6 after seeding. The rate of colony formation was calculated
with the equation: Colony formation rate = (number of
colonies/number of seeded cells) × 100%.
Quantitative reverse transcription-PCR
(qRT-PCR)
mRNAs or miRNAs were reverse transcribed to generate
cDNA using oligo(dT) primers or stem-loop reverse transcriptase
(RT) primers (18), respectively.
Then, U6 snRNA (for miRNA) or GAPDH (for mRNA) was considered as
the endogenous control. Target genes and controls were treated
under the same condition and analyzed by real-time RT-PCR using
SYBR Premix Ex Taq™ (Takara, Dalian, China) according to the
manufacturer’s protocol. The primers used in the present study are
listed in Table I.
 | Table IPrimers and sequences used in the
present study. |
Table I
Primers and sequences used in the
present study.
| Name | Primer | Sequence |
|---|
| U6 | Forward |
5′-GTGCTCGCTTCGGCAGCACATATAC-3′ |
| Reverse |
5′-AAAAATATGGAACGCTCACGAATTTG-3′ |
| GAPDH | Forward |
5′-ATGTCGTGGAGTCTACTGGC-3′ |
| Reverse |
5′-TGACCTTGCCCACAGCCTTG-3′ |
| miR-1246 | Forward |
5′-TATTGCACTCGTCCCCTGCT-3′ |
| Reverse |
5′-GTGCAGGGTCCGAGGT-3′ |
| NFIB | Forward |
5′-AAACCCAGCACTTTGTGTCC-3′ |
| Reverse |
5′-TCTTGGGGAAGAATCCTGTG-3′ |
| miR-1246 ASO | Antisense
miR-1246 |
2′-O-Me-CCTGCTCCAAAAATCCTTT |
Western blot analysis
Protein extracts were prepared with RIPA lysis
buffer in the presence of proteinase inhibitors. Western blot
analysis was performed using antibodies against NFIB (1:600; Cell
Signaling Technology, Danvers, MA, USA) and GAPDH (1:400; Beijing
Biosynthesis Biotechnology Co., Beijing, China). The blots were
then developed using the enhanced chemiluminescence (ECL) reagent
(Amersham Biosciences, USA).
Immunohistochemistry (IHC) staining
The anti-NFIB antibody (1:500; Abcam) was used for
IHC using standard methods.
Flow cytometric analysis
At 48 h after transfection, the HepG2 cells were
detached from the plates by trypsin incubation, rinsed with
phosphate-buffered saline (PBS) and fixed in 70% (v/v) ethanol. The
