Introduction
Salivary adenoid cystic carcinoma (SACC) is a unique
malignant tumor which is unpredictable in nature. The incidence
rate of SACC is ~10% of all salivary gland tumors (1–3), 22%
of all malignant salivary gland tumors (4), and 1% of all head and neck malignant
tumors (2,3). SACC occurs most commonly in minor
salivary glands and the oral cavity (1,3).
Perineural invasion (PNI), a unique pathological entity that
significantly differs from lymphatic and vascular invasion, is
considered to be an essential feature of SACC (5). PNI may also act as a source of
malignant tumor distant metastasis, and the range of PNI is much
wider than local invasion (6–8). PNI
has become an important pathological feature of many malignant
tumors, including pancreatic and prostate cancer,
cholangiocarcinoma and head and neck cancer. PNI is also proven to
be a sign of poor prognosis and a decreased survival omen for these
malignant tumors (9–13).
Epithelial-mesenchymal transition (EMT) refers to
the rapid and reversible changes in cellular morphology and
phenotype characterized by the transformation of epithelial
features into mesenchymal features (14,15).
Numerous lines of evidence have proven that EMT has the capacity of
regulating cell fate and tissue morphology (16–19).
In laboratory studies, EMT has also shown a close relationship with
the process of tumor growth and metastasis (16,18).
However, no direct evidence of EMT has been revealed in the clinic.
Thus, some pathologists have conjectured that EMT exhibits no
correlation with the development and progression of tumors
(20). It is widely acknowledged
that the narrowly defined EMT should be strictly distinguished from
an EMT-like cell phenotype for exploring tumor occurrence and
progression (21). Since EMT
changes result from the poor differentiation of tumor cells, it
seems more appropriate to use ‘EMT-like changes’ to describe the
phenotype of tumor cells in the process of tumor genesis (21). The term ‘EMT-like changes’
emphasizes the renewal of tumor cells and adaptation to a specific
microenvironment instead of a positive de-differentiation process
(21).
p53 is an essential tumor-suppressor gene, and p53
mutations play a critical role in tumor occurrence and progression
(e.g., pancreatic, prostate, and head and neck cancer) (22,23).
Our previous study showed that SACC with PNI exhibited
significantly lower p53 gene expression than SACC without PNI
(24). Chang et al found
that the p53 gene could regulate the EMT process of breast cancer
cells (25). Thus, in the present
study, we hypothesized that p53 plays a crucial role in regulating
PNI activity in SACC through a potential mechanism that is related
to ‘EMT-like changes’.
In the present study, the regulatory role of the p53
gene in SACC and the potential metastatic process was
systematically investigated. We used the RNA interference technique
to downregulate the expression of the p53 gene in SACC-83 cells (a
human SACC cell line), and then examined the changes in cell
phenotype markers, cell cycle, anti-apoptosis and the PNI
capability of SACC-83 cells. Our data showed that downregulation of
p53 gene expression promoted in vitro PNI activity of
SACC-83 cells through ‘EMT-like changes’.
Materials and methods
shRNA preparation
Four p53 shRNAs were designed according to the p53
sequence in the GenBank (NM_006500) following the rules of Tuschl
(26). As shown in Table I, p53 shRNAs contained a unique
19-nt double-stranded human p53 sequence that was presented as an
inverted complementary repeat and separated by a loop of 9-nt
spacer. DNA oligonucleotides targeting p53 were synthesized and
inserted into the linearized pGPU6/GFP/Neo shRNA expression vector
according to the manufacturer’s instructions (Table I). The recombinant vectors were
named pGGNeo-p53-homo-1043-shRNA, pGGNeo-p53-homo-1157-shRNA,
pGGNeo-p53-homo-968-shRNA and pGGNeo-p53-homo-270-shRNA. The
pGGNeo-negative-shRNA contained a nonsense shRNA insert designed
according to the p53-shRNA sequence and was checked by BLAST. The
pGGNeo-GAPDH-shRNA was a positive control vector targeting GAPDH to
test the system error.
 | Table IOligonucleotide sequences of the
p53-specific shRNAs. |
Table I
Oligonucleotide sequences of the
p53-specific shRNAs.
| Genes | Primer sequence
(5′-3′) |
|---|
|
pGGNeo-p53-homo-1043-shRNA |
GCGCACAGAGGAAGAGAATCT
AGATTCTCTTCCTCTGTGCGC |
|
pGGNeo-p53-homo-1157-shRNA |
GAAACCACTGGATGGAGAATA
TATTCTCCATCCAGTGGTTTC |
|
pGGNeo-p53-homo-968-shRNA |
GGAAGACTCCAGTGGTAATCT
AGATTACCACTGGAGTCTTCC |
|
pGGNeo-p53-homo-270-shRNA |
CTACTTCCTGAAAACAACG
CGTTGTTTTCAGGAAGTAG |
|
pGGNeo-negative-shRNA |
TTCTCCGAACGTGTCACGT
ACGTGACACGTTCGGAGAA |
|
pGGNeo-GAPDH-shRNA |
GTATGACAACAGCCTCAAG
CTTGAGGCTGTTGTCATAC |
Cell culture and shRNA transfection
SACC-83 cells are a type of human SACC cell line,
which have been widely used for the investigation of the biological
characteristics of SACC (27). The
SACC-83 cells were cultured in 6-well plates in RPMI-1640 medium
(HyClone, USA) with 10% fetal bovine serum (FBS; Invitrogen,
Carlsbad, CA, USA), 100 U/ml penicillin and 0.1 mg/ml streptomycin
at 37°C in 5% CO2. After incubation for 24 h, cells
(~70% confluency) were treated with various vectors, including
pGGNeo-p53-homo-1043-shRNA, pGGNeo-p53-homo-1157-shRNA,
pGGNeo-p53-homo-968-shRNA, pGGNeo-p53-homo-270-shRNA,
pGGNeo-negative-shRNA and pGGNeo-GAPDH-shRNA which had been
precomplexed with Lipofectamine™ 2000 (Boster, Wuhan, China).
