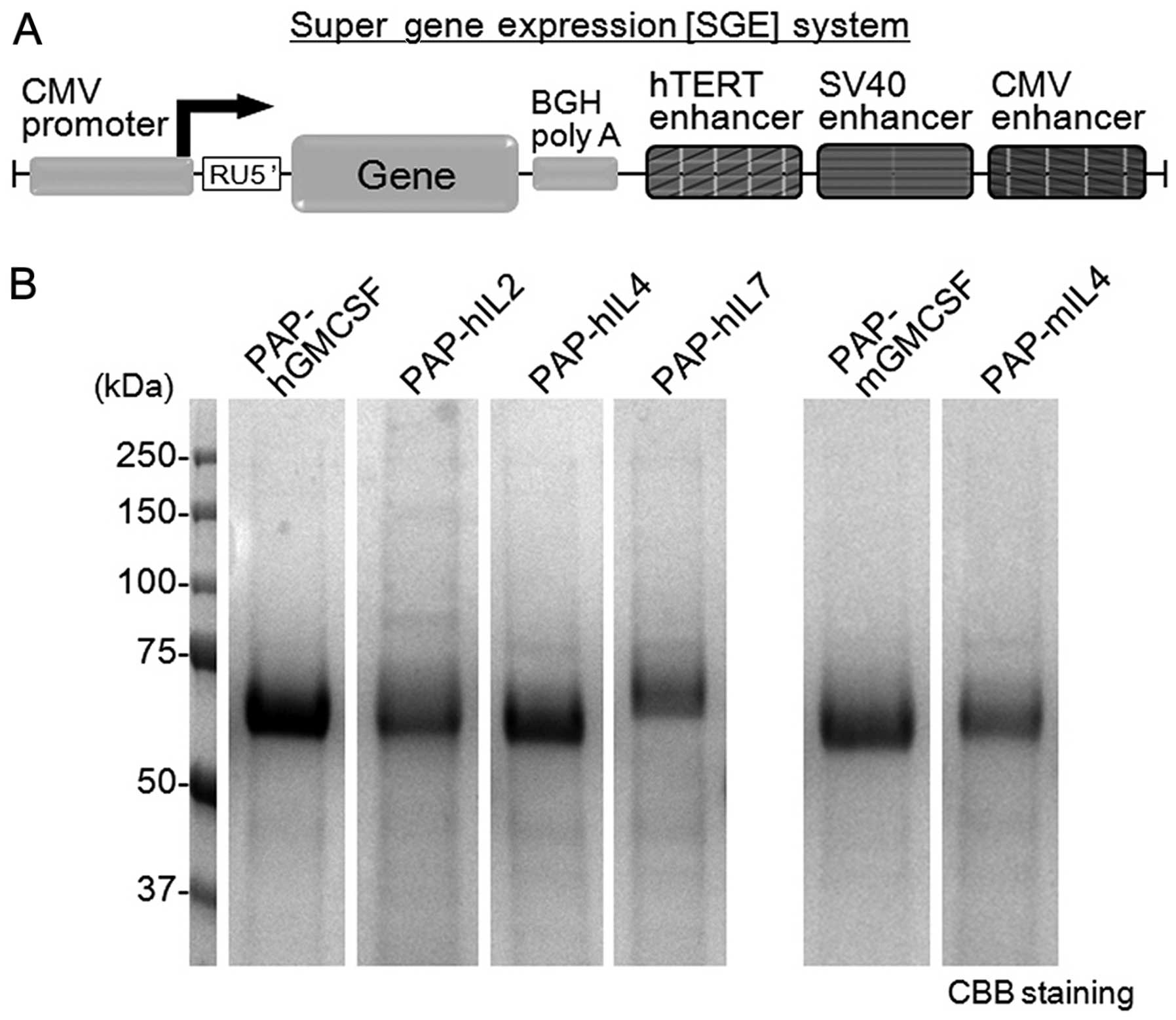
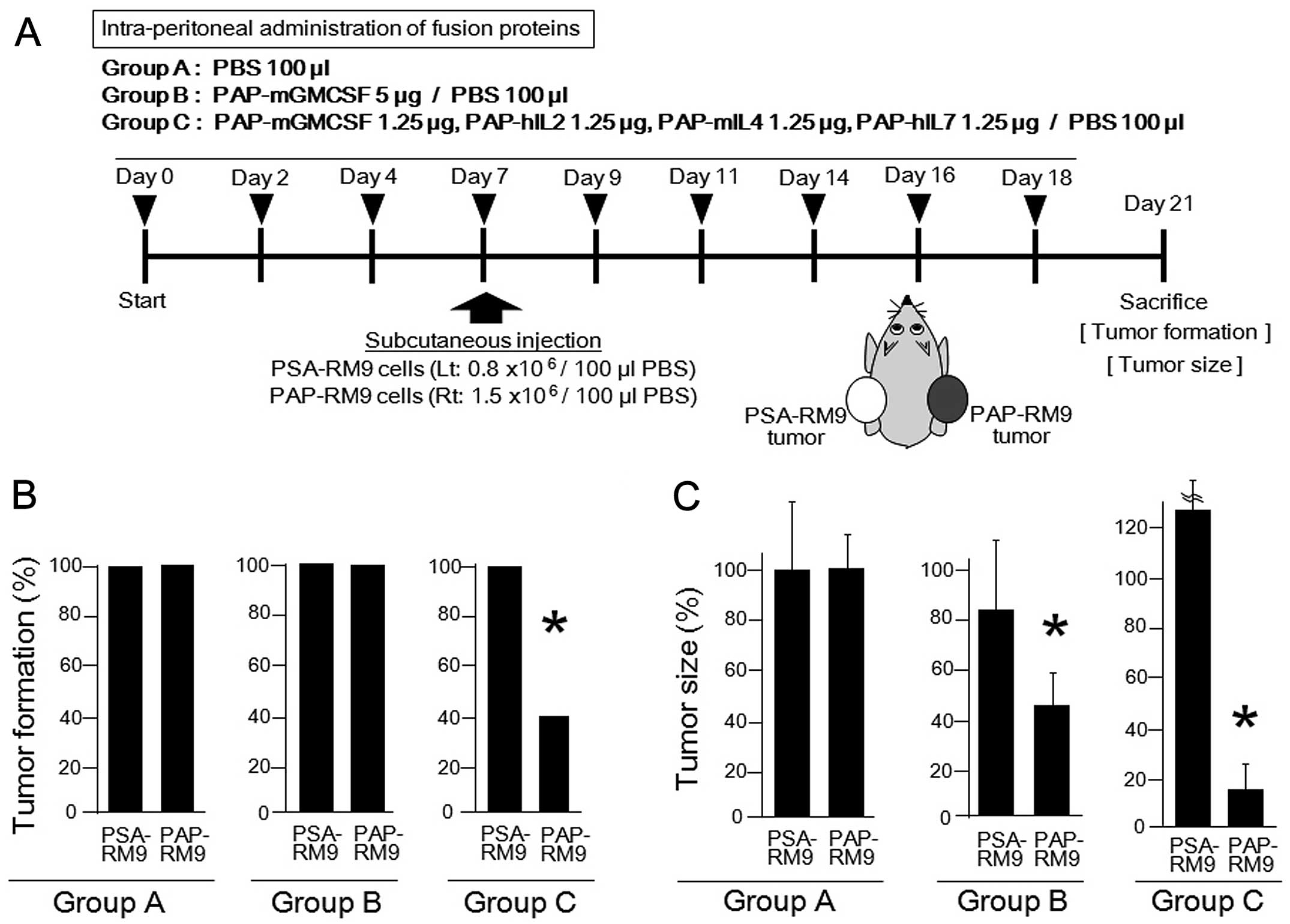
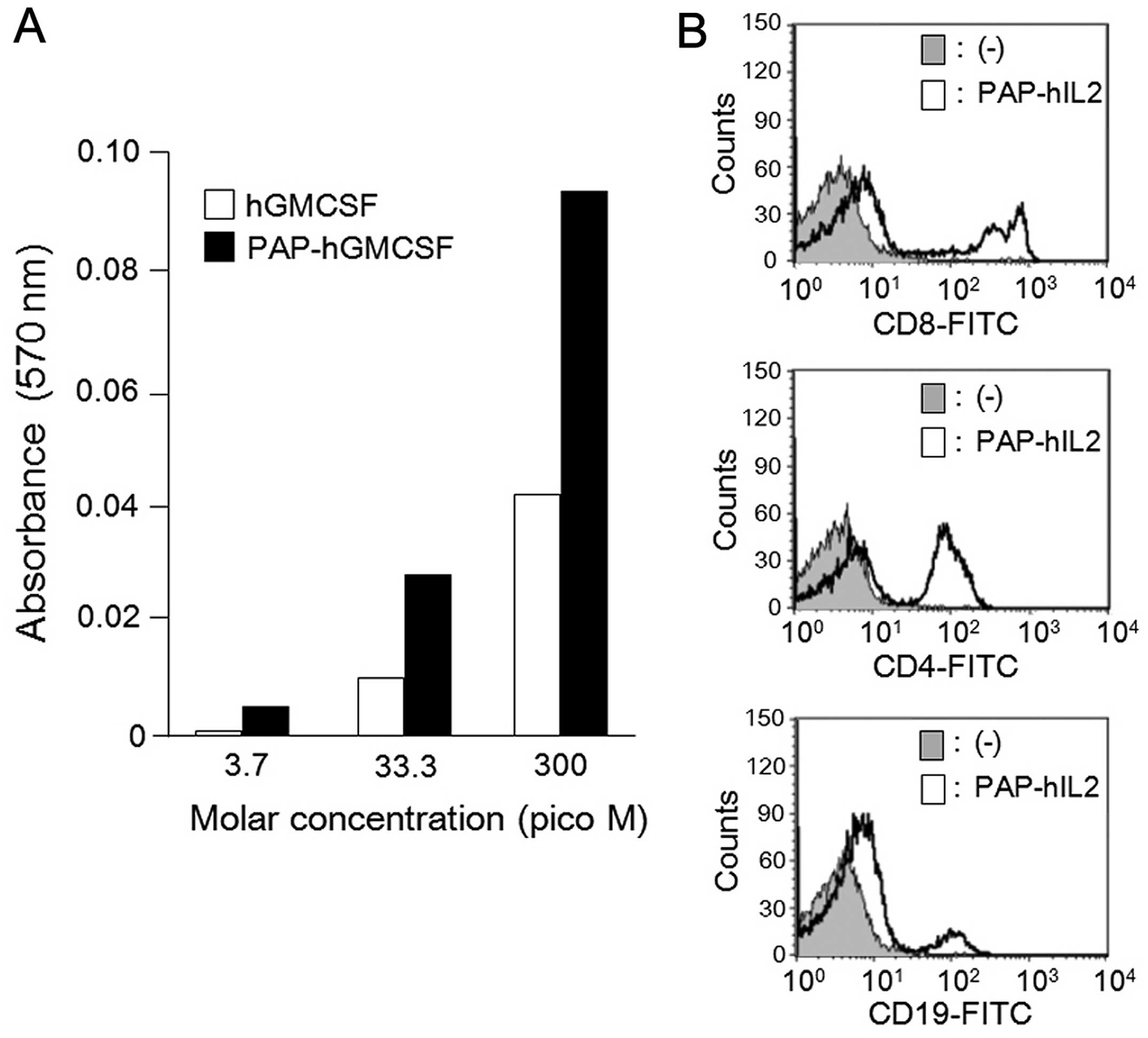
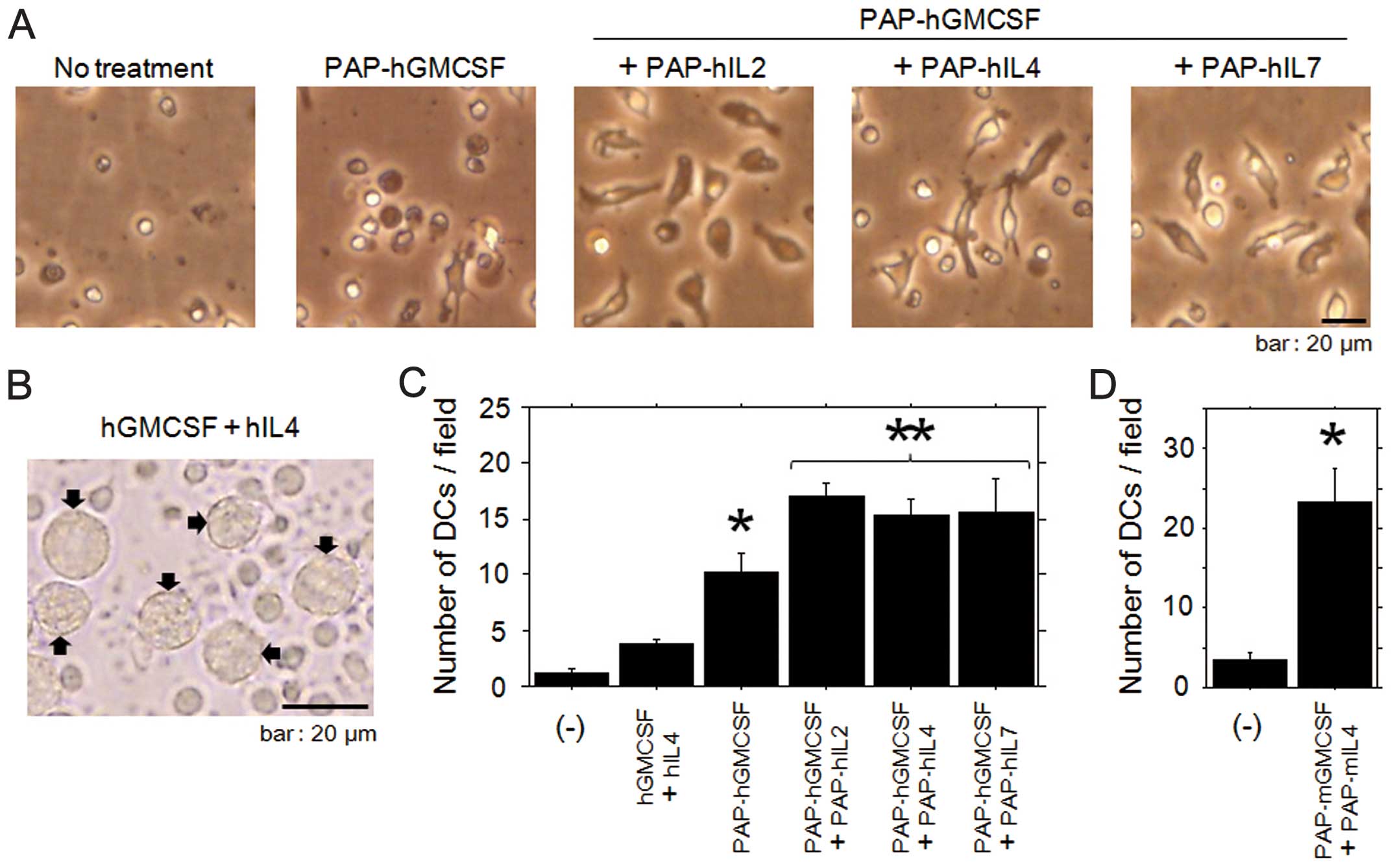
|
1
|
Fong L, Ruegg CL, Brockstedt D, Engleman
EG and Laus R: Induction of tissue-specific autoimmune prostatitis
with prostatic acid phosphatase immunization: implications for
immunotherapy of prostate cancer. J Immunol. 159:3113–3117.