cells were then rehydrated in PBS and incubated with RNase (100
μg/ml) and propidium iodide (PI) (60 μg/ml) (Sigma-Aldrich, St.
Louis, MO, USA). Cells were analyzed using the FACSCalibur system
(BD Biosciences, San Jose, CA, USA).
Statistical analysis
Statistical analysis utilized the two-tailed
Student’s t-test. Statistical significance was set as
p<0.05.
Results
p53 induces the expression of miR-1246 in
HCC cell lines
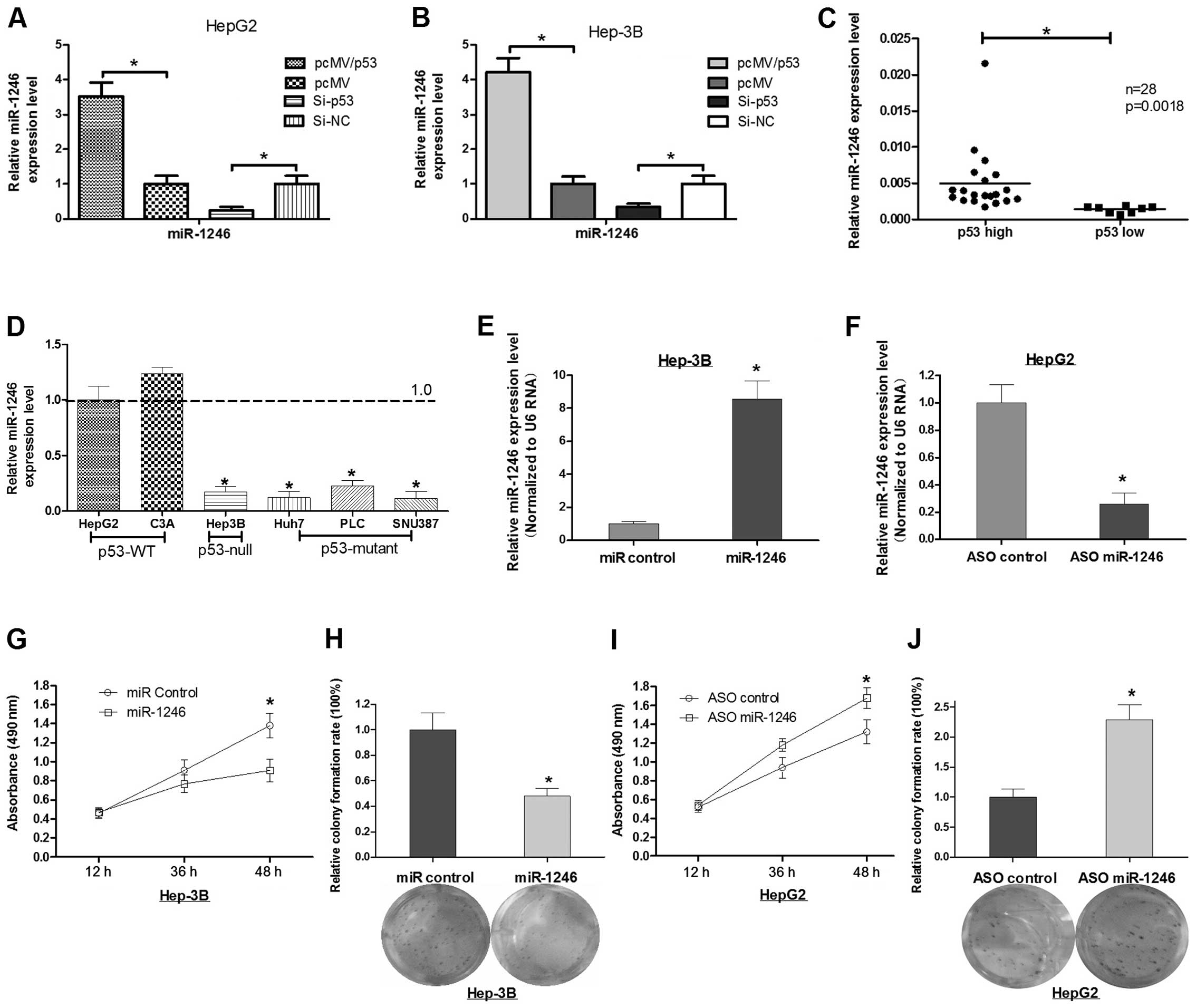
We enforced the expression of p53 in HepG2 or Hep-3B
cells and found that they both had induced expression of miR-1246
(Fig. 1A and B). In contrast, the
knockdown of p53 abrogated the induction of miR-1246 expression in
the two cell lines (Fig. 1A and B).
In addition, we detected the expression of miR-1246 and p53 in 26
HCC tumor tissues, and found that miR-1246 was consistent with the
p53 expression (Fig. 1C). Next, we
detected the miR-1246 expression level in 6 HCC cell lines (two of
them expressed wild-type p53, one of them expressed null p53, and
three of them expressed mutant p53). The finding also suggested
that the expression of miR-1246 was consistent with p53 (Fig. 1D).
Alteration of miR-1246 affects the cell
growth of HCC in vitro
To further explore the role of miR-1246 in HCC, we
transfected either miR-1246 mimics or miR-1246 antisense
oligonucleotides (miR-1246 ASO) into HCC cells. As shown in
Fig. 1E and F, miR-1246 expression
was increased ~8.0-fold in the Hep-3B cells when the miR-1246
mimics were induced, while, miR-1246 ASO resulted in ~74% reduction
in miR-1246 levels in the HepG2 cells. Cell viability of HCC cells
transfected with miR-1246 or miR-1246 ASO was evaluated by the MTT
assay. miR-1246 reduced cell viability at 36 or 48 h after
transfection, whereas miR-1246 ASO increased HCC cell viability
(Fig. 1G and I). In parallel, we
analyzed colony formation and cellular proliferation to assess the
effect of miR-1246 on the proliferative capacity of HCC cells.