Seventy-two hours post shRNA transfection, the cells were used for
real-time PCR, western blotting, flow cytometry and PNI
analyses.
Real-time PCR
The forward and reverse primers corresponding to the
human p53 gene were: 5′-GGTCTGGCCCCTCCTCAGCA-3′ and
5′-TGCCGCCCATGCAGGAACTG-3; total 178 bp. The mRNA of GAPDH was
amplified with forward (5′-ctcatgaccacagtccatgc-3′) and reverse
(5′-ttcagctc tgggatgacctt-3′) primers with a total product length
of 106 bp. Total RNA was extracted using TRIzol reagent
(Invitrogen) according to the manufacturer’s instructions. cDNA was
synthesized with RevertAid™ H Minus First Strand cDNA Synthesis kit
(Fermentas, Hanover, MD, USA). PCR reactions were performed in a
total of 25 μl reaction mixture (1 μl of cDNA, 4 μl of forward and
reverse primers, 12.5 μl of 2X SYBR-Green PCR Master Mix and 7.5 μl
of ddH2O). Data were analyzed with the comparative Ct
method and normalized by actin expression in each sample.
Western blot analysis
Total proteins were extracted with a protein
extraction kit (ProMab, USA). The protein extracts (30 μg/sample)
were subjected to electrophoretic separation by 8 and 10%
Tris-glycine SDS-PAGE and were transferred onto PVDF membranes
(Millipore) after being mixed with 5X loading buffer and boiled for
8 min. The PVDF membranes were blocked in Tris-buffered saline,
0.5% Tween-20 (TBST) containing 5% BSA for 2 h and incubated
overnight at 4°C with primary antibodies to p53 (1:1,000; CST),
vimentin (1:1,000), CK5 (1:1,000), N-cadherin (1:1,000), E-cadherin
(1:1,000) (all from Abcam), C-cadherin (1:1,000; PTG) and GAPDH
(1:1,000; Abcam) in TBST containing 5% BSA. Horseradish
peroxidase-conjugated anti-mouse or anti-rabbit IgG was used as the
secondary antibody. The membranes were then visualized by an ECL
chemiluminescence system (GE ImageQuant 350; GE Healthcare).
Semi-quantitative analysis was performed using the Quantity One
software (Bio-Rad). The experiments were performed in
triplicate.
Flow cytometric analysis
Seventy-two hours after transfection of
pGGNeo-p53-homo-270-shRNA or pGGNeo-negative-shRNA, SACC-83 cells,
at 2×106 for each sample, were harvested by
trypsinization and fixed in 75% pre-cold ethanol at 4°C for 24 h.
Cell pellets were re-suspended in 0.1 mg/ml propidium iodide (PI)
solution (3.8×10−2 sodium citrate, pH=7.0) and fixed in
10 mg/ml RNase solution (both from Sigma, USA) at 37°C for 30 min
in the dark. Cell cycle analyses were performed with a flow
cytometer (Beckman Coulter, Inc., Fullerton, CA, USA). The
experiments were performed in triplicate.
Apoptosis assay
Seventy-two hours after transfection of
pGGNeo-p53-homo-270-shRNA, both transfected and untransfected
SACC-83 cells, at 105–106 for each sample,
were trypsinized, collected and washed twice with pre-cold 1X PBS.
Each sample was re-suspended in 100 μl 5% Annexin V-FITC (ADL, USA)
at 37°C for 15 min in the dark and then fixed in 10 μl PI. Annexin
V-FITC and PI were measured and analyzed by flow cytometry. The
experiments were performed in triplicate.
In vitro PNI assay
The promoting effects of p53 downregulation on PNI
activity in SACC-83 cells were investigated in modified Boyden
chambers. Each Transwell invasion chamber containing polycarbonate
filters (Corning, USA) was coated on the upper surface with 60–80
μl of 3.9 μg/μl basement membrane Matrigel (BD Biosciences, USA) at
37°C for 30 min. Cells (5×104) were suspended in
Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) supplemented
with 1% FBS and added to the upper chamber. The lower chamber
contained 600 μl conditioned medium (incubating NIH3T3 cells in
serum-free DMEM for 24 h) as a chemoattractant (28,29). A
concentration of 25 ng/ml of nerve growth factor (R&D Systems,
USA) which has been proven optimal for increasing the in
vitro PNI activity by our previous study was added into the
conditioned medium (29,30). Cells were incubated at 37°C with 5%
CO2 for 12 h. Then, cells on the upper surface of the
filter were completely removed by a cotton swab. The filter was
then fixed in 95% ethanol and stained with hematoxylin. Cells that
had reached the lower surface of the filter through Matrigel were
counted under a light microscope at a magnification of ×400. We
chose eight fields of vision and counted the number of invaded
cells on the lower surface of the filter. The assays were performed
in triplicate.
Statistical analysis
All data presented here are expressed as the means ±
standard deviation (SD). Statistical analyses were performed using
computer software, Microsoft SPSS version 13.0 for Windows (SPSS,
Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) with
Tukey’s post-hoc analysis was used to determine the
difference between every two groups. P<0.05 was considered to
indicate a statistically significant result.