1997.PubMed/NCBI
|
|
2
|
Fong L, Brockstedt D, Benike C, Breen JK,
Strang G, Ruegg CL and Engleman EG: Dendritic cell-based
xenoantigen vaccination for prostate cancer immunotherapy. J
Immunol. 167:7150–7156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roos AK, Pavlenko M, Charo J, Egevad L and
Pisa P: Induction of PSA-specific CTLs and anti-tumor immunity by a
genetic prostate cancer vaccine. Prostate. 15:217–223. 2005.
View Article : Google Scholar
|
|
4
|
Saha A, Chatterjee SK, Mohanty K, Foon KA
and Bhattacharya-Chatterjee M: Dendritic cell based vaccines for
immunotherapy of cancer. Cancer Ther. 1:299–314. 2003.
|
|
5
|
Matera L: The choice of the antigen in the
dendritic cell-based vaccine therapy for prostate cancer. Cancer
Treat Rev. 36:131–141. 2010. View Article : Google Scholar
|
|
6
|
Drake CG: Prostate cancer as a model for
tumour immunotherapy. Nat Rev Immunol. 10:580–593. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watanabe M, Nasu Y and Kumon H:
Adenovirus-mediated REIC/Dkk-3 gene therapy: Development of an
autologous cancer vaccination therapy (Review). Oncol Lett.
