Introduction
Prostate cancer is the most commonly diagnosed
malignancy in males and frequently metastasizes to the bone marrow
(1) and easily develops resistance
to endocrinotherapy and chemotherapy (2). The mechanisms attributed to tumor
metastasis and resistance are mostly considered to be induced by
the chronic inflammatory microenvironment in prostate tumors, which
is characterized by high levels of IL-2, IL-4, IL-10, TNF-α and
TGF-β (3–7). In addition, the tumor milieu also
contains multiple increased inflammatory mediators such as
chemokines, cytokines, reactive oxygen species and prostaglandin E2
(PGE2), resulting in tumor growth by elevating the expression of
anti-apoptotic proteins such as Bcl2, and by activating
transcription factors including cAMP, NF-κB and STAT3 (8–10).
Despite numerous studies investigating the clinical
significance of local and peripheral blood T lymphocytes (11,12),
the nature of B cell responses in the circulation and in tumor
lesions and functional contributions of antibodies produced in
cancer are still not well explained. Recently, B cells were
reported to mediate immune modulating functions through modulating
the balance of 4 subclasses of immunoglobulin G (IgG) (IgG1, IgG2,
IgG3 and IgG4), that have different physiological functions in the
formation of the immune complex and regulation of the immune
process (13,14). IgG4+ B cells were found
to be infiltrating in lesions of patients with extrahepatic
cholangiocarcinomas and pancreatic cancers (15,16).
Previous studies also indicated abnormalities in the serum level of
IgG4 in patients with melanoma (17,18).
The molecular predilection for antibody class switching to secrete
IgG4 have been regarded to be associated with IL-4 and IL-10
(19,20), but the exact function of B cells and
the relative interaction with inflammatory cytokines in prostate
cancer have not been explored explicitly.
As a Th subset characterized by positive CD4 and
CXCR5, T follicular helper (Tfh) cells engage in promoting the
growth, differentiation and class switching of B cells by secreting
larger amounts of IL-21 in germinal centers (GCs) or outside GCs
(21–24). Recently, Morita et al
(25) distinguished 3 subclasses
(Tfh1, Tfh2 and Tfh17) defined according to the expression of the
CCR6 and CXCR3 chemokine receptors; Tfh1 cells are
CXCR3+CCR6− cells, Tfh2 cells are
CXCR3−CCR6− cells, whereas Tfh17 cells are
CXCR3−CCR6+ cells. Tfh2 and Tfh17 cells could
provide help to B cells via IL-21 production, resulting in
immunoglobulin (Ig) secretion of various isotypes (IgM, IgA, IgG
and IgE for Tfh2 cells). However the functions of different Tfh
subsets in prostate cancer are largely unexplored. Here we found
higher percentages of 3 Tfh subsets and IgG4+ B cells in
patients with prostate cancer and proved a positive correlation
between Tfh2 and IgG4+ B cells by a co-culture system.
Then we verified that IL-4, IL-6, IL-10 and PGE2 could effectively
mediate antibody class switching of B cells, but was not completely
dependent on Tfh cells. These results may be helpful to understand
the interaction between B and T lymphocytes.
Materials and methods
Patients
Thirty new onset patients (male, age ranging from 63
to 75 years) were diagnosed with prostate cancer by digital rectal
examination (DRE), transrectal ultrasound guided automatic biopsy
and detection of prostate-specific antigen (PSA). These patients
were classified into 3 groups according to the prostate cancer
stage and serum levels of PSA (Table
I). Ten healthy volunteers (male, age ranging from 23 to 31
years) were recruited as controls in this study. All of the
participants suffering no systemic disorders or viral infections
and were enrolled at the Department of Urology, China-Japan Union
Hospital of Jilin University from 2011 to 2013. Written informed
consent was obtained from the guardians on behalf of all
participants, and this study was reviewed and approved by the
Ethics Committee of China-Japan Union Hospital of Jilin University,
China.
 | Table ICharacteristics of the study
subjects. |
Table I
Characteristics of the study
subjects.
| Clinical
indices | Group A (n=10) | Group B (n=10) | Group C (n=10) | Healthy controls
(n=10) |
|---|
| Age (years) | 65 (63–69) | 68 (66–71) | 70 (65–75) | 27 (23–31) |
| Clinical stage | ≤T1 | T2 | ≥T3 | - |
| PSA (ng/ml) | 13.84a (5.31–30.05) | 198.72a (8.35–825.41) | 361.48a (64.24–1247.25) | 0.85
(0.73–1.24) |
Lymphocyte stimulation and isolation
Venous blood samples (10 μl) were collected from
individual subjects, and Ficoll-Paque Plus (Amersham Biosciences,
Piscataway, NJ, USA) was used to sort peripheral blood mononuclear
cells (PBMCs) by density-gradient centrifugation. For the isolation
of B-cell subsets, PBMCs were stained in duplicate with anti-CD19
PerCP (BD Biosciences, San Diego, CA, USA), anti-CD27 FITC
(eBioscience, San Diego, CA, USA), anti-CD38 APC (eBioscience) and
anti-IgM PE (BD Biosciences). The frequencies of the different B
cell subsets-naïve B cells (CD19+IgM+),
memory B cells (CD19+CD27+) and mature B
cells (CD19+CD38+) were analyzed by flow
cytometry after stimulation for 5 days in Dulbecco’s modified
Eagle’s medium (DMEM; Gibco-BRL, Gaithersburg, MD, USA) combined
with 10% fetal bovine serum (FBS; Gibco), 2.5 ng/ml TLR9 ligand CpG
2006 ODN (Hycult Biotech, The Netherlands) and 30% supernatant of
Epstein Barr Virus (EBV)-producing B95-8 cells in vitro as
previously described (18,26).