Results
pGGNeo-p53-homo-270-shRNA effectively
downregulates the expression level of p53 in SACC-83 cells
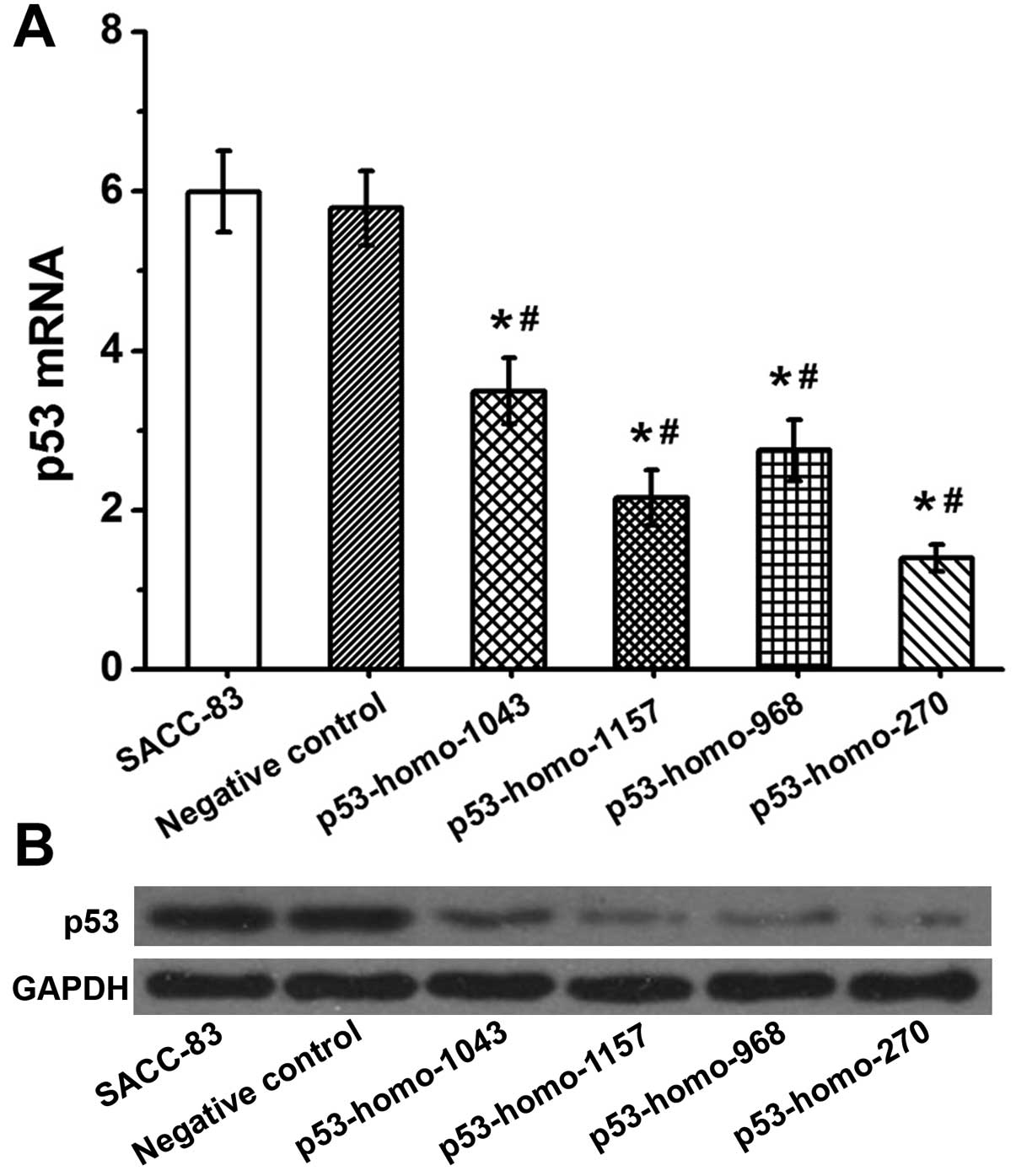
As shown in Fig. 1A,
mRNA p53 expression in the SACC-83 cells transfected with
pGGNeo-p53-homo-1043-shRNA, pGGNeo-p53-homo-1157-shRNA,
pGGNeo-p53-homo-968-shRNA and pGGNeo-p53-homo-270-shRNA p53 mRNA
was relatively decreased by 36, 62, 51 and 78% as compared with the
untransfected SACC-83 cells. Furthermore, western blot analyses
showed reduced expression of p53 proteins in SACC-83 cells
transfected with pGGNeo-p53-homo-1043-shRNA,
pGGNeo-p53-homo-1157-shRNA, pGGNeo-p53-homo-968-shRNA and
pGGNeo-p53-homo-270-shRNA (Fig.
1B). The cells transfected with pGGNeo-p53-homo-270-shRNA
showed the most significant decrease in the expression of p53
protein. These results demonstrated that p53 was downregulated most
specifically and effectively by pGGNeo-p53-homo-270-shRNA. Thus,
cells transfected with pGGNeo-p53-homo-270-shRNA were used in the
present investigation.
