Introduction
Bone cancer pain resulting from primary tumors or
tumors that metastasize to bones is one of the most severe and
intractable types of cancer pain, which reduces the quality of life
of patients (1,2). Bone cancer pain generally consists of
ongoing pain and breakthrough or incident pain (3–5), which
is characterized by pathological symptoms, such as spontaneous
pain, hyperalgesia and allodynia (6,7). The
mechanism underlying the development of bone cancer pain still
remains largely unknown.
Recently, it has been observed that the thermal
hyperalgesia and mechanical hypersensitivity in murine models of
bone cancer pain are associated with increased excitability of
primary afferent neurons that innervate the tumor-bearing limb
(8–10), and reduction of KCNQ/M (Kv7)
potassium channels in dorsal root ganglion (DRG) neurons leads to
the hyperexcitability of primary sensory neurons, which
subsequently causes sensitization of nociceptors and tumor-induced
hyperalgesia under cancer conditions (11), implying that suppression of KCNQ/M
(Kv7) channels in primary DRG neurons plays a crucial role in the
development of peripheral sensitization and cancer-induced bone
pain.
The KCNQ/M (Kv7) channels are a family of five
voltage-gated potassium (K+) channel subunits (Kv7.1 to
Kv7.5) encoded by the KCNQ1–5 genes (12); among those, KCNQ2 and KCNQ3 are
expressed exclusively in the nervous system (13,14)
and co-assembled KCNQ2 and KCNQ3 subunits are thought to constitute
the native M channel in most neurons (15). This channel generates a species of
low-threshold, slowly activating, slowly deactivating and
non-inactivating K+ current (the M current), which acts
as a ‘brake’ to regulate the action potential (AP) firing and the
neuronal excitability (16).
Consequently, suppression of M currents increases the neuronal
excitability (16–18), whereas their enhancement has a
silencing effect (19–22). In support of this notion, acute
inhibition of KCNQ/M channels in primary sensory DRG neurons causes
depolarization of the neuronal resting membrane potential and
increased excitability and ultimately produces inflammatory
nociception in rats (23,24). Transcriptional repression of the M
channel subunit Kv7.2 in DRG neurons contributes to the neuropathic
overexcitability of peripheral sensory fibers and the maintenance
of neuropathic pain in rats suffering from chronic nerve injury
(25).
Apart from peripheral sensitization, central
sensitization is also responsible for the generation of pain
hypersensitivity and the maintenance of persistent pain (26,27).
In fact, sensitization of superficial dorsal horn neurons (28) and dorsal horn wide dynamic range
(WDR) neurons in the spinal cord to mechanical, heat and cold
stimuli have been observed in murine animals with tumor-evoked
hyperalgesia (29,30). On the other hand, functional KCNQ/M
channels have been detected in the dorsal horn of the spinal cord
(31–35) and the central terminals of primary
afferents (36,37). In an in vitro study using
hemisected spinal cord from immature rats, Rivera-Arconada and
Lopez-Garcia (35) reported that
blockade of KCNQ/M channels with XE-991 induced depolarization and
enhanced excitability of dorsal horn neurons, whereas activation of
KCNQ/M channels with retigabine produced hyperpolarization and a
large decrease in the excitability of these neurons. In the present
study, we investigated whether inhibition of KCNQ/M channels in the
spinal cord contributes to the development of bone cancer pain via
sensitization of the dorsal horn WDR neurons. The results indicate
that blockade of KCNQ/M channels in the spinal cord produces
hyperexcitability of dorsal horn WDR neurons and pain
hypersensitivity in normal rats, whereas activation of these
channels inhibits the sensitization of dorsal horn WDR neurons,
while alleviating mechanical allodynia and thermal hyperalgesia in
bone cancer rats. Thus, suppression of KCNQ/M channels in the
spinal cord leads to the sensitization of dorsal horn WDR neurons,
thereby playing an important role in the development of
cancer-induced bone pain.
Materials and methods
Animals
Adult female Sprague-Dawley rats weighing 180 to 220
g at the beginning of the experiment were provided by the
Department of Experimental Animal Sciences, Peking University
Health Science Center. The rats were housed in separated cages with
free access to food and water. The room temperature was kept at
24±1°C under a natural light-dark cycle. All animal experimental
procedures were conducted in compliance with the guidelines of the
International Association for the Study of Pain (38) and were approved by the Animal Care
and Use Committee of Peking University.
Inoculation of tumor cells
A rat model of bone cancer pain was established by
intratibial injections of syngeneic MRMT-1 rat mammary gland
carcinoma cells as previously described (39). Briefly, after the rat was
anesthetized with chloral hydrate (0.3 g/kg, intraperitoneally,
i.p.), the left tibia was carefully exposed and a 23-gauge needle
was inserted into the intra-medullary canal of the bone. It was
then removed and replaced with a long, thin blunt needle attached
to a 10 μl Hamilton syringe containing the medium to be injected. A
volume of 4 μl MRMT-1 rat mammary gland tumor cells
(4×104) or vehicle phosphorylated buffer solution (PBS)
was injected into the tibial bone cavity. After injection, the site
was sealed with bone wax and the wound was closed. None of the
animals showed signs of motor dysfunction after inoculation of the
tumor cells.
Assessment of mechanical allodynia
Mechanical allodynia, as a behavioral sign of bone
cancer pain, was assessed by measuring 50% paw withdrawal threshold
(PWT) as described in our previous studies (11,40).
The 50% PWT in response to a series of von Frey filaments
(Stoelting, Wood Dale, IL, USA) was determined by the Up and Down
method (41). The rat was placed on
a metal mesh floor covered with an inverted clear plastic cage
(18×8×8 cm) and allowed a 20-min period for habituation. Eight von
Frey filaments with approximately equal logarithmic incremental
(0.224) bending forces were chosen (0.41, 0.70, 1.20, 2.00, 3.63,
5.50, 8.50 and 15.10 g). Each trial started with a von Frey force
of 2.00 g delivered perpendicularly to the plantar surface of the
left hind paw for 2 to 3 sec. An abrupt withdrawal of the foot
during stimulation or immediately after the removal of the hair was
recorded as a positive response. Whenever there was a positive or
negative response, the next weaker or stronger filament was
applied, respectively. This procedure was carried out until 6
stimuli after the first change in response had been observed. The
50% PWT was calculated using the following formula: 50% PWT =
10[Xf + kδ], where Xf is the value
of the final von Frey filament used (in log units), k is a value
measured from the pattern of positive/negative responses, and δ =
0.224, which is the average interval (in log units) between the von
Frey filaments (42). If an animal
responded to the lowest von Frey filament, a value of 0.25 g was
assigned. If an animal did not respond to the highest von Frey
filament, the value was recorded as 15.0 g. In rats, mechanical
allodynia is assessed by measuring the 50% PWT to von Frey
filaments and an allodynic rat is defined as displaying a 50% PWT
<4.0 g (i.e., withdrawal in response to non-noxious tactile
stimulus) (43).
Assessment of thermal hyperalgesia
Thermal hyperalgesia of the hind paws was tested as
described in our previous study (11). Rats were allowed to acclimate for a
minimum of 30 min within acrylic enclosures on a clear glass plate
maintained at 30°C. A radiant heat source was focused onto the
plantar surface of the hind paw. Measurements of paw withdrawal
latency (PWL) were taken by a timer that was started by the
activation of the heat source and stopped when withdrawal of the
paw was detected with a photodetector. A maximal cutoff time of 30
sec was used to prevent unnecessary tissue damage. Three
measurements of PWL were taken for each hind paw and were averaged
as the result of each test session. The hind paw was tested
alternately with >5 min intervals between consecutive tests.
Implantation of intrathecal catheter
Under chloral hydrate (0.3 g/kg, i.p.) anesthesia,
implantation of an intrathecal cannula was performed as described
in our previous study (44).
Briefly, a PE-10 polyethylene catheter was implanted between lumbar
(L5) and lumbar (L6) vertebrae to reach the lumbar enlargement of
the spinal cord. The outer part of the catheter was plugged and
fixed onto the skin on closure of the wound. All surgical
procedures were performed under sterile conditions. Rats showing
neurological deficits after the catheter implantation were
euthanized. Drugs or vehicle were intrathecally injected via the
implanted catheter in a 20-μl volume of solution followed by 10 μl
of normal saline (NS) for flushing. Each injection lasted at least
5 min. After the injection, the needle remained in situ for
2 min before being withdrawn.