To purified the Tfh cells, PBMCs were stained with
anti-CD4 APC and anti-CXCR5 PerCP (both from BD Biosciences). The
different Tfh populations were sorted with FACSAria (BD
Biosciences) according to the expression of CXCR3 and CCL6 within
the CD4+CXCR5+ cell population, and defined
as Tfh1 (CXCR3+CCR6−), Tfh2
(CXCR3−CCR6−) and Tfh17
(CXCR3−CCR6+) (25). Sorted Tfh populations were
stimulated for 5 days with plate-bound CD3 (1 μg/ml) and CD28 (10
μg/ml) mAbs (both from Invitrogen Life Technologies, Carlsbad, CA,
USA) in vitro. Data were collected using a FACSAria
analytical instrument, and analysis was performed by FlowJo
software (v7.6).
Cell co-culture
Sorted Tfh populations (2.5×105
cells/well) were co-cultured with B cells (5×104
cells/well each for 5 days for Ig measurements) in DMEM
supplemented with 10% FBS in the presence of EBV and CpG 2006 ODN
in 24-well plates (Corning Inc., Corning, NY, USA) precoated with
CD3/CD28 mAbs. The concentrations of total IgG and its subclasses
(IgG1, IgG2, IgG3 and IgG4) were determined by ELISA (Uscn Life
Science, Wuhan, China) as described previously (17,18).
Analysis of mRNA levels by RT-PCR
Total RNA was extracted from Tfh (2.5×105
cells) populations co-cultured with B cells (5×104
cells/well) before or after IL-4, IL-6, IL-10 and PGE2 treatment,
using TRIzol (Invitrogen) according to the manufacturer’s
instructions. RNA pellets were stored in sterile ribonuclease-free
water. Reverse transcription was carried out using 1 μg total RNA,
0.5 μg oligo(dT) and Superscript II enzyme (Invitrogen). The
gene-specific primers for RT-PCR were listed as follows: CXCR3
forward, 5′-ACACCTTCCTG CTCCACCTA-3′ and reverse,
5′-GTTCAGGTAGCGGTCAA AGC-3′; CCR6 forward, 5′-ACAAAGCCATCCGTGTA
ATC-3′ and reverse, 5′-TTCTGAACTTCTGCCCAATAA-3′; GAPDH forward,
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse,
5′-TTGATTTTGGAGGGATCTCG-3′.
Western blot analysis
Total proteins were extracted from Tfh populations
using lysis buffer (Beyourtime, Wuhan, China) supplemented with 1%
protease inhibitor mixture (Sigma-Aldrich, St. Louis, MO, USA).
Equal amounts of protein per sample were separated by 10% SDS-PAGE
gel electrophoresis and transferred to PVDF membranes (Invitrogen).
Membranes were incubated with Abs against CXCR3 and CCR6 (Abcam,
Cambridge, MA, USA) followed by an animal-matched horseradish
peroxidase-conjugated secondary antibody respectively (Santa Cruz
Biotechnology, Santa Cruz, CA, USA). The densitometry score was
determined with Quantity One software (V4.6).
Statistical analysis
All data are expressed as mean ± SD, and the
Student’s t-test was performed to analyze the results of the gene
expression profiling assays. P-value <0.05 was considered to
indicate a statistically significant result.
Results
Distribution of IgG secreted by B cells
is switched toward the IgG4 subclass in the process of prostate
cancer
It has been found that the level of IgG4 antibodies
is obviously higher in melanoma lesions and is closely correlated
with tumor severity (27,28). However, the distribution of IgG
subclasses in prostate cancer is still unknown. To examine the
proportions of IgG subclasses produced in prostate cancer, B cells
from patient peripheral blood were cultured with CpG 2006 ODN and
EBV for 5-day stimulation, and then IgG1, IgG2, IgG3, IgG4 and
nonspecific IgG titers in the supernatants were measured by ELISA.