shRNA-mediated downregulation of p53
induces ‘EMT-like changes’ in SACC-83 cells
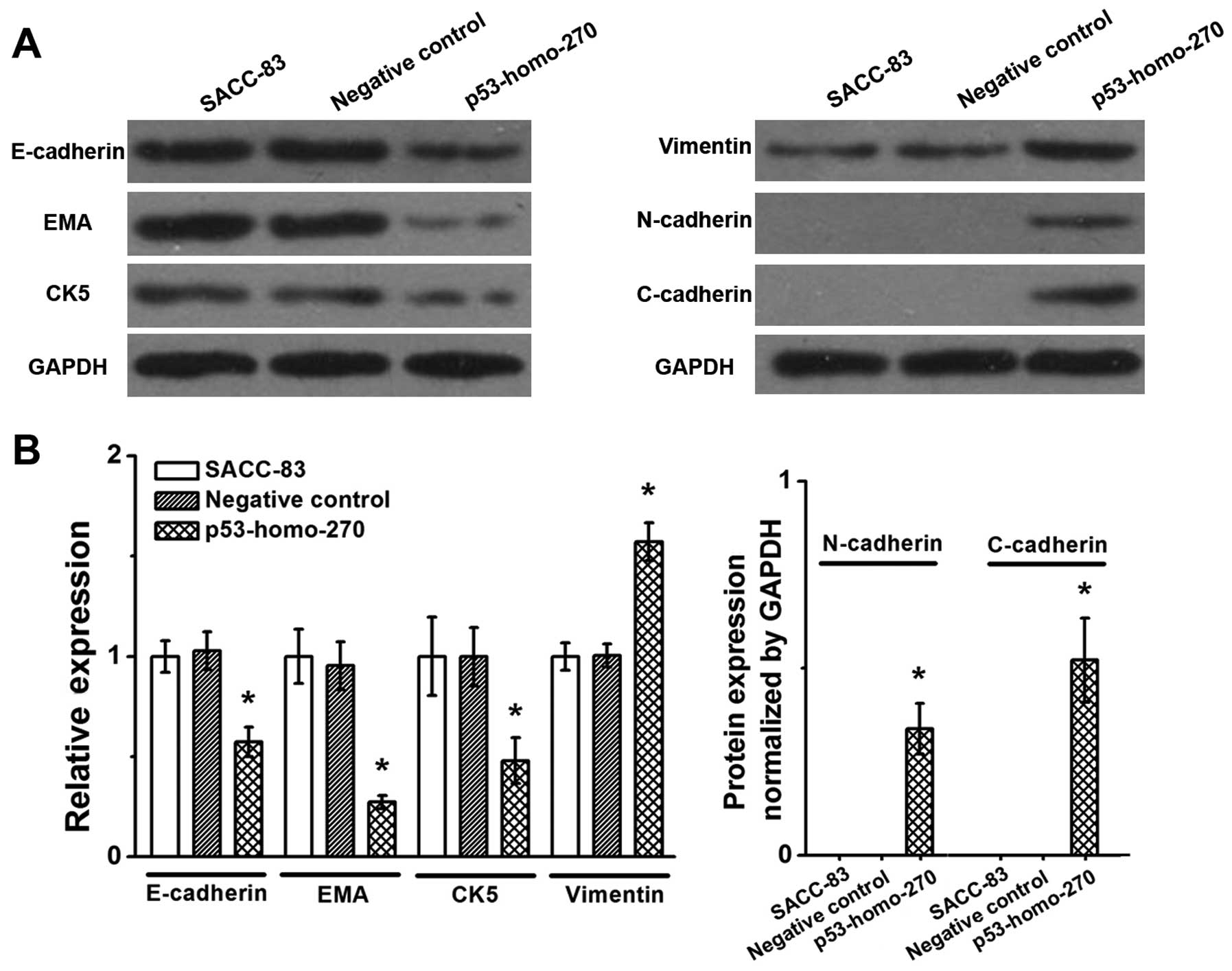
As shown in Fig. 2,
expression levels of epithelial cell markers, including E-cadherin,
EMA and CK5 were significantly decreased in the experimental group
(p53-homo-270) as compared with the negative or blank control group
(P<0.05). The experimental group also showed significant
increases in the expression levels of mesenchymal cell markers,
including vimentin, N-cadherin and C-cadherin (P<0.05). These
results suggest that pGGNeo-p53-homo-270-shRNA-mediated
downregulation of p53 induced ‘EMT-like changes’ in the SACC-83
cells.