Compared with the control group, transfection with miR-1246 was
~50% less than that of Hep3B cells transfected with the miR-control
(Fig. 1H). Conversely, the colony
formation rate of the HepG2 cells after transfection with miR-1246
ASO was increased 2.3-fold when compared with the control group
(Fig. 1J). These results indicate
that miR-1246 inhibits the cell proliferation of HCC cells.
miR-1246 targets NFIB and negatively
regulates its expression
miR-1246 has been demonstrated to play an essential
role in the regulation of growth and apoptosis of cells (17–21).
To determine the mechanism of miR-1246-mediated cell proliferation
and apoptosis regulation in HCC cells, we next identified target
genes that could be responsible for the effect of miR-1246. The
oncogene NFIB, which was predicted to have two miR-1246 binding
sites in its 3′-untranslated region (3′UTR) (Fig. 2A), was chosen for further study. To
confirm whether miR-1246 could bind to this predicted region and
suppress the protein expression of NFIB, we constructed a
luciferase reporter vector in which the 3′UTR fragment of NFIB,
including the putative binding site, was inserted downstream of the
luciferase coding region. HepG2 cells were transfected with the
reporter vector together with either miR-1246 ASO or miR-1246. As
shown in Fig. 2B, the intensity of
luciferase was higher in the miR-1246-blocked group as compared to
the control group, whereas ectopic expression of miR-1246 decreased
the intensity of luciferase intensity compared with the control
group. In addition, we constructed two other luciferase reporter
vectors containing mutations in the miR-1246 binding sites
(Fig. 2A). Neither blocking
miR-1246 with ASO nor overexpressing miR-1246 had any effect on the
intensity of fluorescence from the vector with the mutated miR-1246
binding region (Fig. 2B). These
results showed that miR-1246 binds directly to the 3′UTR of
NFIB.
To determine whether miR-1246 negatively regulates