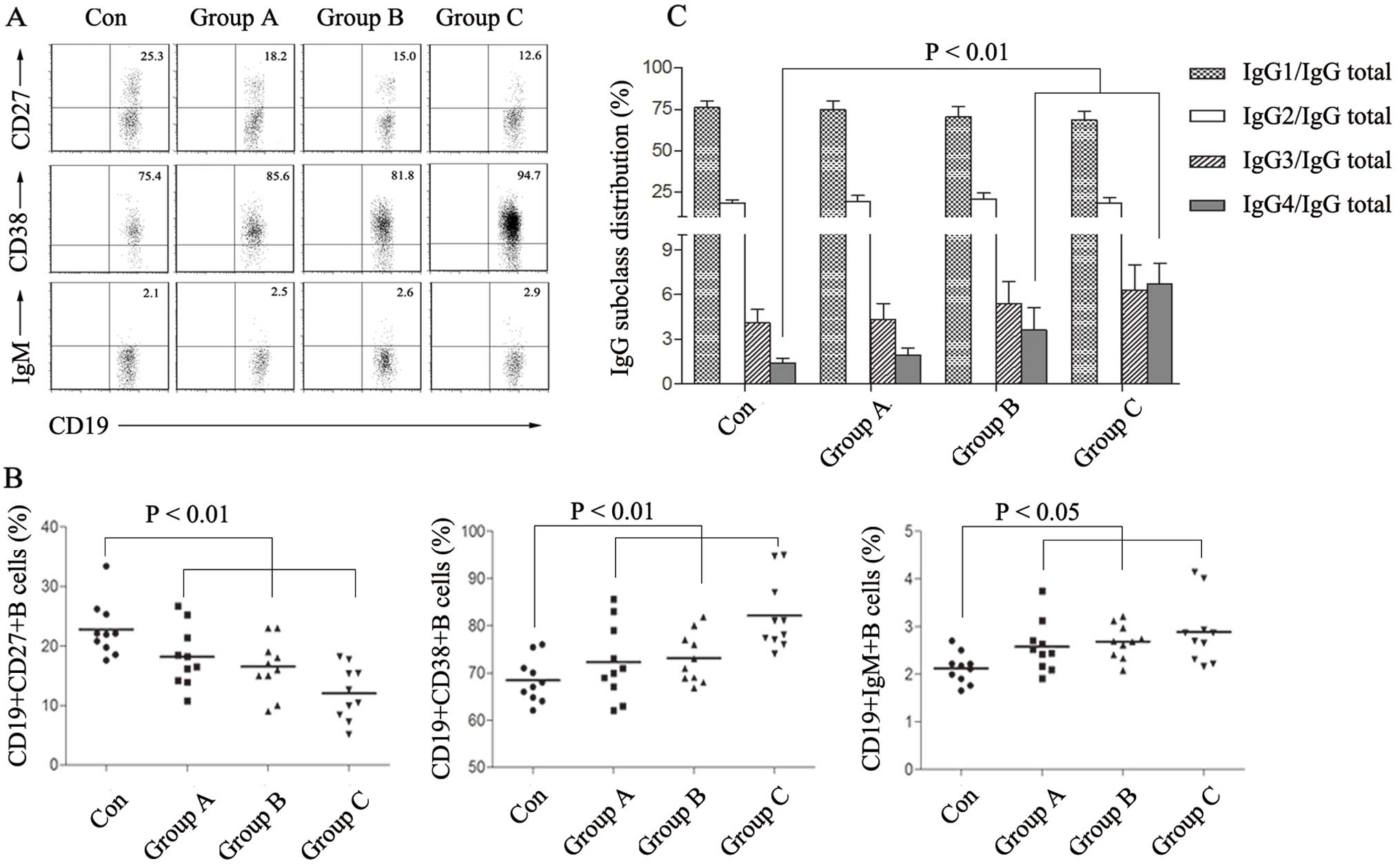
We found a significantly increased frequency of mature
CD19+CD38+ B cells and a decreased level of
memory CD19+CD27+ B cells in the prostate
cancer groups compared with these frequencies in the healthy
controls, while the CD19+IgM+ naïve B cells
were not obviously changed (Fig. 1A and
B). As assessed using ELISA analysis, each IgG subset/IgG total
ratio was IgG1: 76.2±4.1%; IgG2: 18.3±2.1%; IgG3: 4.1±0.9%; IgG4:
1.4±0.3% in the healthy controls; IgG1: 74.7±5.5%; IgG2: 19.1±3.9%;
IgG3: 4.3±1.1%; IgG4: 1.9±0.5% in group A; IgG1: 70.5±6.0%; IgG2:
20.5±3.7%; IgG3: 5.4±1.5%; IgG4: 3.6±1.5% in group B and IgG1:
68.6±5.5%; IgG2: 18.4±3.4%; IgG3: 6.3±1.7%; IgG4: 6.7±1.4% in group
C respectively (Fig. 1C). The
results showed an obvious increase in mature B cells in peripheral
circulating blood and the majority of them mainly tended to secret
IgG4 antibodies. The high level of IgG4+ B cells may
participate in tumor invasion and metastasis.
Percentage of circulating Tfh2 are
upregulated in patients with prostate cancer
Tfh cells are a subset of T cells characterized by
increased expression of molecules including CXCR5, PD-1, ICOS,
CD40L and IL-21, specialized in facilitating B cell growth,
differentiation and class switching. Tfh cells generally localize
in GCs, however they have been proven to exist in human peripheral
blood to regulate the immune system as well (22,29).