Downregulation of p53 by
pGGNeo-p53-homo-270-shRNA increases the survival of SACC-83
cells
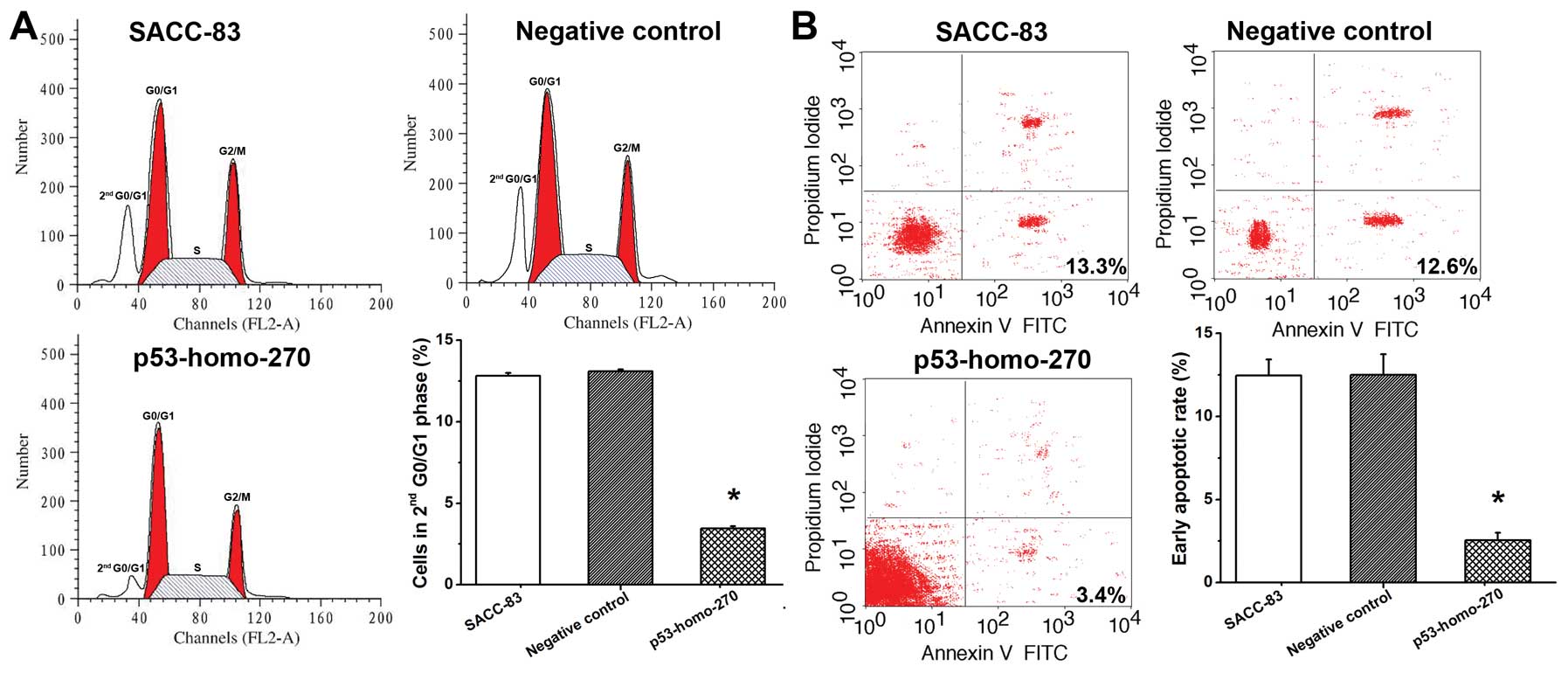
Seventy-two hours after transfection with
pGGNeo-p53-homo-270-shRNA, the cell cycles distribution of the
SACC-83 cells was examined to evaluate cell survival. Our results
showed that cells in the control and negative control group
resulted in cycling of ~12 and 13% of cells in the second G0/G1
phase, respectively (Fig. 3A). The
percentage of cells in the second G0/G1 phase was decreased to ~3%
in the experimental group (Fig.
3A). These data suggest that downregulation of p53 promoted the
anti-apoptotic ability of SACC-83 cells by modulating the second
G0/G1 cell cycle regulator. As shown in Fig. 3B, the early apoptotic rates in the
control and negative control group were 13.3 and 12.6%,
respectively. In the experimental group, the early apoptotic rate
was significantly reduced to 3.4% (Fig.
3B). These findings were consistent with the cell cycle
results, indicating that downregulation of p53 promoted the
anti-apoptotic ability and thus increased cell survival of SACC-83
cells.
Downregulation of p53 by
pGGNeo-p53-homo-270-shRNA increases in vitro PNI of SACC-83
cells
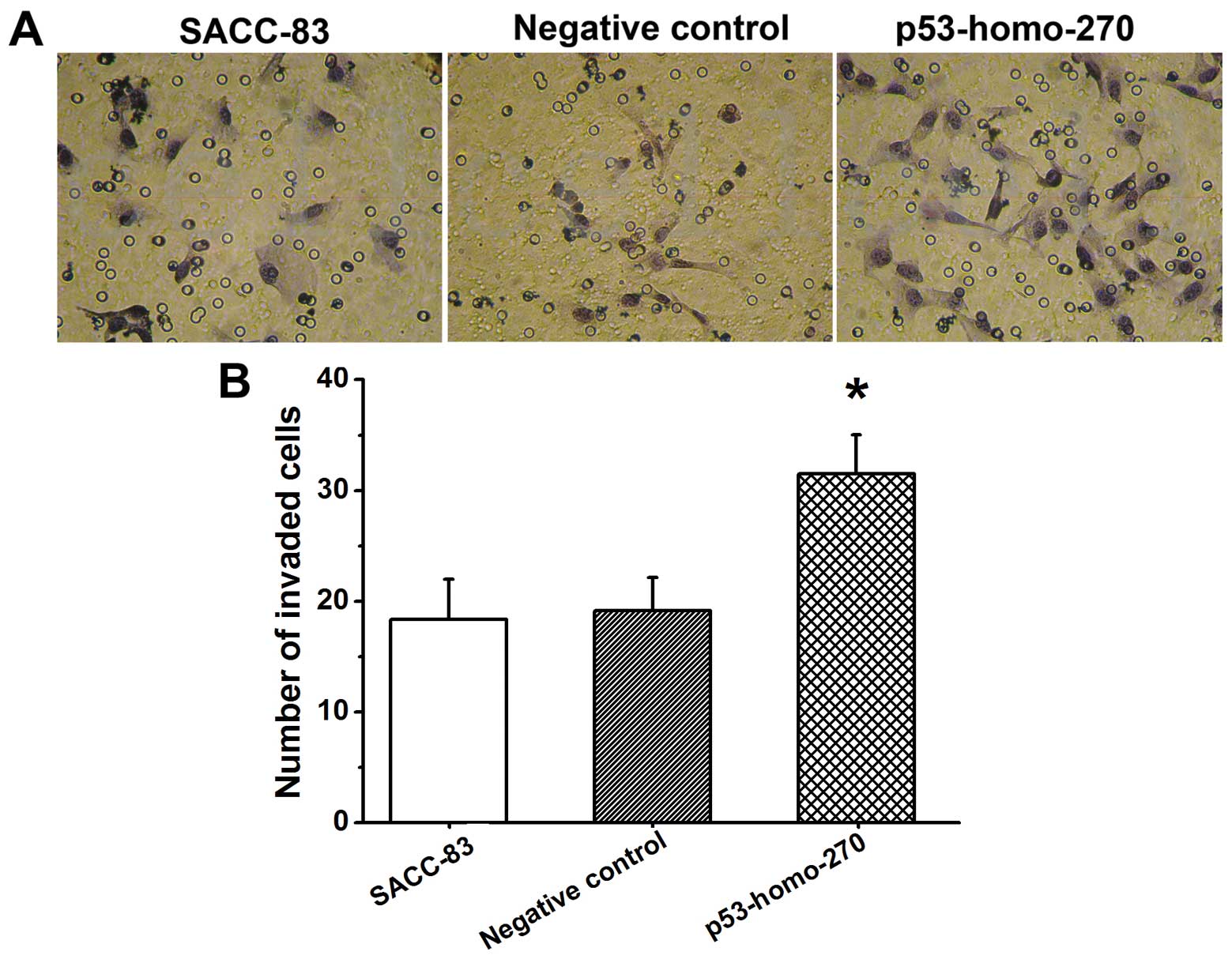
PNI activity of the SACC-83 cells transfected with
pGGNeo-p53-homo-270-shRNA was assayed in vitro using
modified Boyden chambers. As compared with the control group
(SACC-83) and negative control group, SACC-83 cells in the
experimental group (p53-homo-270) showed significantly higher PNI
activity (P<0.01) (Fig. 4).