Measurement of drug effects on pain
behaviors
The first behavioral experiment was performed to
examine whether inhibition of KCNQ/M channels in the spinal cord
with a potent blocker XE-991 would produce pain hypersensitivity in
normal rats. XE-991 (at 0.9 μg/μl) was intrathecally administered
to the animal on day 7 after intrathecal cannula implantation and
the effects of XE-991 on ipsilateral PWT and PWL of the rats were
measured at 0.5, 1.5, 2.5, and 3.5 h after drug injection,
respectively. The second experiment was carried out to determine
whether activation of spinal KCNQ/M channels by using its specific
opener retigabine (RTG) would attenuate the hyperalgesic behaviors
in a rat model of bone cancer pain, and whether the effects of
retigabine could be reversed by the KCNQ/M-channel blocker XE-991.
On day 14 after tumor cell inoculation, rats exhibiting bone cancer
pain, confirmed by measuring the mechanical allodynia and thermal
hyperalgesia, were intrathecally administered with RTG, RTG plus
XE-991, or vehicle DMSO. Here, RTG was used at a concentration of
8.5 μg/μl and XE-991 at 0.9 μg/μl, and the effects of RTG on
ipsilateral PWT and PWL of rat were measured at 0.5, 1.5, 2.5 and
3.5 h after drug injection, respectively. To examine the long-term
effects of intrathecal RTG on bone cancer-induced pain
hypersensitivity in the MRMT-1-inoculated rats, RTG (at 8.5 μg/μl)
was intrathecally administered to the rats twice per day at a
30-min interval, lasted for 3 days, and the effect of RTG on both
PWT and PWL was tested on day 4 after drug injection.
Assessment of locomotor function
Inclined-plate test was used for the assessment of
locomotor function. Rat was placed crosswise to the long axis of an
inclined plate. The initial angle of the inclined plate was 50
degrees. The angle was then adjusted in 5-degree increments. The
maximum angle of the plate on which the rat maintained its body
position for 5 sec without falling was determined according to the
method reported by Rivlin and Tator (45). In this study, inclined-plate test
was performed for all behavioral experiments in which drugs were
intrathecally administrated to rats.
Electrophysiological studies
Surgery
Each rat was initially anesthetized with urethane
(1.2–1.5 g/kg, i.p.). The trachea was cannulated to allow
mechanical ventilation with room air. A catheter was inserted into
the right jugular vein for continuous infusion of Tyrode’s solution
[in mmol/l: NaCl 137, KCl 2.7, CaCl2 1.4,
MgCl2 1.0, NaHCO3 6.0,
NaH2PO4 2.1, D-(+)-glucose 6.5] with a pH of
7.4 at 1.0–1.5 ml/h. The rectal temperature was maintained at
36.5–37.5°C via a feedback controlled under a body heating pad. A
pair of bipolar silver hook electrodes was placed under sciatic
nerve immediately proximal to the trifurcation for the electrical
stimulation. The vertebral column was rigidly fixed in the frame
with two clamps. The lumbar enlargement of the spinal cord was
exposed by laminectomy at vertebrae T13 and L1 and the dura
covering the lumbosacral spinal segments was carefully removed. A
small well was built with 3% agar on the dorsal spinal cord at the
recording segment to allow application of drugs or vehicles
(44,46). The exposed spinal tissue was covered
with warm (37°C) saline solution. After surgery, the animal was
artificially ventilated with a small animal ventilator and
continuous anesthesia was maintained with urethane (0.10–0.15
g/kg/h) during the whole experiment. The depth of anesthesia was
monitored by examination of pupillary size and reflexes. The
physiological condition of the animal was monitored by recording
the electrocardiogram (330–460 beats/min), end-expiratory
CO2 (3.5–4.5%), and rectal temperature (36.5–37.5°C) and
was maintained within the range indicated (44).
Extracellular recording
Single-unit extracellular recordings were conducted
from the lumbar dorsal horn neurons within 1,200 μm of the dorsal
surface of the spinal cord with 2–5 MΩ parylene-coated tungsten
microelectrode (Friedrick Haer & Co. Inc., Bowdoinham, ME,
USA). The microelectrode was inserted perpendicularly into the
dorsal horn from a point about midway between the midline and the
medial edge of the dorsal root entry zone. During electrode
advancement, electrical pulses (0.5 Hz, 0.3 msec pulse width, 0.4
mA) were applied to the ipsilateral sciatic nerve as search stimuli
so that a neuron with no spontaneous firing could be identified.
Once a single unit was identified, the receptive field and response
characteristics were determined by a range of mechanical stimuli of
various intensities, including brushing or touching the skin with a
cotton brush, light pressure with a probe and pinching a fold of
skin with toothed forceps. A neuron responding to innocuous tactile
stimuli, light pressure and noxious pinch in a graded manner was
identified as a WDR neuron and was selected for further
investigation (44). The recorded
signals were first amplified with an AC pre-amplifier and then
filtered with a band pass filter at 500–1,000 Hz. The filtered
signals were displayed on an oscilloscope and fed to a Pentium
computer via a CED 1401 interface for off-line analysis using the
Spike2 software (Cambridge Electronic Design, Cambridge, UK).
Spikes appearing 45–300 msec after stimulus were defined as C-fiber
responses, i.e. responses in the WDR neurons evoked by C-fiber
activation (44). Single cell
recording was confirmed on the basis of amplitude and shape of the
action potentials. In the following electrophysiological studies,
only one cell was studied in each animal and each animal received
only one dose of RTG, XE-991 or vehicle.
Measurement of drug effects
The electrophysiological experiments were designed
to investigate the effects of spinal administration of XE-991 and
RTG on the activities of WDR neurons in naive rats and in rats with
bone cancer pain, respectively. In this experiment, a train of 10
stimuli (0.5 Hz, 0.5-msec pulse width, with a pulse current of 2×
C-fiber response threshold), which was used as the test stimulus,
was applied repeatedly to the sciatic nerve at a 5-min interval,
and post-stimulus histograms from the responses of WDR neurons were
generated by the Spike2 software. After six stable control
responses were recorded, XE-991 at the dose of 0.4 mM (33) or RTG at the dose of 5 mM (32) in a 50 μl volume of solution, or the
equal volume of vehicle, was applied topically to the dorsal
surface of the spinal cord. The post-drug responses evoked by the
same test stimulus as described above were measured at 5 min
intervals for up to 60 min. In the present study, only C-fiber
responses of WDR neurons, which are highly related to nociceptive
transmission (44,47) were examined and analyzed. All of the
C-fiber responses were expressed as percentages of the mean
response value of six pre-drug consecutive trains of test
stimuli.
Chemical preparation and
application
Retigabine
(N-(2-amino-4-(4-fluorobenzylamino)-phenyl)carbamic acid ethyl
ester, Melone Phamaceutical Co., Ltd., Dalian, China) and XE-991
(10,10-bis(4-pyridinyl-methyl)-9(10H)-anthracenone,
Tocris, Ellisville, MO, USA) were dissolved in DMSO(dimethyl
sulfoxide; Sigma Co.) as a 0.5 M stock solution, stored at −20°C
and diluted to the desired concentrations with normal saline just
before the experiments. The final concentration of DMSO was
<0.5%. All other chemicals or reagents were obtained from Sigma,
Invitrogen or Abcam except as mentioned in the text.
Statistical analysis
Statistical analyses were performed with GraphPad
Prism 5 for Windows (GraphPad Software, La Jolla, CA, USA). All
data are expressed as mean ± SEM. The two-tailed Student t-test was
used for the comparison of the mean values between the two groups.
One-way analysis of variance (ANOVA) followed by the Dunnett
multiple comparison test or two-way ANOVA followed by Bonferroni
post-hoc test was used for multiple comparisons. Differences with
P<0.05 were considered statistically significant. The area under
the time-course curve (AUC) during the analysis time was used for
assessing the summed effects of XE-991 and retigabine as previously
described (44).