Here, we identified CD4+CXCR5+ T cells as Tfh
cells and divided Tfh cells into 3 different subsets:
CXCR3+CCR6− Tfh1 cells,
CXCR3−CCR6− Tfh2 cells and
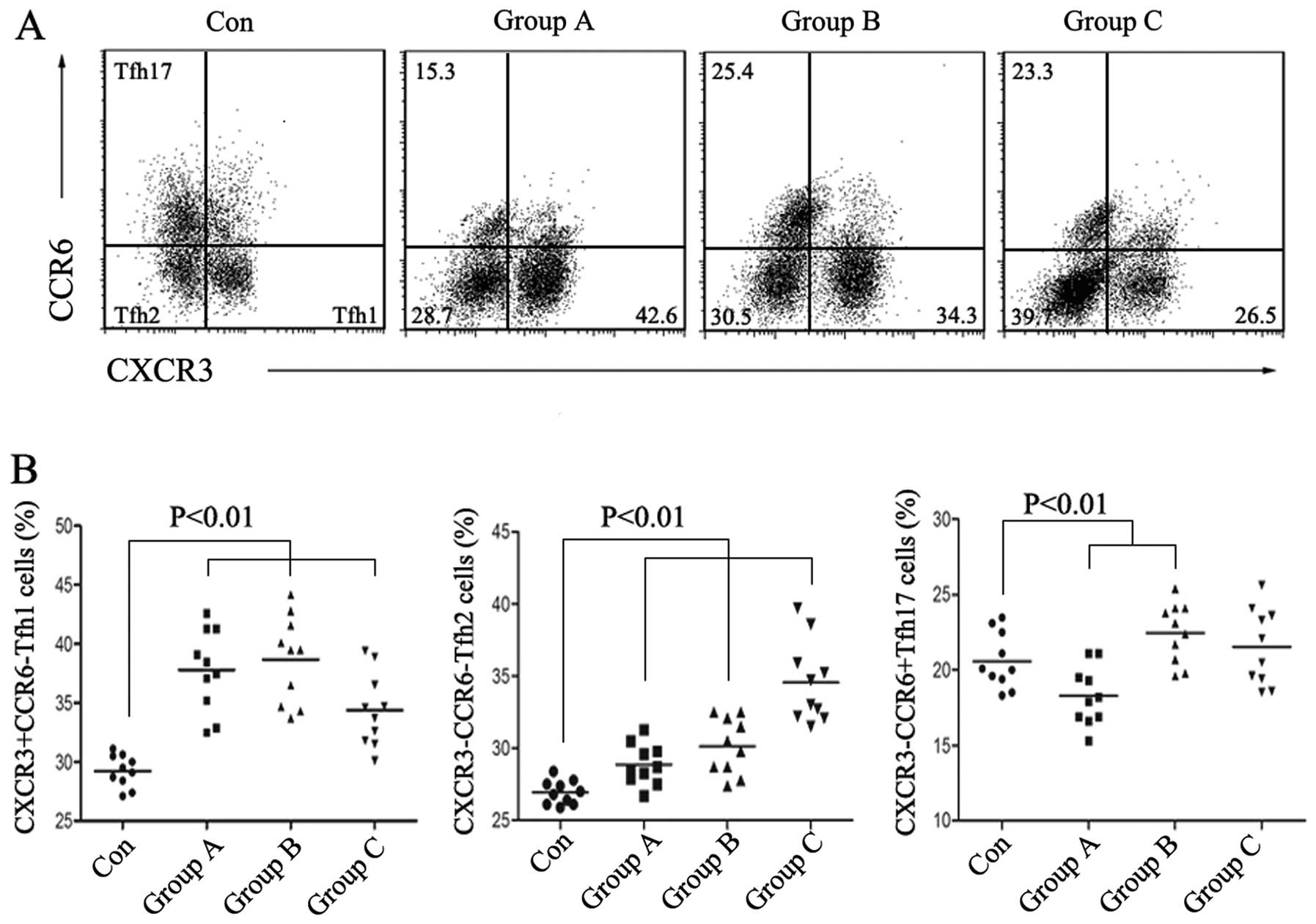
CXCR3−CCR6+ Tfh17 cells and then carried out
analysis by flow cytometry. We observed that the percentage of Tfh2
cells was positively increased along with prostate cancer
progression, while that of Tfh17 changed irregularly and Tfh1
showed a sharply decrease in group C (Fig. 2A and B). The results imply that Tfh2
cells participate in the development of prostate cancer.