These findings indicate that the downregulation of p53 promoted the
PNI activity of SACC-83 cells.
Discussion
p53 is a recognized tumor-suppressor gene, located
on the short arm of human chromosome 17p13 which shows loss of
heterozygosity (LOH) status in most tumors. The p53 gene can
activate the transcription of cyclin-dependent kinase inhibitor
p21, and then induce G1 phase arrest of tumor cells cycling through
p21. This process has been investigated and widely recognized in
previous research (31). The p53
gene can also inhibit malignant growth by promoting the apoptosis
of tumor cells (32). Many studies
have also confirmed that the p53 gene can inhibit the process of
malignant transformation and malignant proliferation of tumor cells
(33–37).
EMT is considered to be closely related to tumor
development, malignant tumor local invasion and distant metastasis
(15–20). Generally, tumor cells always exhibit
poor differentiation, which may result in various differentiation
errors (e.g., EMT) during the tumor cell differentiation process.
Thus, it seems more appropriate to use ‘EMT-like changes’ to
describe the tumor cell phenotype in the process of tumor genesis
(22). In addition, ‘EMT-like
changes’ also stimulate other properties of tumor cells during
tumor progression, such as enhanced migration activity, elevated
anti-apoptotic capability and increased invasive activity (21,22).
The present study focused on depicting the regulatory mechanisms of
‘EMT-like changes’ and clarifying the role of ‘EMT-like changes’ in
tumor development and metastasis. To our knowledge, this is the
first report to investigate the relationship between ‘EMT-like
changes’ and PNI. The regulatory role of the p53 gene in EMT or
‘EMT-like changes’ also remains poorly understood. Chang et
al reported that p53 acted as a key factor in the regulation of
the EMT process of breast cancer (25). Considering that the mutation rate of
the p53 gene in head and neck cancer is high (50–70%), we
hypothesized in the present study that downregulation of p53 may
induce ‘EMT-like changes’ in SACC and thus enhance its PNI
activity.
In the present study, we used the RNA interference
technique to reduce p53 gene expression in SACC-83 cells, and
subsequently detected alterations in ‘EMT-like change’ markers. We
found that pGGNeo-p53-homo-270-shRNA effectively reduced the
expression of p53. Our western blot analyses demonstrated that
downregulation of the p53 gene in SACC-83 cells caused
significantly reduced expression levels of epithelial cell markers
(E-cadherin, CK5 and EMA), and significantly increased expression
levels of mesenchymal cell markers (vimentin, N-cadherin and
C-cadherin). These results revealed obvious ‘EMT-like changes’ and
suggest that downregulation of p53 induced ‘EMT-like changes’ in
the SACC-83 cells.
Seventy-two hours after transfection with
pGGNeo-p53-homo-270-shRNA, we examined the cell cycle distribution
of SACC-83 cells with ‘EMT-like changes’. We found that the number
of SACC-83 cells in the second G0/G1 phase cell cycle was
significantly reduced, indicating that downregulation of p53
promoted the anti-apoptotic activity of SACC-83 cells. Furthermore,
the Annexin V-FITC and PI apoptosis experiment was performed to
evaluate the anti-apoptotic activity of SACC-83 cells with
‘EMT-like changes’. Our results showed that the number of early
apoptotic cells was significantly decreased. Thus, these findings
suggest that ‘EMT-like changes’ induced by p53 downregulation could
increase the anti-apoptotic activity of SACC-83 cells and thus
promote cell survival. We also examined the in vitro PNI
capacity of SACC-83 cells with ‘EMT-like changes’. Our results
indicated that ‘EMT-like changes’ induced by p53 gene
downregulation enhanced the in vitro PNI capability of the
SACC-83 cells. Together, these findings in the present study
revealed that downregulation of the p53 gene induced ‘EMT-like
changes’ in SACC-83 cells which increased the in vitro PNI
activity in these cells.