Results
Increase in excitability of dorsal horn
WDR neurons in the spinal cord in bone cancer rats
First, we examined the C-fiber responses of dorsal
horn WDR neurons, which are highly related to nociceptive
transmission (44,47) in rats with cancer-induced bone pain.
We found that the C-fiber responses of WDR neurons were
significantly increased in the MRMT-1 tumor cell inoculated rats
compared to the naive and PBS-treated rats (Fig. 1). As shown in Fig. 1D, the total spike count of the
electrically evoked C-fiber responses in 10 stimuli was
significantly increased in the MRMT-1 rats compared to the naive
and PBS-treated rats (P<0.001, two-way ANOVA). As summarized in
the AUC of C-fiber responses, the AUC (0–55 min of the analysis
time) of the C-fiber responses in the MRMT-1-inoculated rats was
also increased prominently (11,700±590.7) compared to the naive
(3,343±463.7) and the PBS-treated rats (3,991±318.2) (P<0.001,
one-way ANOVA; Fig. 1E).
Representative examples illustrating the C-fiber responses of
dorsal horn WDR neurons in the naive, PBS- and MRMT-1-inoculated
rats are shown in Fig. 2A–C
respectively. These results suggest that hyperexcitability of
dorsal horn WDR neurons emerges in the tumor cell inoculated rats,
which likely underlies the development of central sensitization and
cancer-induced bone pain.
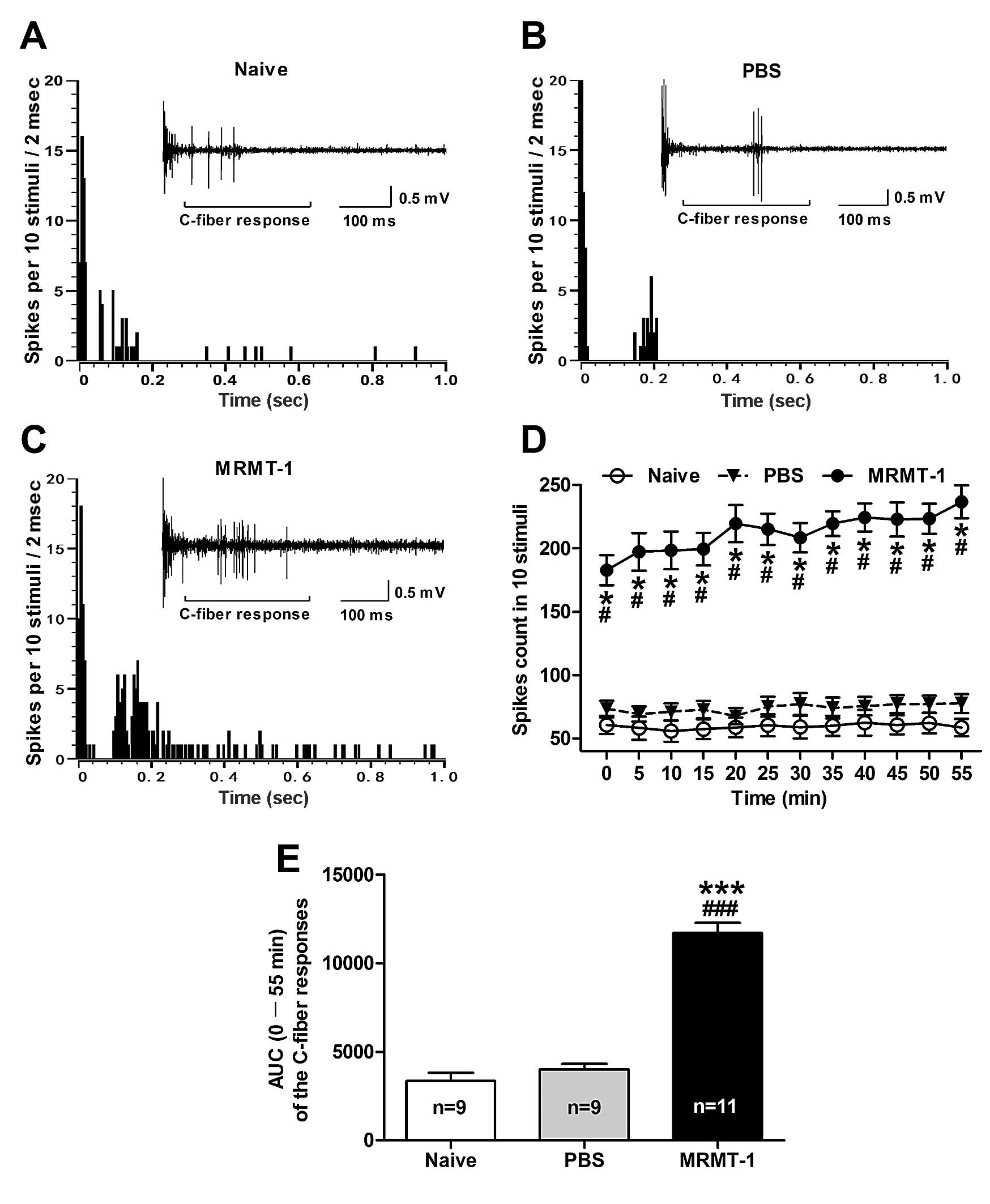 | Figure 1Alterations of the C-fiber responses
of dorsal horn WDR neurons in bone cancer rats. (A–C)
Representative C-fiber responses of dorsal horn WDR neurons in
naive (A), PBS (B) and MRMT-1 tumor cell (C) inoculated rats.
Panels illustrate the post-stimulus histogram of the electrically
evoked neuronal responses. Inset shows the original recordings of
the first electrically evoked neuronal responses. (D) The total
spike count of the electrically evoked C-fiber responses in 10
stimuli. */#P<0.001, compared to the naive and the
PBS group, respectively, two-way ANOVA followed by Bonferroni
post-hoc test, n=9–11/group. (E) AUC (0–55 min) of the C-fiber
responses. ***/###P<0.001, compared to the naive and
the PBS group, respectively, one-way ANOVA followed by the Dunnett
multiple comparison test, n=9–11/group. AUC, area under the
time-course curve. WDR, wide dynamic range; PBS, phosphorylated
buffer solution; ANOVA, analysis of variance. |
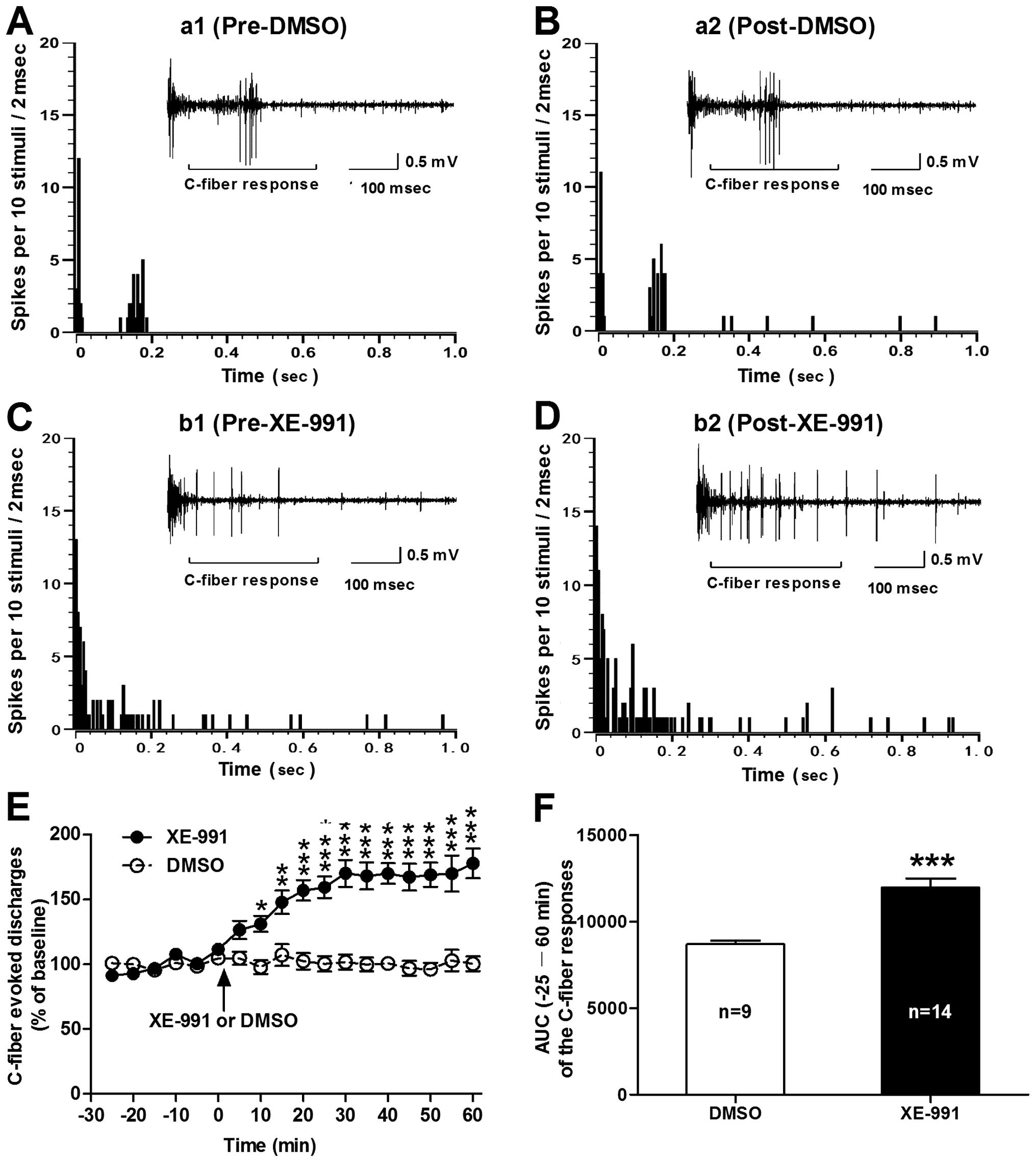 | Figure 2Effects of spinal administration of
XE-991 on the C-fiber responses of dorsal horn WDR neurons in
normal rats. (A–D) Post-stimulus histograms of the electrically
evoked neuronal responses in a dorsal horn WDR neuron before (a1,
DMSO; b1, XE-991) and after (a2, DMSO; b2, XE-991) drug
application. Inset shows the original recordings of the first
electrically evoked neuronal responses corresponding to each
time-point respectively. (E) Analysis of the C-fiber evoked
discharges before and after drug (XE-991 or DMSO) application. Note
that spinal administration of XE-991 (0.4 mM) induced a significant
increase in C-fiber responses of dorsal horn WDR neurons in normal
rats. *P<0.05, **P<0.01 and
***P<0.001, compared to control DMSO, two-way ANOVA
followed by Bonferroni post-hoc test, n=9–14/group). (F) AUC
(−25–60 min) of the C-fiber responses. ***P<0.001,
XE-991 vs. DMSO, two-tailed unpaired t-test, n=9 DMSO and 14
XE-991. WDR, wide dynamic range; ANOVA, analysis of variance; AUC,