Tfh2 promotes the production of
IgG4+ B cells
Considerating the effect of Tfh cells on promoting B
cell maturation, we next investigated the different functions of
the 3 Tfh subtypes. We co-cultured peripheral blood B cells from
healthy volunteers with correspondingly purified Tfh1, Tfh2 and
Tfh17 cells on 24-well plates precoated with CD3 and CD28 mAbs. In
contrast, B cells cultured with no stimulus were set to be the
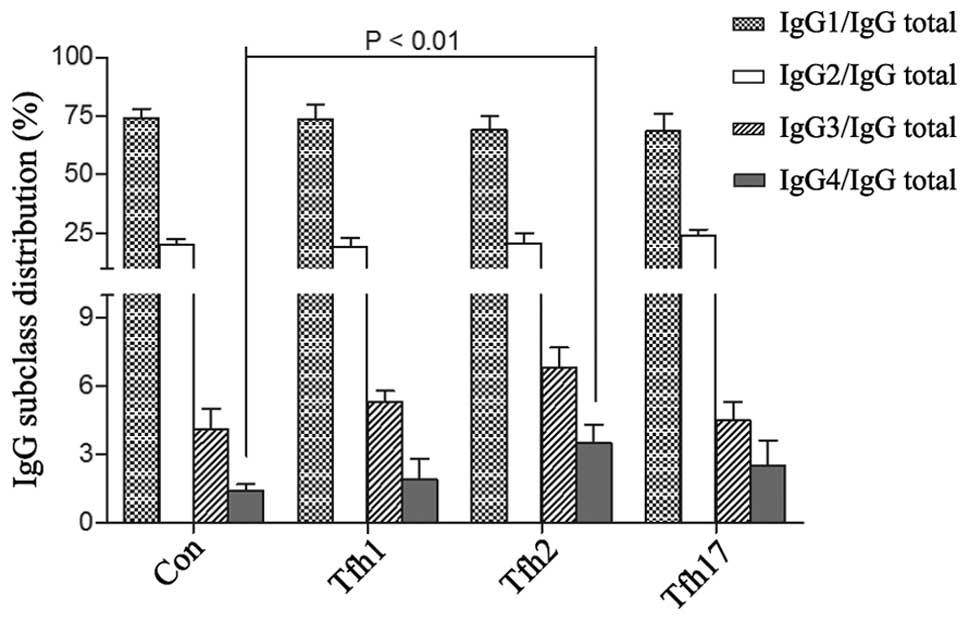
negative control. After stimulating B cells with CpG 2006 ODN and
EBV for 5 days, we detected an increased IgG4/IgG total ratio in
blood B cells cultured with Tfh2 (IgG1: 69.2±6.0%; IgG2: 20.5±4.7%;
IgG3: 6.8±0.9%; IgG4: 3.5±0.8%), while the IgG4/IgG total ratio
remained low when B cells were cultured with Tfh1 (IgG1: 73.7±6.5%;
IgG2: 19.1±3.9%; IgG3: 5.3±0.5%; IgG4: 1.9±0.9%) and Tfh17 (IgG1:
68.6±7.5%; IgG2: 24.0±2.8%; IgG3: 4.5±0.8%; IgG4: 2.5±1.1%)
(Fig. 3). Thus in Tfh cells, Tfh2
may act as a regulator to improve polarized expression of IgG4
secreted by B cells.
Inflammatory cytokines significantly
improve B cell polarization, inducing IgG4 secretion
Prostate cancer easily develops to become immune
tolerant, which is related to the cancer microenvironment
characterized by high levels of cytokines such as IL-4, IL-6,
IL-10, nitric oxide (NO), TGF-β and PGE2. Taking into account the
upregulated IgG4+ B cells in patients with malignant
prostate cancer, we explored the above soluble factors that may
contribute to the polarization of B cells for producing IgG4
antibodies and investigated whether the effect of these cytokines
was mediated by Tfh2. We cultured B cells with
CD4+CXCR5+ Tfh cells from volunteers in DMEM
supplemented with CpG 2006 ODN and EBV on a 24-well plate procoated
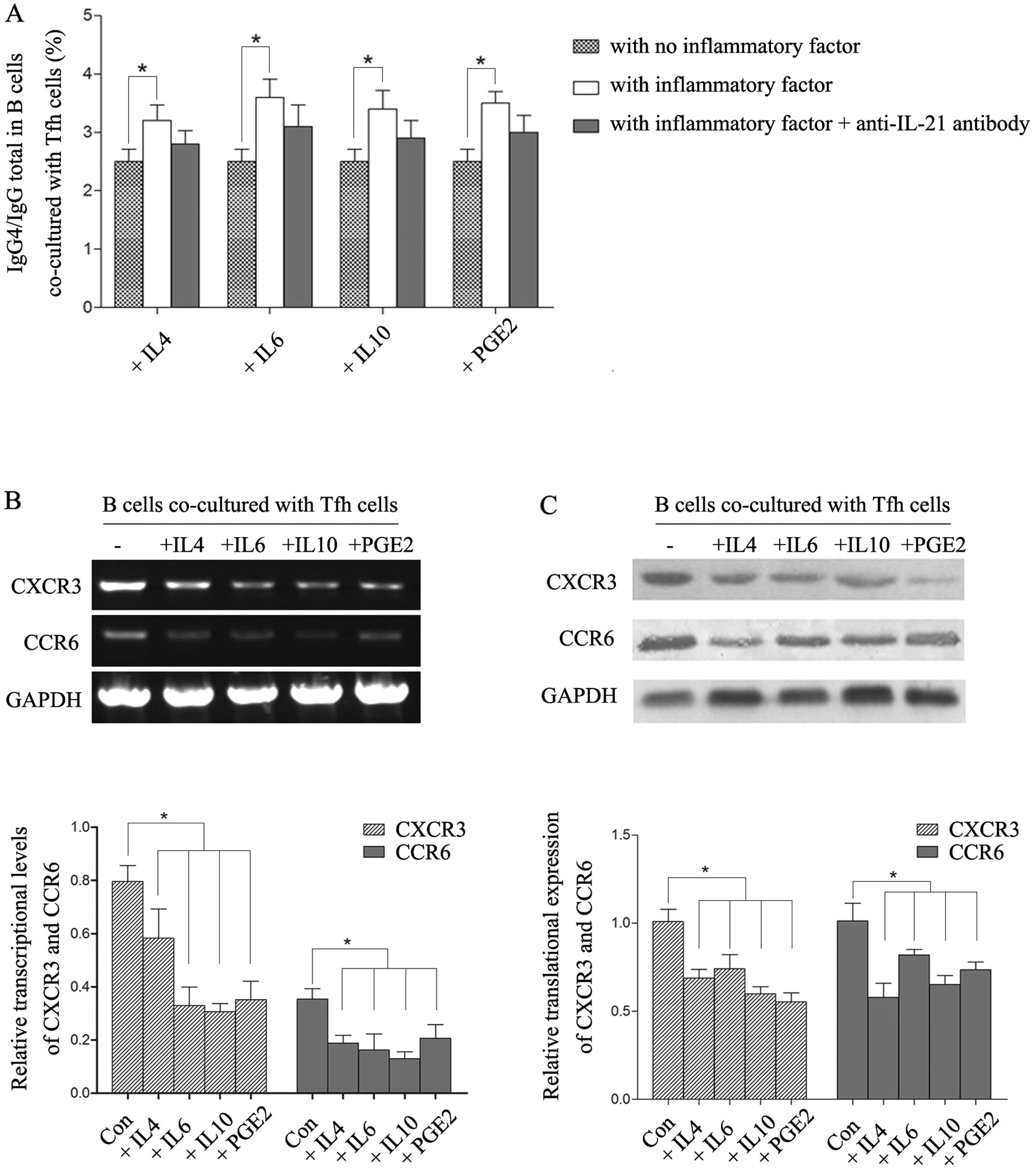
with CD3/CD28 mAbs, and then added recombinant human IL-4, IL-6,
IL-10 and PGE2 respectively into the medium. The ratio of IgG4/IgG
total was obviously increased in all IL-4, IL-6, IL-10 and PGE2
treatment groups compared with the ratio in the
non-cytokine-treated control (P<0.01). Although the IgG4 ratio
was decreased after adding the anti-IL-21 antibody which could
inhibit Tfh cell functions, the change was not statistically
significant (Fig. 4A).
Subsequently, we collected Tfh cells of each group and found that
the expressions levels of CXCR3 and CCR6 were both significantly
downregulated at the transcriptional level and translational level
in the cytokine-treated groups (Fig. 4B
and C), which indicated the polarization of the Tfh2 subset.
Thus, IL4, IL6, IL-10 and PGE2 facilitated B cells to produce the
IgG4 subclass by downregulating CXCR3 and CCR6 to induce Tfh2
cells. Yet, there may exist other regulatory pathways of these
inflammatory suppressors to increase the proportion of
IgG4+ B cells which is not completely dependent on Tfh
cells.
Discussion