SACC is a common malignant salivary gland tumor
characterized by PNI. PNI has an important impact on clinical
treatment and prognosis. Therefore, it is imperative to study the
mechanism of PNI of SACC, thus providing a potentially new strategy
for the clinical treatment of SACC. Since a replication-defective
p53 gene virus (Ad5CMV P53) was reported in 1994 (38), p53-based gene therapy for tumors has
gained extensive attention. Roth et al directly injected a
retroviral vector containing the wild-type p53 gene into patients
with non-small cell lung cancer under control of the actin promoter
and achieved satisfactory therapeutic effects (39). Moreover, many clinical trials
regarding p53 have also been completed, including its application
in esophageal cancer (40). Several
trials have reached the third stage, yet p53 gene therapy has not
yet received final approval from the FDA (41). In the present study, we found that
the downregulation of the p53 gene could induce ‘EMT-like changes’
in SACC-83 cells, and thus these ‘EMT-like changes’ promoted in
vitro PNI activity of SACC-83 cells. Our findings suggest that
the p53 gene may be used as a new target gene for the clinical
treatment of SACC.
Acknowledgements
The present study was sponsored by the National
Natural Science Foundation of China (grant nos. 81072230 and
30772428).
References
|
1
|
Khan AJ, DiGiovanna MP, Ross DA, Sasaki
CT, Carter D, Son YH and Haffty BG: Adenoid cystic carcinoma: a
retrospective clinical review. Int J Cancer. 96:149–158. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chummun S, McLean NR, Kelly CG, Dawes PJ,
Meikle D, Fellows S and Soames JV: Adenoid cystic carcinoma of the
head and neck. Br J Plast Surg. 54:476–480. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wiseman SM, Popat SR, Rigual NR, Hicks WL
Jr, Orner JB, Wein RO, McGary CT and Loree TR: Adenoid cystic
carcinoma of the paranasal sinuses or nasal cavity: a 40-year
review of 35 cases. Ear Nose Throat J. 81:510–517. 2002.PubMed/NCBI
|
|
4
|
Hotte SJ, Winquist EW, Lamont E, MacKenzie
M, Vokes E, Chen EX, Brown S, Pond GR, Murgo A and Siu LL: Imatinib
mesylate in patients with adenoid cystic carcinoma of the salivary
glands expressing c-kit: a Princess Margaret Hospital phase II
consortium study. J Clin Oncol. 23:585–590. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fordice J, Kershaw C, El-Naggar A and
Goepfert H: Adenoid cystic carcinoma of the head and neck:
predictors of morbidity and mortality. Arch Otolayrngol Head Neck
Surg. 125:149–152. 1999. View Article : Google Scholar
|
|
6
|
Batsakis JG: Nerves and neurotropic
carcinomas. Ann Otol Rhinol Laryngol. 94:426–427. 1985.PubMed/NCBI
|
|
7
|
Hassan MO and Maksem J: The prostatic
perineural space and its relation to tumor spread: an
ultrastructural study. Am J Surg Pathol. 4:143–148. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodin AE, Larson DL and Roberts DK: Nature
of the perineural space invaded by prostatic carcinoma. Cancer.
20:1772–1779. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ozaki H, Hiraoka T, Mizumoto R, Matsuno S,
Matsumoto Y, Nakayama T, Tsunoda T, Suzuki T, Monden M, Saitoh Y,
Yamauchi H and Ogata Y: The prognostic significance of lymph node
metastasis and intrapancreatic perineural invasion in pancreatic
cancer after curative resection. Surg Today. 29:16–22. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Law WL and Chu KW: Anterior resection for
rectal cancer with mesorectal excision: a prospective evaluation of
622 patients. Ann Surg. 240:260–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beard CJ, Chen MH, Cote K, Loffredo M,
Renshaw AA, Hurwitz M and D’Amico AV: Perineural invasion is
associated with increased relapse after external beam radiotherapy
for men with low-risk prostate cancer and may be a marker for
occult, high-grade cancer. Int J Radiat Oncol Biol Phys. 58:19–24.
2004. View Article : Google Scholar
|
|
12
|
Su CH, Tsay SH, Wu CC, Shyr YM, King KL,
Lee CH, Lui WY, Liu TJ and P’eng FK: Factors influencing
postoperative morbidity, mortality, and survival after resection
for hilar cholangiocarcinoma. Ann Surg. 223:384–394. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duraker N, Sişman S and Can G: The
significance of perineural invasion as a prognostic factor in
patients with gastric carcinoma. Surg Today. 33:95–100. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Savagner P: Leaving the neighborhood:
molecular mechanisms involved during epithelial-mesenchymal
transition. Bioessays. 23:912–923. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jechlinger M, Grunert S, Tamir IH, Janda
E, Lüdemann S, Waerner T, Seither P, Weith A, Beug H and Kraut N:
Expression profiling of epithelial plasticity in tumor progression.
Oncogene. 22:7155–7169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi Y and Massagué J: Mechanisms of TGF-β
signaling from cell membrane to the nucleus. Cell. 113:685–700.
2003. View Article : Google Scholar
|
|
18
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: new insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hennessy BT, Gonzalez-Angulo AM,
Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J,
Sahin A, Agarwal R, Joy C, Liu W, Stivers D, Baggerly K, Carey M,
Lluch A, Monteagudo C, He X, Weigman V, Fan C, Palazzo J,
Hortobagyi GN, Nolden LK, Wang NJ, Valero V, Gray JW, Perou CM and
Mills GB: Characterization of a naturally occurring breast cancer
subset enriched in epithelial-to-mesenchymal transition and stem
cell characteristics. Cancer Res. 69:4116–4124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tarin D, Thompson EW and Newgreen DF: The
fallacy of epithelial mesenchymal transition in neoplasia. Cancer
Res. 65:5996–6001. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klymkowsky MW and Savagner P:
Epithelial-mesenchymal transition: a cancer researcher’s conceptual
friend and foe. Am J Pathol. 174:1588–1593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Greenblatt MS, Bennett WP, Hollstein M and
Harris CC: Mutations in the p53 tumor suppressor gene: clues to
cancer etiology and molecular pathogenesis. Cancer Res.