NFIB expression at the mRNA or protein level, we assessed
endogenous NFIB expression in HepG2 or Hep3B cells with altered
miR-1246 expression. Both cell lines were transfected with miR-1246
or miR-1246 ASO to overexpress or block miR-1246, respectively.
Notably, miR-1246 showed an inability to alter NFIB expression at
the mRNA level in both cell groups transfected with miR-1246 or
miR-1246 ASO (Fig. 2C). However,
miR-1246 downregulated NFIB protein expression in the LO2, HepG2
and C3A cells, while miR-1246 ASO induced increased NFIB protein
expression in the Hep-3B, Huh7 and PLC cells (Fig. 2D). These results suggest that
miR-1246 regulates endogenous NFIB expression by targeting mRNAs
and triggering translation repression.
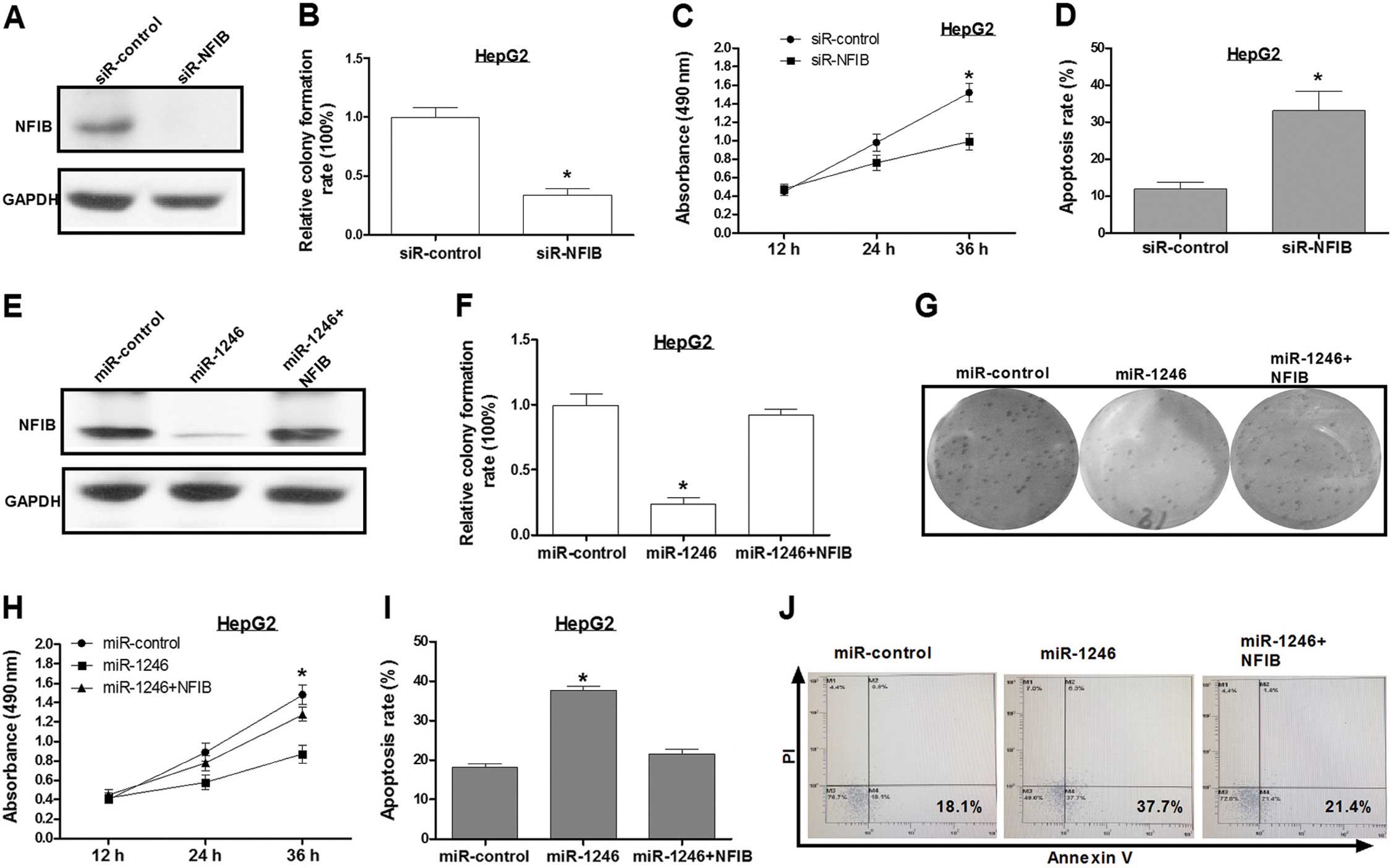
Knockdown of NFIB inhibits HCC cell
growth and promotes its apoptosis
Sequence-specific small interfering RNAs (siRNAs)
can effectively suppress gene expression. An siRNA targeting NFIB
(siR-NFIB) was used for the present study. Western blot assay
showed that the level of NFIB expression was reduced by ~90% in the
HepG2 cells that were transfected with siR-NFIB, as compared to
HepG2 cells transfected with a control siRNA (Fig. 3A). Inhibition of NFIB expression
inhibited cell growth and increased HepG2 cell apoptosis as
compared to the control group (Fig.
3B–D), which was consistent with the effects of miR-1246
overexpression.
Overexpression of NFIB abolishes the
regulation of cell apoptosis caused by miR-1246
To further explore the miR-1246-induced HCC cell
proliferation inhibition mediated by NFIB, we transfected HepG2
cells with the miR-control, miR-1246 or miR-1246/NFIB expression
plasmid (pcDNA3/NFIB), respectively. Western blotting confirmed
that the NFIB expression vector was effective (Fig. 3E). Based on the results, ectopic
expression of NFIB reversed the effects of miR-1246 on HCC cell
phenotypes (Fig. 3F–J). These
results revealed that NFIB was a key mediator in the
miR-1246-induced cell growth inhibition in HCC.