Studies have focused on B cell development, and the
multiple functions of B cells were explored including modulation of
humoral immunity, activation of T lymphocytes by antigen
presentation and regulation of the tissue immune microenvironment
by crosstalk with other soluble cytokines. The main mediators of B
cells are different immune globulin subtypes (IgA, IgG, IgM and
IgE) and IgG was proven to be essential in the regulation of
tumorigenesis, invasion and metastasis (30,31).
The proportion of the IgG subclass (IgG1, IgG2, IgG3 and IgG4) is
65, 25, 6 and 4% of the total IgG, respectively, in healthy adult
serum, but these proportions were found to be altered in certain
diseases such as various types of cancer (14,32).
In the present study, we observed an abnormal
distribution of 4 IgG subclasses existing in patients with prostate
cancer as well. The ratio of IgG4/IgG total was positively
increased in the patient groups (6.7±1.4% in clinical stage ≥T3
group, 3.6±1.5% in stage T2 group, 1.9±0.5% in stage ≤T2 group and
1.4±0.3% in healthy controls, respectively). In contrast, the
IgG1/IgG total ratio was gradually decreased with the progression
of cancer. The result indicated a quite abnormal polarization
toward IgG4 in patients with aggressive prostate cancer, implying a
potential interaction between IgG4+ B cells and features
involved in tumor progression.
Considerating the development of B cells, we further
detected Tfh cells, which were considerate as the main helpers to
promote B cell proliferation and maturation. Since the percentage
of Tfh2 cells increased positively in the progression of prostate
cancer, we concluded there existed a polarization of Tfh cells for
producing the Tfh2 subset. To illustrate the different effects of
Tfh subsets, we co-cultured B cells with Tfh1, Tfh2 and Tfh17 cells
respectively, and tested the IgG distribution. As we predicted, the
IgG4/IgG total ratio was obvious higher in the Tfh2 co-cultured
group than that in the Tfh1 or Tfh17 co-cultured group. Thus in Tfh
cells, Tfh2 may act as a key regulator to improve B cell
polarization, resulting in antibody class switching to IgG4.
The immune tolerant environment in the interior of
malignant tumors has been recognized as a key characteristic which
promotes tumor proliferation or invasion and what is more important
facilitates the escape from immune surveillance (33,34).
Various immune cell types have been shown to be related to immune
tolerance in different diseases or experiments, including
regulatory T cells (Tregs), myeloid-derived suppressor cells
(MDSCs), tumor-associated macrophages (TAMs), nature killer T (NKT)
cells and regulatory dendritic cells (DCs). These immune response
inhibitors act mainly by suppressing Th1 and Th2 responses through
IFN-γ dependent NO or PGE2 production, secretion of IL-4/6/10,
inducing Treg cells and mutually interacting with each other
(11,35–38).