area under the time-course curve. |
Blockade of spinal KCNQ/M channels with
XE-991 induces hyperexcitability of dorsal horn WDR neurons in
normal rats
Next, we investigated whether repressed KCNQ/M
channels in the spinal cord contribute to the hyperexcitability of
dorsal horn WDR neurons. We found that spinal administration of
XE-991 (at 0.4 mM), a potent KCNQ/M channel blocker, induced a
significant increase in C-fiber responses of dorsal horn WDR
neurons in the normal rats (Fig.
2). The enhanced effect of XE-991 on C-fiber responses of WDR
neurons began at 10 min post-XE-991 (131.09±6.2% of baseline),
maintained for 60 min (177.74±11.5% of baseline) until termination
of the experiment. On the contrary, spinal administration of
vehicle DMSO had no significant effect on the C-fiber responses of
WDR neurons (P<0.05–0.001, two-way ANOVA; Fig. 2E). As summarized in the AUC of the
C-fiber responses, the AUC (−25–60 min of the analysis time) was
also increased markedly in the XE-991 group (11,970±521.4) compared
to the DMSO group (8,689±223.2) (P<0.001, XE-991 vs. DMSO,
two-tailed unpaired t-test; Fig.
2F). Representative examples illustrating the C-fiber responses
of dorsal horn WDR neurons before and after administration of
XE-991 or DMSO are shown in Fig.
2A–D. These results imply that repression of spinal KCNQ/M
channels may induce enhanced excitability of dorsal horn WDR
neurons in normal rats.
Intrathecal administration of XE-991
produces mechanical allodynia and thermal hyperalgesia in normal
rats
Given that blockade of spinal KCNQ/M channels with
XE-991 induces hyperexcitability of dorsal horn WDR neurons, we
hypothesized that intrathecal administration of XE-991 would
produce pain hypersensitivity in normal rats. As our expectation,
spinal application of XE-991 produced a statistical decrease in
ipsilateral PWT (in g) from 0.5 h after drug injection (4.45±0.65
XE-991 vs. 15.10±0.01 DMSO, P<0.001) and lasted for 3.5 h
(5.07±0.69 XE-991 vs. 14.21±0.44 DMSO, P<0.001) until
termination of the experiment (two-way ANOVA, n=11/group; Fig. 3A). As summarized in the AUC of PWT,
the AUC (0–3.5 h of the analysis time) was significantly decreased
in the XE-991 group (17.85±1.79) compared to the DMSO group
(50.70±1.11) (P<0.001, two-tailed unpaired t-test; Fig. 3C). Similarly, intrathecal injection
of XE-991 also produced a remarkable decrease in ipsilateral PWL
(in sec) from 0.5 h (11.19±0.91 XE-991 vs. 21.58±1.11 DMSO,
P<0.001) to 3.5 h (10.74±1.22 XE-991 vs. 21.93±0.8 DMSO,
P<0.001) following drug administration (two-way ANOVA,
n=9/group; Fig. 3B). The AUC (0–3.5
h of the analysis time) of PWL was prominently decreased in the
XE-991 group (38.92±2.58) compared to the DMSO group (74.35±2.79)
(P<0.001, two-tailed unpaired t-test, Fig. 3D). Moreover, the inclined-plate test
was performed at 30 min before and 4 h after drug injection to
assess the effect of XE-991 on the locomotor function of the rats.
The results showed that intrathecal injection of XE-991 at our
experimental dose had no obvious damage to the locomotor function
of the rats (P>0.05, two-tailed unpaired t-test, pre-drug vs.
post-drug, n=11/group, Fig. 3E).
These data indicate that suppression of spinal KCNQ/M channels also
produces mechanical allodynia and thermal hyperalgesia in normal
rats.