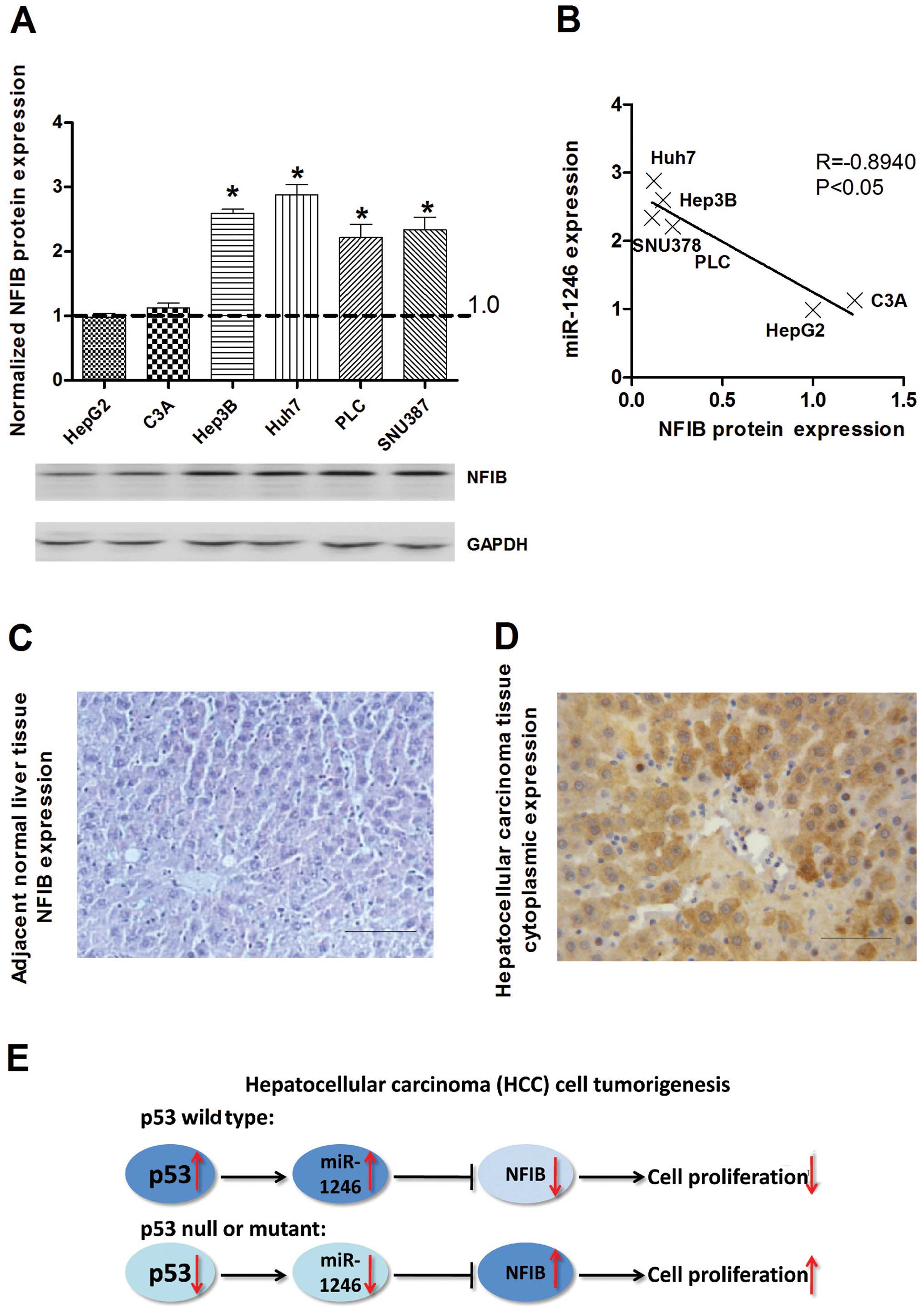
NFIB is inversely expressed with miR-1246
in HCC
To explore the relevance of NFIB in relation to
miR-1246 in human HCC cells, the expression of NFIB was evaluated
in HCC cells by western blotting using the anti-NFIB antibody. The
expression of NFIB was increased in all mutant or null
p53-containing HCC cell lines with p53 wild-type cells, which was
inversely correlated with miR-1246 (Fig. 4A and B). In addition, IHC staining
showed predominantly cytoplasmic localization of NFIB, and the
expression of NFIB was markedly increased in all malignant tumor
cells from the examined archival HCC samples relative to the
adjacent non-tumor tissues (Fig. 4C and
D). In total, we found that the expression of miR-1246 could be
induced by wild-type p53 in the HCC cell lines. Overexpression of
miR-1246 inhibited the proliferation of HCC cells by targeting
NFIB. In contrast, the expression of miR-1246 was reduced in the
mutant or null p53 HCC cells, and downregulation of miR-1246
resulted in upregulation of NFIB, subsequently promoting HCC cell
proliferation (Fig. 4E).
Discussion
Hepatocellular carcinoma (HCC) is one of the most
common types of cancers, and ranks as the fifth most leading cause
of cancer-related deaths worldwide (22). Deregulated miRNA expression has been
closely associated with the abnormal expression of cellular genes
that regulate apoptosis, proliferation, the cell cycle and
metastasis, leading to the development and progression of HCC
(23–26). It has been reported that miR-1246 is
a p53 target miRNA, as p53 inhibited DYRK1A expression through the
induction of miR-1246 in non-small cell lung cancer cells (18). We aimed to ascertain whether
miR-1246 is also induced by p53 and plays a role as a tumor
suppressor in HCC cells. To address this issue, we first examined
miR-1246 expression after overexpressing or the knockdown of p53 in
HCC cells by real-time RT-PCR, as previously described (27). We then examined miR-1246 expression
in p53 wild-type, mutant and null p53-containing HCC cell lines.