Taking into account the upregulated IgG4+ B cells and
Tfh2 cells in patients with aggressive prostate cancer, we
hypothesized that these inflammatory suppressors could improve the
alteration of the subtypes in B or Tfh cells during cancer
development. By co-culturing B cells with Tfh cells in
vitro, IL-4, IL-6, IL-10 and PGE2 were added into the medium,
respectively. We observed an obviously increase in the level of the
ratio IgG4/IgG total in each IL-4/IL-6/IL-10 and PGE2 treated
group, which confirmed the ability of these anti-inflammatory
cytokines to induce the concentration of IgG4 antibodies. However,
inhibition of Tfh cell functions using the anti-IL-21 antibody was
not effective in preventing the upregulation of IgG4 stimulated by
IL-4, IL-6, IL-10 and PGE2. Meanwhile, we found that the expression
levels of CXCR3 and CCR6 were decreased after IL-4/6/10/PGE2
treatment, which implied the polarization of the Tfh2 subset. Thus,
the above inflammatory suppressors may contribute to the
polarization of B cells for producing IgG4 antibodies by
downregulating CXCR3 and CCR6 to promote the Tfh2 cell proportion.
Yet, there still may exist other regulatory pathways of these
cytokines causing an increase in the proportion of IgG4+
B cells, which is not completely dependent on Tfh cells.
In conclusion, these data provide additional
evidence of the B cell response to the immunesuppressiven
environment by evaluating IgG4 antibodies and established the
relationship between IgG4+ B cells and Tfh2 cells.
Although alterations in the specific T/B subtypes were confirmed to
be strongly associated with tumor proliferation and malignancy, the
definite mechanisms such as immunoregulatory signaling pathways
remain obscure. Clarification of the lymphocyte functions in the
inflammatory microenvironment of tumors may be of potential
therapeutic value.
Acknowledgements
We are grateful to Dr Zhang and Professor Wang for
the clinical sample collection and clinical information
support.
Abbreviations:
|
DCs
|
regulatory dentritic cells
|
|
DRE
|
digital rectal examination
|
|
GCs
|
germinal centers
|
|
IgG
|
immunoglobulin G
|
|
MDSCs
|
myeloid-derived suppressor cells
|
|
NKT cells
|
nature killer T cells
|
|
NO
|
nitric oxide
|
|
PGE2
|
prostaglandin E2
|
|
PSA
|
prostate-specific antigen
|
|
TAMs
|
tumor-associated macrophages
|
|
Tfh cells
|
T follicular helper cells
|
|
Tregs
|
regulatory T cells
|
References
|
1
|
Jemal A, Thomas A, Murray T and Thun M:
Cancer statistics, 2002. CA Cancer J Clin. 52:23–47. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heinlein CA and Chang C: Androgen receptor
in prostate cancer. Endocr Rev. 25:276–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Marzo AM, Platz EA, Sutcliffe S, Xu J,
Grönberg H, Drake CG, Nakai Y, Isaacs WB and Nelson WG:
Inflammation in prostate carcinogenesis. Nat Rev Cancer. 7:256–269.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sfanos KS and De Marzo AM: Prostate cancer
and inflammation: the evidence. Histopathology. 60:199–215. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sethi G, Shanmugam MK, Ramachandran L,
Kumar AP and Tergaonkar V: Multifaceted link between cancer and
inflammation. Biosci Rep. 32:1–15. 2012. View Article : Google Scholar
|
|
7
|
Salman H, Ori Y, Bergman M, Djaldetti M
and Bessler H: Human prostate cancer cells induce inflammatory
cytokine secretion by peripheral blood mononuclear cells. Biomed
Pharmacother. 66:330–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sciarra A, Mariotti G, Salciccia S, Autran
Gomez A, Monti S, Toscano V and Di Silverio F: Prostate growth and
inflammation. J Steroid Biochem Mol Biol. 108:254–260. 2008.
View Article : Google Scholar
|
|
10
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sha W, Olesch C, Hanaka H, Rådmark O,
Weigert A and Brüne B: Necrosis in DU145 prostate cancer spheroids
induces COX-2/mPGES-1-derived PGE2 to promote tumor growth and to
inhibit T cell activation. Int J Cancer. 133:1578–1588. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Donkor MK, Sarkar A, Savage PA, Franklin
RA, Johnson LK, Jungbluth AA, Allison JP and Li MO: T cell
surveillance of oncogene-induced prostate cancer is impeded by T
cell-derived TGF-β1 cytokine. Immunity. 35:123–134. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jefferis R: Isotype and glycoform
selection for antibody therapeutics. Arch Biochem Biophys.
526:159–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Papadea C and Check IJ: Human
immunoglobulin G and immunoglobulin G subclasses: biochemical,
genetic and clinical aspects. Crit Rev Clin Lab Sci. 27:27–58.
1989. View Article : Google Scholar
|
|
15
|
Harada K, Shimoda S, Kimura Y, Sato Y,
Ikeda H, Igarashi S, Ren XS, Sato H and Nakanuma Y: Significance of
(IgG4)-positive cells in extrahepatic cholangiocarcinoma: molecular
mechanism of IgG4 reaction in cancer tissue. Hepatology.