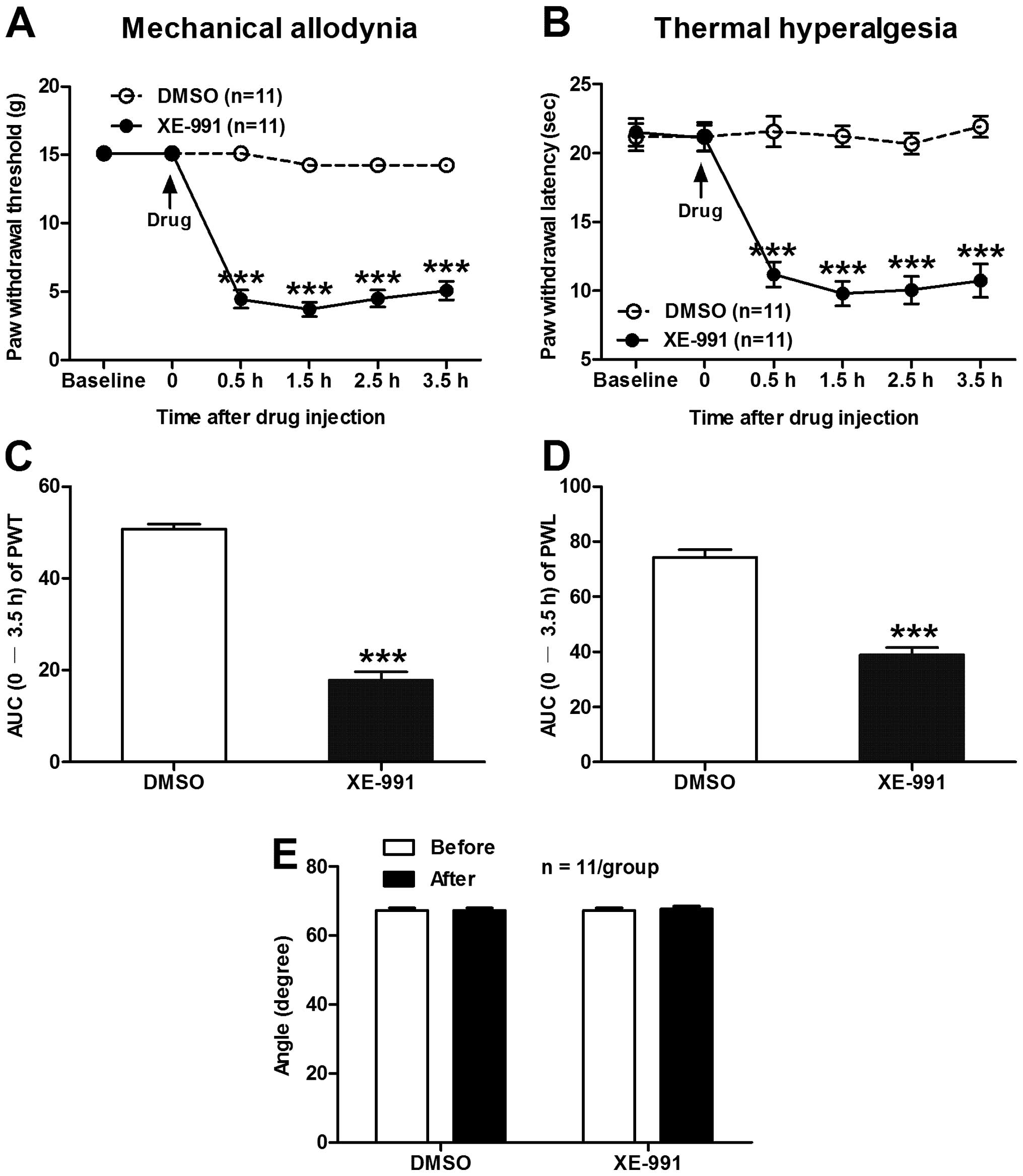 | Figure 3Effects of intrathecal administration
of XE-991 on ipsilateral PWT and PWL in normal rats. (A and B) The
time course of spinal application of XE-991 (0.9 μg/μl) on PWT (A)
and PWL (B). Note that intrathecal administration of XE-991
produced obvious mechanical allodynia and thermal hyperalgesia in
normal rats. ***P<0.001, compared to the vehicle DMSO
group, two-way ANOVA followed by Bonferroni post-hoc test,
n=11/group. (C and D) Area under the time-course curve (AUC) of PWT
(C) and PWL in A and B, respectively. ***P<0.001,
compared to the vehicle DMSO group, two-tailed unpaired t-test,
n=11/group. (E) Effects of spinal XE-991 on the locomotor function
of rats measured by the angle of the inclined-plate at which the
animal begins to fall. Note that intrathecal injection of XE-991
had no significant damage on the locomotor function of the rats
(P>0.05, pre-drug vs. post-drug, two-tailed unpaired t-test,
n=11/group). PWT, paw withdrawal threshold; PWL, paw withdrawal
latency; ANOVA, analysis of variance; AUC, area under the
time-course curve. |
Activation of spinal KCNQ/M channels with
retigabine reduces the bone cancer-induced hyperexcitability of
dorsal horn WDR neurons in MRMT-1-inoculated rats
To further determine whether the enhanced
excitability of dorsal horn WDR neurons in rats with bone cancer
pain depends on the repression of spinal KCNQ/M channels, we tested
the effects of RTG, a selective KCNQ/M channel opener, on C-fiber
responses of WDR neurons in MRMT-1-inoculated rats. As shown in
Fig. 4, spinal administration of
RTG (at 5 mM) significantly reduced the enhanced excitability of
dorsal horn WDR neurons in bone cancer rats. The inhibitory effect
of RTG on C-fiber responses of WDR neurons began at 10 min post-RTG
(83.82±4.5% of baseline), lasted for 60 min (66.23±3.2% of
baseline) until termination of the experiment. In contrast, spinal
administration of vehicle DMSO had no significant effect on the
C-fiber responses of WDR neurons in the MRMT-1-inoculated rats
(P<0.01–0.001, RTG vs. DMSO, two-way ANOVA; Fig. 4E). As summarized in AUC of the
C-fiber responses, the AUC (−25–60 min of the analysis time) was
significantly decreased in the RTG group (6,978±148.5) compared to
the DMSO group (8,648±151.6) (P<0.001, RTG vs. DMSO, two-tailed
unpaired t-test; Fig. 4F).
Representative examples illustrating the C-fiber responses of
dorsal horn WDR neurons before and after administration of RTG or
DMSO in the MRMT-1-inoculated rats are shown in Fig. 4A–D. The results indicate that
activation of spinal KCNQ/M channels with RTG may inhibit the bone
cancer-induced hyperexcitability of dorsal horn WDR neurons in
MRMT-1-inoculated rats.
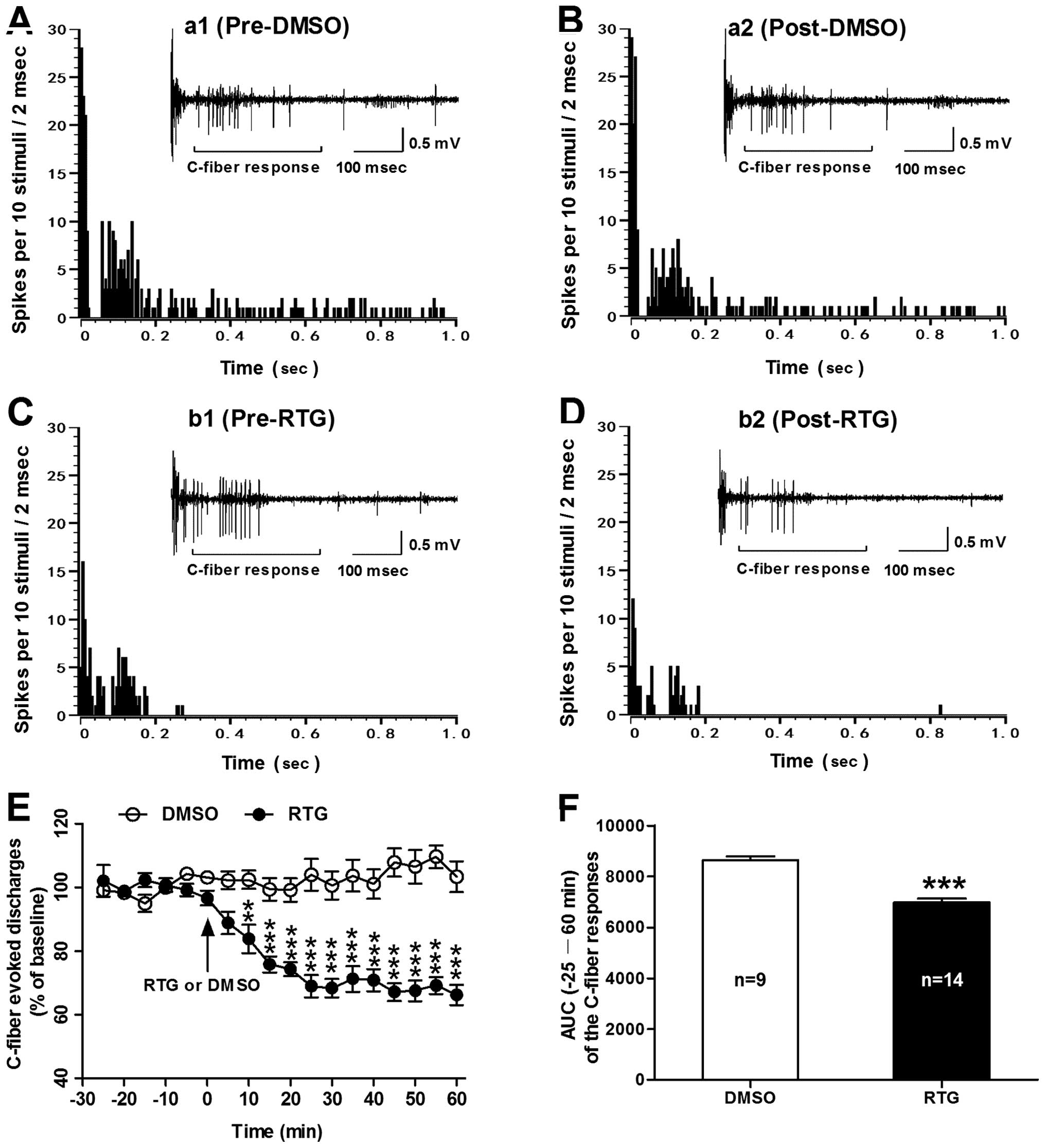 | Figure 4Effects of spinal administration of
RTG on C-fiber responses of dorsal horn WDR neurons in bone cancer
rats. (A–D) Post-stimulus histograms of the electrically evoked
neuronal responses in a dorsal horn WDR neuron before (a1, DMSO;
b1, RTG) and after (a2, DMSO; b2, RTG) drug application. Inset
shows the original recordings of the first electrically evoked
neuronal responses corresponding to each time point respectively.