7:595–601. 2014.PubMed/NCBI
|
|
8
|
Saif JM, Vadakekolathu J, Rane SS,
McDonald D, Ahmad M, Mathieu M, Pockley AG, Durrant L, Metheringham
R, Rees RC and McArdle SE: Novel prostate acid phosphatase-based
peptide vaccination strategy induces antigen-specific T-cell
responses and limits tumour growth in mice. Eur J Immunol.
44:994–1004. 2014. View Article : Google Scholar
|
|
9
|
Peshwa MV, Shi JD, Ruegg C, Laus R and van
Schooten WC: Induction of prostate tumor-specific CD8+
cytotoxic T-lymphocytes in vitro using antigen-presenting cells
pulsed with prostatic acid phosphatase peptide. Prostate.
36:129–138. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, Xu Y, Frohlich MW and Schellhammer PF; IMPACT Study
Investigators. Sipuleucel-T immunotherapy for castration-resistant
prostate cancer. N Engl J Med. 363:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Lorenzo G, Ferro M and Buonerba C:
Sipuleucel-T (Provenge®) for castration-resistant
prostate cancer. BJU Int. 110:E99–E104. 2012. View Article : Google Scholar
|
|
12
|
McNeel DG, Dunphy EJ, Davies JG, Frye TP,
Johnson LE, Staab MJ, Horvath DL, Straus J, Alberti D, Marnocha R,
Liu G, Eickhoff JC and Wilding G: Safety and immunological efficacy
of a DNA vaccine encoding prostatic acid phosphatase in patients
with stage D0 prostate cancer. J Clin Oncol. 27:4047–4054. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Machlenkin A, Paz A, Bar Haim E,
Goldberger O, Finkel E, Tirosh B, Volovitz I, Vadai E, Lugassy G,
Cytron S, Lemonnier F, Tzehoval E and Eisenbach L: Human CTL
epitopes prostatic acid phosphatase-3 and six-transmembrane
epithelial antigen of prostate-3 as candidates for prostate cancer
immunotherapy. Cancer Res. 65:6435–6442. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsueda S, Takedatsu H, Yao A, Tanaka M,
Noguchi M, Itoh K and Harada M: Identification of peptide vaccine
candidates for prostate cancer patients with HLA-A3 supertype
alleles. Clin Cancer Res. 11:6933–6943. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burch PA, Breen JK, Buckner JC, Gastineau
DA, Kaur JA, Laus RL, Padley DJ, Peshwa MV, Pitot HC, Richardson
RL, Smits BJ, Sopapan P, Strang G, Valone FH and Vuk-Pavlović S:
Priming tissue-specific cellular immunity in a phase I trial of
autologous dendritic cells for prostate cancer. Clin Cancer Res.
6:2175–2182. 2000.PubMed/NCBI
|
|
16
|
Small EJ, Fratesi P, Reese DM, Strang G,
Laus R, Peshwa MV and Valone FH: Immunotherapy of
hormone-refractory prostate cancer with antigen-loaded dendritic
cells. J Clin Oncol. 18:3894–3903. 2000.PubMed/NCBI
|
|
17
|
Zou GM and Tam YK: Cytokines in the
generation and maturation of dendritic cells: recent advances. Eur
Cytokine Netw. 13:186–199. 2002.PubMed/NCBI
|
|
18
|
Liao W, Lin JX and Leonard WJ: IL-2 family
cytokines: new insights into the complex roles of IL-2 as a broad
regulator of T helper cell differentiation. Curr Opin Immunol.
23:598–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marçais A, Viel S, Grau M, Henry T, Marvel
J and Walzer T: Regulation of mouse NK cell development and
function by cytokines. Front Immunol. 4:4502013. View Article : Google Scholar :
|
|
20
|
Watanabe M, Sakaguchi M, Kinoshita R, Kaku
H, Ariyoshi Y, Ueki H, Tanimoto R, Ebara S, Ochiai K, Futami J, Li
SA, Huang P, Nasu Y, Huh NH and Kumon H: A novel gene expression
system strongly enhances the anticancer effects of a
REIC/Dkk-3-encoding adenoviral vector. Oncol Rep. 31:1089–1095.