56:157–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cipponi A, Mercier M, Seremet T, Baurain
JF, Théate I, van den Oord J, Stas M, Boon T, Coulie PG and van
Baren N: Neogenesis of lymphoid structures and antibody responses
occur in human melanoma metastases. Cancer Res. 72:3997–4007. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karagiannis P, Gilbert AE, Josephs DH, Ali
N, Dodev T, Saul L, Correa I, Roberts L, et al: IgG4 subclass
antibodies impair antitumor immunity in melanoma. J Clin Invest.
123:1457–1474. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gilbert AE, Karagiannis P, Dodev T, Koers
A, Lacy K, Josephs DH, Takhar P, et al: Monitoring the systemic
human memory B cell compartment of melanoma patients for anti-tumor
IgG antibodies. PLoS One. 6:e193302011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Platts-Mills TA, Woodfolk JA, Erwin EA and
Aalberse R: Mechanisms of tolerance to inhalant allergens: the
relevance of a modified Th2 response to aller gens from domestic
animals. Springer Semin Immunopathol. 25:271–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Satoguina JS, Weyand E, Larbi J and
Hoerauf A: T regulatory-1 cells induce IgG4 production by B cells:
role of IL-10. J Immunol. 174:4718–4726. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Crotty S: Follicular helper CD4 T cells
(TFH). Annu Rev Immunol. 29:621–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Breitfeld D, Ohl L, Kremmer E, Ellwart J,
Sallusto F, Lipp M and Förster R: Follicular B helper T cells
express CXC chemokine receptor 5, localize to B cell follicles and
support immunoglobulin production. J Exp Med. 192:1545–1552. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McHeyzer-Williams LJ, Pelletier N, Mark L,
Fazilleau N and McHeyzer-Williams MG: Follicular helper T cells as
cognate regulators of B cell immunity. Curr Opin Immunol.
21:266–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davidson A and Aranow C: Lupus nephritis:
lessons from murine models. Nat Rev Rheumatol. 6:13–20. 2010.
View Article : Google Scholar
|
|
25
|
Morita R, Schmitt N, Bentebibel SE,
Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, et al:
Human blood CXCR5(+) CD4(+) T cells are counterparts of T
follicular cells and contain specific subsets that differentially
support antibody secretion. Immunity. 34:108–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Traggiai E, Becker S, Subbarao K,
Kolesnikova L, Uematsu Y, Gismondo MR, Murphy BR, Rappuoli R and
Lanzavecchia A: An efficient method to make human monoclonal
antibodies from memory B cells: potent neutralization of SARS
coronavirus. Nat Med. 10:871–875. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Neild GH, Rodriguez-Justo M, Wall C and
Connolly JO: Hyper-IgG4 disease: report and characterization of a
new disease. BMC Med. 4:232006. View Article : Google Scholar
|
|
28
|
Stone JH, Zen Y and Deshpande V:
IgG4-related disease. N Engl J Med. 366:539–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bentebibel SE, Schmitt N, Banchereau J and
Ueno H: Human tonsil B-cell lymphoma 6 (BCL6)-expressing
CD4+ T-cell subset specialized for B-cell help outside
germinal centers. Proc Natl Acad Sci USA. 108:E488–E497. 2011.
View Article : Google Scholar
|
|
30
|
Aalberse RC and Schuurman J: IgG4 breaking
the rules. Immunology. 105:9–19. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aalberse RC, Stapel SO, Schuurman J and
Rispens T: Immunoglobulin G4: an odd antibody. Clin Exp Allergy.
39:469–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
French M: Serum IgG subclasses in normal
adults. Monogr Allergy. 19:100–107. 1986.PubMed/NCBI
|
|
33
|
Andreu P, Johansson M, Affara NI, Pucci F,
Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, Naldini
L, de Visser KE, De Palma M and Coussens LM: FcRgamma activation
regulates inflammation-associated squamous carcinogenesis. Cancer
Cell. 17:121–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
DiLillo DJ, Yanaba K and Tedder TF: B
cells are required for optimal CD4+ and CD8+
T cell tumor immunity: therapeutic B cell depletion enhances B16
melanoma growth in mice. J Immunol. 184:4006–4016. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao E, Wang L, Dai J, Kryczek I, Wei S,
Vatan L, Altuwaijri S, Sparwasser T, Wang G, Keller ET and Zou W:
Regulatory T cells in the bone marrow microenvironment in patients
with prostate cancer. Oncoimmunology. 1:152–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
English K, Tonlorenzi R, Cossu G and Wood
KJ: Mesoangioblasts suppress T Cell proliferation through IDO and
PGE-2-dependent pathways. Stem Cells Dev. 22:512–523. 2013.
View Article : Google Scholar :
|
|
37
|
Chen YL, Chang MC, Chen CA, Lin HW, Cheng
WF and Chien CL: Depletion of regulatory T lymphocytes reverses the
imbalance between pro- and anti-tumor immunities via enhancing
antigen-specific T cell immune responses. PLoS One. 7:e471902012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miyara M and Sakaguchi S: Natural
regulatory T cells: mechanisms of suppression. Trends Mol Med.
13:108–116. 2007. View Article : Google Scholar : PubMed/NCBI
|