(E) Analysis of the C-fiber evoked discharges before and after drug
(RTG or DMSO) application in MRMT-1 tumor cell inoculated rats.
Note that spinal administration of RTG (5 mM) significantly reduced
the enhanced excitability of dorsal horn WDR neurons in the bone
cancer rats. **P<0.01, ***P<0.001,
compared to the vehicle DMSO group, two-way ANOVA followed by
Bonferroni post-hoc test, n=9–14/group). (F) AUC (−25–60 min) of
the C-fiber responses. ***P<0.001, RTG vs. DMSO,
two-tailed unpaired t-test, n=9–14/group. RTG, retigabine; WDR,
wide dynamic range; ANOVA, analysis of variance; AUC, area under
the time-course curve. |
Intrathecal administration of retigabine
alleviates mechanical allodynia and thermal hyperalgesia in bone
cancer rats
Based on the aforementioned findings that activation
of spinal KCNQ/M channels with RTG inhibits the sensitization of
dorsal horn WDR neurons in bone cancer rats, we speculated that
intrathecal administration of RTG would attenuate the bone
cancer-induced pain hypersensitivity in the MRMT-1-inoculated rats.
As shown in Fig. 5, spinal
application of RTG rescued the bone cancer-induced decrease in
ipsilateral PWT and PWL in the MRMT-1-inoculated rats. The
decreased PWT (in g) was restored from 0.5 h (10.39±1.13 RTG vs.
2.92±0.57 DMSO, P<0.001) to 2.5 h (6.32±0.59 RTG vs. 2.99±0.80
DMSO, P<0.05) after intrathecal injection of RTG compared to
vehicle DMSO (two-way ANOVA, n=9/group; Fig. 5A). The AUC (0–3.5 h of the analysis
time) of PWT was significantly decreased in the RTG group
(24.47±2.46) compared to the DMSO group (10.17±2.34) (P<0.01,
one-way ANOVA, n=9/group; Fig. 5C).
Moreover, the inhibitory effect of RTG on the reduction of PWT in
bone cancer rats was almost completely blocked by the
KCNQ/M-channel antagonist XE-991 (Fig.
5A and C). Similarly, RTG also inhibited the reduction of PWL
in the bone cancer rats, and this inhibitory effect of RTG was also
blocked by XE-991 (Fig. 5B and D).
To examine the long-term effects of intrathecal RTG on bone
cancer-induced pain hypersensitivity in the MRMT-1-inoculated rats,
RTG at 8.5 μg/μl was intrathecally administrated to rats twice/day
at a 30-min interval, for 3 days, and the effect of RTG on both PWT
and PWL was tested on day 4 after drug injection. The results
showed that repeated administration of RTG for 3 days produced a
long-term anti-allodynic and anti-hyperalgesic effects in the bone
cancer rats. As shown in Fig. 5E and
F, both the decreased PWT (7.59±0.70 vs. 3.10±0.56 g, RTG vs.
DMSO, P<0.001) and PWL (15.40±1.26 vs. 9.13±0.97 s, RTG vs.
DMSO, P<0.001) in the MRMT-1-treated rats were significantly
restored on day 4 after repeated application of RTG compared to
vehicle DMSO (two-way ANOVA, n=9/group). Similarly, XE-991 blocked
the inhibitory effects of RGT on both the decreased PWT and PWL in
the bone cancer rats (Fig. 5E and
F), and no significant damage was observed in the locomotor
function of rats after repeated application of RTG (Fig. 5G). These results suggest that
activation of the spinal KCNQ/M channels by RGT alleviates both
mechanical allodynia and thermal hyperalgesia in bone cancer
rats.
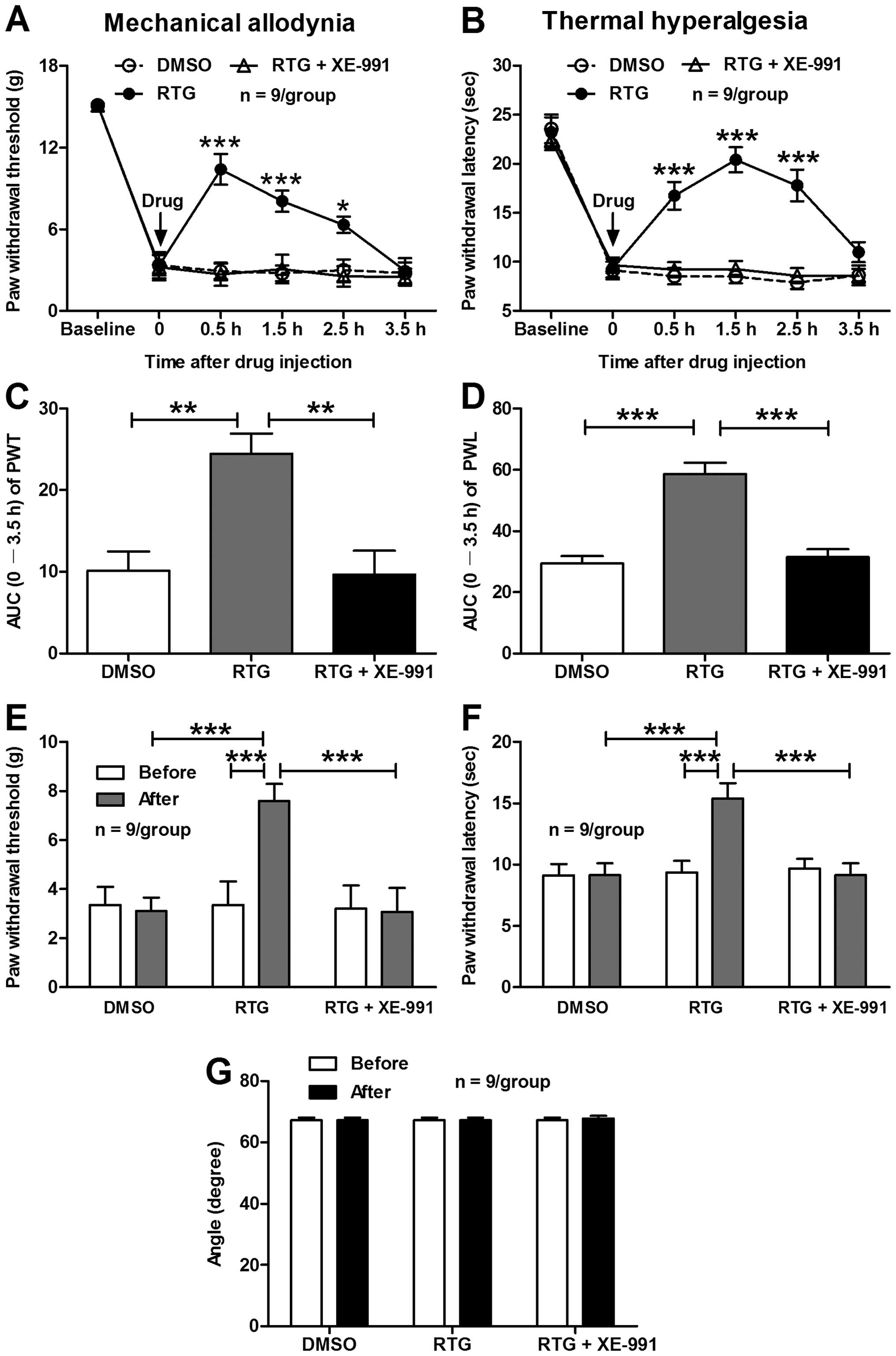 | Figure 5Effects of intrathecal administration
of RTG on ipsilateral PWT and PWL in bone cancer rats. (A and B)
The time course of spinal application of RTG (8.5 μg/μl) on PWT (A)
and PWL (B). Note that RTG significantly inhibited the bone
cancer-induced reduction of ipsilateral PWT (A) and PWL (B) in
MRMT-1-inoculated rats and all the effects of RTG were blocked by
XE-991. *P<0.05 and ***P<0.001,
compared to the vehicle DMSO group, two-way ANOVA followed by
Bonferroni post-hoc test, n=9/group. (C and D) Area under the
time-course curve (AUC) of PWT and PWL in A and B, respectively.