54:4855–4878. 1994.PubMed/NCBI
|
|
23
|
Brosh R and Rotter V: When mutants gain
new powers: news from the mutant p53 field. Nat Rev Cancer.
9:701–713. 2009.PubMed/NCBI
|
|
24
|
Chen W, Zhang HL, Shao XJ, Jiang YG, Zhao
XG, Gao X, Li JH, Liu BL and Sun MY: Gene expression profile of
salivary adenoid cystic carcinoma associated with perinerual
invasion. Tohoku J Exp Med. 212:319–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang CJ, Chao CH, Xia W, Yang JY, Xiong
Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH and Hung MC: p53
regulates epithelial-mesenchymal transition and stem cell
properties through modulating miRNAs. Nat Cell Biol. 13:317–323.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tuschl T: Expanding small RNA
interference. Nat Biotechnol. 20:446–448. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong L, Ge XY, Wang YX, Yang LQ, Li SL, Yu
GY, Gao Y and Fu J: Transforming growth factor-β and
epithelial-mesenchymal transition are associated with pulmonary
metastasis in adenoid cystic carcinoma. Oral Oncol. 49:1051–1058.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zou W, Yang H, Hou X, Zhang W, Chen B and
Xin X: Inhibition of CD147 gene expression via RNA interference
reduces tumor cell invasion, tumorigenicity and increase
chemosensitivity to paclitaxel in HO-8910pm cells. Cancer Lett.
248:211–218. 2007. View Article : Google Scholar
|
|
29
|
Chen W, Zhang HL, Jiang YG, Li JH, Liu BL
and Sun MY: Inhibition of CD146 gene expression via RNA
interference reduces in vitro perineural invasion on ACC-M cells. J
Oral Pathol Med. 38:198–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang L, Sun M, Jiang Y, Yang L, Lei D, Lu
C, Zhao Y, Zhang P, Yang Y and Li J: Nerve growth factor and
tyrosine kinase A in human salivary adenoid cystic carcinoma:
expression patterns and effects on in vitro invasive behavior. J
Oral Maxillofac Surg. 64:636–641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Giono LE and Manfredi JJ: The p53 tumor
suppressor participates in multiple cell cycle checkpoints. J Cell
Physiol. 209:13–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen L, Park SM, Tumanov AV, Hau A, Sawada
K, Feig C, Turner JR, Fu YX, Romero IL, Lengyel E and Peter ME:
CD95 promotes tumour growth. Nature. 465:492–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eliyahu D, Michalovitz D, Eliyahu S,
Pinhasi-Kimhi O and Oren M: Wild-type p53 can inhibit
oncogene-mediated focus formation. Proc Natl Acad Sci USA.
86:8763–8767. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baker SJ, Markowitz S, Fearon ER, Willson
JK and Vogelstein B: Suppression of human colorectal carcinoma cell
growth by wild-type p53. Science. 249:912–915. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Diller L, Kassel J, Nelson CE, Gryka MA,
Litwak G, Gebhardt M, Bressac B, Ozturk M, Baker SJ, Vogelstein B,
et al: p53 functions as a cell cycle control protein in
osteosarcomas. Mol Cell Biol. 10:5772–5781. 1990.PubMed/NCBI
|
|
36
|
Michalovitz D, Halevy O and Oren M:
Conditional inhibition of transformation and of cell proliferation
by a temperature-sensitive mutant of p53. Cell. 62:671–680. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martinez J, Georgoff I, Martinez J and
Levine AJ: Cellular localization and cell cycle regulation by a
temperature-sensitive p53 protein. Genes Dev. 5:151–159. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang WW, Fang X, Mazur W, French BA,
Georges RN and Roth JA: High-efficiency gene transfer and
high-level expression of wild-type p53 in human lung cancer cells
mediated by recombinant adenovirus. Cancer Gene Ther. 1:5–13.
1994.PubMed/NCBI
|
|
39
|
Roth JA, Nguyen D, Lawrence DD, Kemp BL,
Carrasco CH, Ferson DZ, Hong WK, Komaki R, Lee JJ, Nesbitt JC,
Pisters KM, Putnam JB, Schea R, Shin DM, Walsh GL, Dolormente MM,
Han CI, Martin FD, Yen N, Xu K, Stephens LC, McDonnell TJ,
Mukhopadhyay T and Cai D: Retrovirus-mediated wild-type p53 gene
transfer to tumors of patients with lung cancer. Nat Med.
2:985–991. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shimada H, Matsubara H, Shiratori T,
Shimizu T, Miyazaki S, Okazumi S, Nabeya Y, Shuto K, Hayashi H,
Tanizawa T, Nakatani Y, Nakasa H, Kitada M and Ochiai T: Phase I/II
adenoviral p53 gene therapy for chemoradiation resistant advanced
esophageal squamous cell carcinoma. Cancer Sci. 97:554–561. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brown CJ, Lain S, Verma CS, Fersht AR and
Lane DP: Awakening guardian angels: drugging the P53 pathway. Nat
Rev Cancer. 9:862–873. 2009. View Article : Google Scholar : PubMed/NCBI
|