The results showed that the expression of miR-1246 was correlated
with the expression of wild-type p53. Then, we determined the
effect of miR-1246 on HCC cells by gain- and loss-of-function
approaches. MTT and colony formation assays showed that miR-1246
inhibited the growth of HCC cells. Thus, we inferred that miR-1246
may be a growth-inhibition factor in HCC.
The fundamental function of miRNAs is to regulate
their targets by direct cleavage of mRNAs or by inhibition of
translation (18), depending on the
degree of complementarity with the 3′UTR of their target genes. The
putative targets of miR-1246 were predicted using the miRanda,
PicTar and TargetScan target algorithms, which may be correlated
with the phenotype of the HCC cell lines caused by the alteration
of miR-1246. Among them, the oncogene nuclear factor I/B (NFIB) was
identified as a novel direct target of miR-1246. qRT-PCR and
western blot assay showed that miR-1246 decreased the NFIB
expression at the protein but not the mRNA level in the HCC cell
lines compared with the control. In addition, NFIB expression was
upregulated in the HCC tissues compared to that in the normal
tissues. We confirmed that miR-1246 can bind directly to the NFIB
3′UTR and negatively regulates NFIB gene expression by a luciferase
reporter assay. We then confirmed the oncogenic role of NFIB in the
HCC cells, and found that knockdown of NFIB by siRNA promoted HCC
cell apoptosis, whereas ectopic expression of NFIB effectively
alleviated the miR-1246-induced apoptosis of HCC cells.
Collectively, these findings indicate that miR-1246 may exert its
effects in HCC mainly by targeting NFIB. However, Chen et al
recently reported that miR-1246 promotes SiHa cervical cancer cell
proliferation, invasion and migration through suppression of its
target gene thrombospondin 2 (20),
which is paradoxical with our results in HCC. One possible
explanation is that miRNAs have different functions in different
types of tissues; for instance, miR-9 is upregulated in breast
cancer cells (28), yet
downregulated in human ovarian cancer (29).
Recent studies found that NFIB regulates cell
viability and proliferation during transformation, and is an
oncogene in small cell lung cancer (30). Furthermore, silencing of the NFIB
gene resulted in reduced proliferation and an increase in the
apoptotic signaling pathway, suggesting a potential value of NFIB
as a novel target in ER-negative breast cancer (31). These results are consistent with our
studies in HCC cells.
In conclusion, we found that p53 induced the
expression of miR-1246 in HCC cell lines. Overexpression of
miR-1246 promoted the cell apoptosis of HCC cells possibly through
inhibition of NFIB. Thus, identification of miR-1246 and its target
gene NFIB may help us to understand the molecular mechanism of
tumorigenesis in HCC and may propose a new p53-miR-1246-NFIB
pathway in HCC cancer.
References
|
1
|
Collavin L, Lunardi A and Del Sal G:
p53-family proteins and their regulators: hubs and spokes in tumor
suppression. Cell Death Differ. 17:901–911. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qian Y and Chen X: Tumor suppression by
p53: making cells senescent. Histol Histopathol. 25:515–526.
2010.PubMed/NCBI
|
|
3
|
Menendez D, Inga A and Resnick MA: The
expanding universe of p53 targets. Nat Rev Cancer. 9:724–737. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hermeking H: The miR-34 family in cancer
and apoptosis. Cell Death Differ. 17:193–199. 2010. View Article : Google Scholar
|
|
5
|
Hermeking H: MicroRNAs in the p53 network:
micromanagement of tumour suppression. Nat Rev Cancer. 12:613–626.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shin S, Cha HJ, Lee EM, et al: MicroRNAs
are significantly influenced by p53 and radiation in HCT116 human
colon carcinoma cells. Int J Oncol. 34:1645–1652. 2009.PubMed/NCBI
|
|
7
|
He L, He X, Lowe SW and Hannon GJ:
microRNAs join the p53 network - another piece in the
tumour-suppression puzzle. Nat Rev Cancer. 7:819–822. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng Z, Zhang C, Wu R and Hu W: Tumor
suppressor p53 meets microRNAs. J Mol Cell Biol. 3:44–50. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang W and Cohen SM: The Hippo pathway
acts via p53 and microRNAs to control proliferation and
proapoptotic gene expression during tissue growth. Biol Open.