2014.PubMed/NCBI
|
|
21
|
Sakaguchi M, Watanabe M, Kinoshita R, Kaku
H, Ueki H, Futami J, Murata H, Inoue Y, Li SA, Huang P, Putranto
EW, Ruma IM, Nasu Y, Kumon H and Huh NH: Dramatic increase in
expression of a transgene by insertion of promoters downstream of
the cargo gene. Mol Biotechnol. 56:621–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watanabe M, Kashiwakura Y, Huang P, Ochiai
K, Futami J, Li SA, Takaoka M, Nasu Y, Sakaguchi M, Huh NH and
Kumon H: Immunological aspects of REIC/Dkk-3 in monocyte
differentiation and tumor regression. Int J Oncol. 34:657–663.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kitamura T, Tojo A, Kuwaki T, Chiba S,
Miyazono K, Urabe A and Takaku F: Identification and analysis of
human erythropoietin receptors on a factor-dependent cell line,
TF-1. Blood. 73:375–380. 1989.PubMed/NCBI
|
|
24
|
Klampfer L, Zhang J and Nimer SD: GM-CSF
rescues TF-1 cells from growth factor withdrawal-induced, but not
differentiation-induced apoptosis: the role of BCL-2 and MCL-1.
Cytokine. 11:849–855. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu X, Park SH, Thompson TC and Lane DP:
ras-Induced hyperplasia occurs with mutation of p53, but activated
ras and myc together can induce carcinoma without p53 mutation.
Cell. 70:153–161. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen J, Watanabe M, Huang P, Sakaguchi M,
Ochiai K, Nasu Y, Ouchida M, Huh NH, Shimizu K, Kashiwakura Y, Kaku
H and Kumon H: REIC/Dkk-3 stable transfection reduces the malignant
phenotype of mouse prostate cancer RM9 cells. Int J Mol Med.
24:789–794. 2009.PubMed/NCBI
|
|
27
|
Charley B, Petit E, Leclerc C and Stefanos
S: Production of porcine interleukin-2 and its biological and
antigenic relationships with human interleukin-2. Immunol Lett.
10:121–126. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chazen GD, Pereira GM, LeGros G, Gillis S
and Shevach EM: Interleukin 7 is a T-cell growth factor. Proc Natl
Acad Sci USA. 86:5923–5927. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barata JT, Silva A, Abecasis M, Carlesso
N, Cumano A and Cardoso AA: Molecular and functional evidence for
activity of murine IL-7 on human lymphocytes. Exp Hematol.
34:1133–1142. 2006.PubMed/NCBI
|
|
30
|
Kradin RL, Xia W, Pike M, Byers HR and
Pinto C: Interleukin-2 promotes the motility of dendritic cells and
their accumulation in lung and skin. Pathobiology. 64:180–186.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bykovskaja SN, Buffo MJ, Bunker M, Zhang
H, Majors A, Herbert M, Lokshin A, Levitt ML, Jaja A, Scalise D,
Kosiban D, Evans C, Marks S and Shogan J: Interleukin-2 induces
development of denditric cells from cord blood CD34+
cells. J Leukoc Biol. 63:620–630. 1998.PubMed/NCBI
|
|
32
|
Civallero M, Barni S, Nano R and Capelli
E: Dendritic cells and interleukin-2: cytochemical and
ultrastructural study. Histol Histopathol. 15:1077–1085.
2000.PubMed/NCBI
|
|
33
|
Lynch DH, Namen AE and Miller RE: In vivo
evaluation of the effects of interleukins 2, 4 and 7 on enhancing
the immunotherapeutic efficacy of anti-tumor cytotoxic T
lymphocytes. Eur J Immunol. 21:2977–2985. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mackensen A, Lindemann A and Mertelsmann
R: Immunostimulatory cytokines in somatic cells and gene therapy of
cancer. Cytokine Growth Factor Rev. 8:119–128. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sakaguchi M, Kataoka K, Abarzua F,
Tanimoto R, Watanabe M, Murata H, Than SS, Kurose K, Kashiwakura Y,
Ochiai K, Nasu Y, Kumon H and Huh NH: Overexpression of REIC/Dkk-3
in normal fibroblasts suppresses tumor growth via induction of
interleukin-7. J Biol Chem. 284:14236–14244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iwamoto H, Ojima T, Hayata K, Katsuda M,
Miyazawa M, Iida T, Nakamura M, Nakamori M, Iwahashi M and Yamaue
H: Antitumor immune response of dendritic cells (DCs) expressing
tumor-associated antigens derived from induced pluripotent stem
cells: in comparison to bone marrow-derived DCs. Int J Cancer.
134:332–341. 2014. View Article : Google Scholar
|