**P<0.01 and ***P<0.001, one-way ANOVA
followed by the Dunnett multiple comparison test, n=9/group. (E and
F) Effects of repeated application of RTG on ipsilateral PWT (E)
and PWL (F) in MRMT-1-inoculated rats. Note that repeated
administration of RTG for 3 days produced long-term anti-allodynic
and anti-hyperalgesic effects in bone cancer rats.
***P<0.001, two-way ANOVA followed by Bonferroni
post-hoc test, n=9/group. (G) Effects of repeated application of
RTG on the locomotor function of rats measured by the angle of the
inclined-plate at which the animal begins to fall. Note that
repeated application of RTG had no significant damage on the
locomotor function of rats (P>0.05, pre-drug vs. post-drug,
two-tailed unpaired t-test, n=9/group). RTG, retigabine; PWT, paw
withdrawal threshold; PWL, paw withdrawal latency; ANOVA, analysis
of variance; AUC, area under the time-course curve. |
Discussion
As extension of our previous findings showing that
inoculation of tumor cells into the tibial canal in rats induces
enhanced excitability of primary sensory DRG neurons, which is
responsible for the peripheral sensitization and tumor-induced
hyperalgesia under cancer conditions (10), our present study provides
electrophysiological evidence confirming the sensitization of
dorsal horn WDR neurons (i.e., central sensitization) in MRMT-1
tumor cell inoculated rats, which is considered as another
underlying mechanism for the development of cancer-induced bone
pain (7,29). A marked increase in the C-fiber
responses of WDR neurons to electrical stimulation of the sciatic
nerve in bone cancer rats was observed. This is consistent with a
previous study showing that, in a mouse model of bone cancer pain
established by implantation of fibrosarcoma cells into the
calcaneus bone, WDR, but not high threshold (HT), nociceptive
dorsal horn neurons in tumor-bearing mice exhibited sensitization
to mechanical, heat, and cold stimuli that probably contributed to
tumor-evoked hyperalgesia (29). In
the present study it was suggested that although peripheral
sensitization including the sensitization of primary sensory
neurons participate in the generation of bone cancer pain (1,8–10),
central sensitization, especially the sensitization of dorsal horn
WDR neurons, likely plays a more important role in tumor-evoked
hyperalgesia and the maintenance of cancer-induced bone pain
(7,27,29,30,48).
To clarify the pathogenic mechanisms underlying the
sensitization of dorsal horn WDR neurons in bone cancer rats, we
focused on KCNQ/M (Kv7) potassium channels, a family of
voltage-gated potassium channels that is considered as a ‘brake’ to
regulate the action potential firing and the neuronal excitability
(16). Recently, we have found that
inhibition of KCNQ/M channels in DRG neurons with XE-991 causes a
robust increase in neuronal excitability that is associated with an
obvious mechanical allodynia in naive rats (11). Consistent with our previous
findings, the present study revealed that blockade of spinal KCNQ/M
channels by local administration of XE-991, a potent KCNQ/M channel
blocker, induced a significant increase in C-fiber responses of
dorsal horn WDR neurons in normal rats, implying that repression of
spinal KCNQ/M channels likely induces sensitization of dorsal horn
WDR neurons that is highly related to the pathogenesis of pain
hypersensitivity under cancer conditions (48,49).
In support of this notion, we discovered that intrathecal
administration of XE-991 produced mechanical allodynia and thermal
hyperalgesia in normal rats, suggesting that inhibition of spinal
KCNQ/M channels and its associated sensitization of dorsal horn WDR
neurons probably contributed to the pathogenesis of pain
hypersensitivity under cancer conditions. Moreover, we provide
additional evidence showing that activation of spinal KCNQ/M
channels by retigabine, a selective KCNQ/M channel opener, not only
inhibited the bone cancer-induced hyperexcitability of dorsal horn
WDR neurons, but also alleviated mechanical allodynia and thermal
hyperalgesia in bone cancer rats, and all of these effects of
retigabine were blocked by KCNQ/M-channel antagonist XE-991. These
results are line with previous findings showing that in hemisected
spinal cord from immature rats, blockade of KCNQ/M channels with
XE-991 induced depolarization and enhanced excitability of dorsal
horn neurons, whereas activation of KCNQ/M channels with retigabine
produced hyperpolarization and a large decrease in the excitability
of these neurons (35). Retigabine,
applied to the dorsal spinal cord, inhibited C and A-δ
fiber-mediated responses of dorsal horn neurons both in normal rats
and in rats subjected to spinal nerve ligation (32).
In conclusion, the present study showed that
inoculation of MRMT-1 tumor cells into the tibial canal in rats
induced sensitization of dorsal horn WDR neurons in the spinal
cord. Blockade of spinal KCNQ/M channels produced hyperexcitability
of dorsal horn WDR neurons and pain hypersensitivity in normal
rats, whereas activation of these channels inhibited the
sensitization of dorsal horn WDR neurons, meanwhile alleviating
mechanical allodynia and thermal hyperalgesia in bone cancer rats.
Thus, suppression of KCNQ/M channels in the spinal cord leads to
the sensitization of dorsal horn WDR neurons, thereby playing an
important role in the development of cancer-induced bone pain.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (81371237, 31171063
and 81072951), the Beijing Natural Science Foundation (7112079),
the Special Foundation for Public Welfare Profession Scientific
Research Program from the Ministry of Health of the People’s
Republic of China (201302013-01) and the ‘973’ Program of the
Ministry of Science and Technology of China (2013CB531905).
References
|
1
|
Jimenez-Andrade JM, Mantyh WG, Bloom AP,
Ferng AS, Geffre CP and Mantyh PW: Bone cancer pain. Ann NY Acad
Sci. 1198:173–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knopp KL, Nisenbaum ES and Arneric SP:
Evolving cancer pain treatments: rational approaches to improve the
quality of life for cancer patients. Curr Pharm Biotechnol.
12:1627–1643. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Middlemiss T, Laird BJ and Fallon MT:
Mechanisms of cancer-induced bone pain. Clin Oncol (R Coll Radiol).
23:387–392. 2011. View Article : Google Scholar
|
|
4
|
Paice JA and Ferrell B: The management of
cancer pain. CA Cancer J Clin. 61:157–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
von Gunten CF: Pathophysiology of pain in
cancer. J Pediatr Hematol Oncol. 33(Suppl 1): S12–S18. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luger NM, Mach DB, Sevcik MA and Mantyh
PW: Bone cancer pain: from model to mechanism to therapy. J Pain
Symptom Manage. 29:S32–S46. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Urch C: The pathophysiology of
cancer-induced bone pain: current understanding. Palliat Med.