2:822–828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liao JM, Cao B, Zhou X and Lu H: New
insights into p53 functions through its target microRNAs. J Mol
Cell Biol. 6:206–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tarasov V, Jung P, Verdoodt B, et al:
Differential regulation of microRNAs by p53 revealed by massively
parallel sequencing: miR-34a is a p53 target that induces apoptosis
and G1-arrest. Cell Cycle. 6:1586–1593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang TC, Wentzel EA, Kent OA, et al:
Transactivation of miR-34a by p53 broadly influences gene
expression and promotes apoptosis. Mol Cell. 26:745–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raver-Shapira N, Marciano E, Meiri E, et
al: Transcriptional activation of miR-34a contributes to
p53-mediated apoptosis. Mol Cell. 26:731–743. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bommer GT, Gerin I, Feng Y, et al:
p53-mediated activation of miRNA34 candidate tumor-suppressor
genes. Curr Biol. 17:1298–1307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Corney DC, Flesken-Nikitin A, Godwin AK,
Wang W and Nikitin AY: MicroRNA-34b and microRNA-34c are targets of
p53 and cooperate in control of cell proliferation and
adhesion-independent growth. Cancer Res. 67:8433–8438. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He L, He X, Lim LP, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao JM, Zhou X, Zhang Y and Lu H:
miR-1246: a new link of the p53 family with cancer and Down
syndrome. Cell Cycle. 11:2624–2630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Liao JM, Zeng SX and Lu H: p53
downregulates Down syndrome-associated DYRK1A through miR-1246.
EMBO Rep. 12:811–817. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W, Wu YF, Xu RH, Lu H, Hu C and Qian H:
miR-1246 releases RTKN2-dependent resistance to UVB-induced
apoptosis in HaCaT cells. Mol Cell Biochem. 394:299–306. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Yao D, Zhao S, et al: MiR-1246
promotes SiHa cervical cancer cell proliferation, invasion, and
migration through suppression of its target gene thrombospondin 2.
Arch Gynecol Obstet. 290:725–732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu LJ, Jiang T, Zhao W, et al: Parallel
mRNA and microRNA profiling of HEV71-infected human neuroblastoma
cells reveal the up-regulation of miR-1246 in association with DLG3
repression. PLoS One. 9:e952722014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
23
|
Huang S and He X: The role of microRNAs in
liver cancer progression. Br J Cancer. 104:235–240. 2011.
View Article : Google Scholar :
|
|
24
|
Wu N, Liu X, Xu X, et al: MicroRNA-373, a
new regulator of protein phosphatase 6, functions as an oncogene in
hepatocellular carcinoma. FEBS J. 278:2044–2054. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou L, Yang ZX, Song WJ, et al:
MicroRNA-21 regulates the migration and invasion of a stem-like
population in hepatocellular carcinoma. Int J Oncol. 43:661–669.
2013.PubMed/NCBI
|
|
26
|
Zhou J, Lu S, Yang S, et al: MicroRNA-127
post-transcriptionally downregulates Sept7 and suppresses cell
growth in hepatocellular carcinoma cells. Cell Physiol Biochem.
33:1537–1546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen C, Ridzon DA, Broomer AJ, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma L, Young J, Prabhala H, et al: miR-9, a
MYC/MYCN-activated microRNA, regulates E-cadherin and cancer
metastasis. Nat Cell Biol. 12:247–256. 2010.PubMed/NCBI
|
|
29
|
Tang H, Yao L, Tao X, et al: miR-9
functions as a tumor suppressor in ovarian serous carcinoma by
targeting TLN1. Int J Mol Med. 32:381–388. 2013.PubMed/NCBI
|
|
30
|
Dooley AL, Winslow MM, Chiang DY, et al:
Nuclear factor I/B is an oncogene in small cell lung cancer. Genes
Dev. 25:1470–1475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moon HG, Hwang KT, Kim JA, et al: NFIB is
a potential target for estrogen receptor-negative breast cancers.
Mol Oncol. 5:538–544. 2011. View Article : Google Scholar : PubMed/NCBI
|