18:267–274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hamamoto DT, Khasabov SG, Cain DM and
Simone DA: Tumor-evoked sensitization of C nociceptors: a role for
endothelin. J Neurophysiol. 100:2300–2311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao J, Pan HL, Li TT, Zhang YQ, Wei JY
and Zhao ZQ: The sensitization of peripheral C-fibers to
lysophosphatidic acid in bone cancer pain. Life Sci. 87:120–125.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng Q, Fang D, Cai J, Wan Y, Han JS and
Xing GG: Enhanced excitability of small dorsal root ganglion
neurons in rats with bone cancer pain. Mol Pain. 8:242012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng Q, Fang D, Liu M, Cai J, Wan Y, Han
JS and Xing GG: Suppression of KCNQ/M (Kv7) potassium channels in
dorsal root ganglion neurons contributes to the development of bone
cancer pain in a rat model. Pain. 154:434–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wulff H, Castle NA and Pardo LA:
Voltage-gated potassium channels as therapeutic targets. Nat Rev
Drug Discov. 8:982–1001. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Biervert C, Schroeder BC, Kubisch C,
Berkovic SF, Propping P, Jentsch TJ and Steinlein OK: A potassium
channel mutation in neonatal human epilepsy. Science. 279:403–406.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klinger F, Gould G, Boehm S and Shapiro
MS: Distribution of M-channel subunits KCNQ2 and KCNQ3 in rat
hippocampus. Neuroimage. 58:761–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang HS, Pan Z, Shi W, et al: KCNQ2 and
KCNQ3 potassium channel subunits: molecular correlates of the
M-channel. Science. 282:1890–1893. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brown DA and Passmore GM: Neural KCNQ
(Kv7) channels. Br J Pharmacol. 156:1185–1195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marrion NV: Control of M-current. Annu Rev
Physiol. 59:483–504. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen W, Hamilton SE, Nathanson NM and
Surmeier DJ: Cholinergic suppression of KCNQ channel currents
enhances excitability of striatal medium spiny neurons. J Neurosci.
25:7449–7458. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fritch PC, McNaughton-Smith G, Amato GS,
et al: Novel KCNQ2/Q3 agonists as potential therapeutics for
epilepsy and neuropathic pain. J Med Chem. 53:887–896. 2010.
View Article : Google Scholar
|
|
20
|
Gu N, Vervaeke K, Hu H and Storm JF:
Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the
somatic medium after-hyperpolarization and excitability control in
CA1 hippocampal pyramidal cells. J Physiol. 566:689–715. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hansen HH, Ebbesen C, Mathiesen C, et al:
The KCNQ channel opener retigabine inhibits the activity of
mesencephalic dopaminergic systems of the rat. J Pharmacol Exp
Ther. 318:1006–1019. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hansen HH, Andreasen JT, Weikop P, Mirza
N, Scheel-Krüger J and Mikkelsen JD: The neuronal KCNQ channel
opener retigabine inhibits locomotor activity and reduces forebrain
excitatory responses to the psychostimulants cocaine,
methylphenidate and phencyclidine. Eur J Pharmacol. 570:77–88.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Linley JE, Rose K, Patil M, Robertson B,
Akopian AN and Gamper N: Inhibition of M current in sensory neurons
by exogenous proteases: a signaling pathway mediating inflammatory
nociception. J Neurosci. 28:11240–11249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu B, Linley JE, Du X, Zhang X, Ooi L,
Zhang H and Gamper N: The acute nociceptive signals induced by
bradykinin in rat sensory neurons are mediated by inhibition of
M-type K+ channels and activation of
Ca2+-activated Cl- channels. J Clin Invest.
120:1240–1252. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rose K, Ooi L, Dalle C, Robertson B, Wood
IC and Gamper N: Transcriptional repression of the M channel
subunit Kv7.2 in chronic nerve injury. Pain. 152:742–754. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Woolf CJ: Central sensitization:
implications for the diagnosis and treatment of pain. Pain.
152:S2–S15. 2011. View Article : Google Scholar
|
|
27
|
Latremoliere A and Woolf CJ: Central
sensitization: a generator of pain hypersensitivity by central
neural plasticity. J Pain. 10:895–926. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Donovan-Rodriguez T, Dickenson AH and Urch
CE: Superficial dorsal horn neuronal responses and the emergence of
behavioural hyperalgesia in a rat model of cancer-induced bone
pain. Neurosci Lett. 360:29–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khasabov SG, Hamamoto DT, Harding-Rose C
and Simone DA: Tumor-evoked hyperalgesia and sensitization of
nociceptive dorsal horn neurons in a murine model of cancer pain.
Brain Res. 1180:7–19. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Urch CE, Donovan-Rodriguez T and Dickenson
AH: Alterations in dorsal horn neurones in a rat model of
cancer-induced bone pain. Pain. 106:347–356. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dedek K, Kunath B, Kananura C, Reuner U,
Jentsch TJ and Steinlein OK: Myokymia and neonatal epilepsy caused
by a mutation in the voltage sensor of the KCNQ2 K+
channel. Proc Natl Acad Sci USA. 98:12272–12277. 2001. View Article : Google Scholar
|
|
32
|
Passmore GM, Selyanko AA, Mistry M, et al:
KCNQ/M currents in sensory neurons: significance for pain therapy.
J Neurosci. 23:7227–7236. 2003.PubMed/NCBI
|
|
33
|
Passmore GM, Reilly JM, Thakur M,
Keasberry VN, Marsh SJ, Dickenson AH and Brown DA: Functional
significance of M-type potassium channels in nociceptive cutaneous
sensory endings. Front Mol Neurosci. 5:632012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rivera-Arconada I, Martinez-Gomez J and
Lopez-Garcia JA: M-current modulators alter rat spinal nociceptive
transmission: an electrophysiological study in vitro.
Neuropharmacology. 46:598–606. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rivera-Arconada I and Lopez-Garcia JA:
Effects of M-current modulators on the excitability of immature rat
spinal sensory and motor neurones. Eur J Neurosci. 22:3091–3098.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rivera-Arconada I and Lopez-Garcia JA:
Retigabine-induced population primary afferent hyperpolarisation in
vitro. Neuropharmacology. 51:756–763. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Roza C and Lopez-Garcia JA: Retigabine,
the specific KCNQ channel opener, blocks ectopic discharges in
axotomized sensory fibres. Pain. 138:537–545. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Medhurst SJ, Walker K, Bowes M, et al: A
rat model of bone cancer pain. Pain. 96:129–140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu M, Yang H, Fang D, et al: Upregulation
of P2X3 receptors by neuronal calcium sensor protein VILIP-1 in
dorsal root ganglions contributes to the bone cancer pain in rats.
Pain. 154:1551–1568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dixon WJ: Efficient analysis of
experimental observations. Annu Rev Pharmacol Toxicol. 20:441–462.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zimmermann M: Pathobiology of neuropathic
pain1. Eur J Pharmacol. 429:23–37. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Qu XX, Cai J, Li MJ, et al: Role of the
spinal cord NR2B-containing NMDA receptors in the development of
neuropathic pain. Exp Neurol. 215:298–307. 2009. View Article : Google Scholar
|
|
45
|
Rivlin AS and Tator CH: Objective clinical
assessment of motor function after experimental spinal cord injury
in the rat. J Neurosurg. 47:577–581. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xing GG, Liu FY, Qu XX, Han JS and Wan Y:
Long-term synaptic plasticity in the spinal dorsal horn and its
modulation by electroacupuncture in rats with neuropathic pain. Exp
Neurol. 208:323–332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lu Y and Perl ER: Selective action of
noradrenaline and serotonin on neurones of the spinal superficial
dorsal horn in the rat. J Physiol. 582:127–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yanagisawa Y, Furue H, Kawamata T, et al:
Bone cancer induces a unique central sensitization through synaptic
changes in a wide area of the spinal cord. Mol Pain. 6:382010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gordon-Williams RM and Dickenson AH:
Central neuronal mechanisms in cancer-induced bone pain. Curr Opin
Support Palliat Care. 1:6–10. 2007. View Article : Google Scholar
|



















