Introduction
Gastric cancer is one of the most common malignant
tumors worldwide, and its incidence is fourth among malignant
tumors, ranking second in the leading causes of death (1). Surgical resection was the standard
treatment for early gastric cancer. In China, the majority of
patients are diagnosed at an advanced stage, representing lost
opportunities for radical surgery. Systemic chemotherapy is the
main treatment for such patients (2). Traditional chemotherapy for gastric
cancer includes large doses of cytotoxic drugs that last 2–3 weeks.
However, although these agents have a tumor suppressive effect,
they are accompanied by heavy toxic side effects. In addition,
their high-dose and intermittent-dosing patterns make remnants of
tumor cells grow between chemotherapeutic intervals, inducing
acquired drug resistance and resulting in treatment failure
(3). To avoid problems associated
with traditional chemotherapy, attempts have been made to switch
from ‘maximum tolerable dose (MTD)’ to ‘minimal effective dose
(MED)’ as a new delivery mode to reduce toxicity and for
synergistic purposes.
Bertolini et al (3) demonstrated ‘metronomic chemotherapy’
as a mode of drug administration with sustainability, using
low-dose high-frequency cytotoxic drugs (4). In breast, ovarian, prostate, and
colorectal cancer animal models and clinical trials, metronomic
chemotherapy was demonstrated to have superior efficacy to MTD, and
the side effects were significantly less than those of traditional
chemotherapy (5–7). Preclinical studies confirmed that
metronomic chemotherapy selectively affected tumor-derived vascular
endothelia and angiogenesis (8,9).
However, whether metronomic chemotherapy exert an anti-angiogenesis
effect has not yet been reported. Notably, the VEGF-neutralizing
antibody bevacizumab was unsuccessful as a first-line treatment for
gastric cancer in two large clinical studies, while the anti-VEGFR
monoclonal antibody ramucizumab demonstrated positive results
during and after second-line treatment for gastric cancer,
indicating that anti-angiogenesis was the key factor affecting
treatment success or failure (10,11).
Fluorouracil (Fu)-based chemotherapy has been widely
used as chemotherapy for gastric cancer (12). Large clinical studies of REAL-2 and
ML17032 demonstrated the value of oral Fu drugs, including
capecitabine, as a first-line treatment of gastric cancer (13,14).
The CLASSIC and ARTIST studies demonstrated the value of
postoperative adjuvant chemotherapy (radiotherapy) for gastric
carcinoma (15,16). In addition, oral administration more
easily accommodated smaller doses, as compared to high-frequency
‘metronomic chemotherapy’. A preliminary study from our research
group found that metronomic capecitabine inhibited cell
proliferation in colon cancer cells, and its tumor suppressor
effect was achieved by anti-angiogenesis (9).
The aim of the present study was to evaluate whether
capecitabine chemotherapy with a metronomic pattern may play an
inhibitory role in the growth of gastric cancer cells in
vitro and in vivo and whether anti-angiogenesis was
involved in this inhibitory effect.
Materials and methods
Ethics statement
The human gastric cancer cell lines and human
umbilical vein endothelial cells (HUVEC) were conserved by the
Shanghai Institute of Digestive Surgery as described previously
(9,17). The use of these cell lines was
approved by the Ethics Committee of Ruijin Hospital, Shanghai
Jiaotong University School of Medicine. The mouse experiments were
approved by the Animal Care and Use Committee and conducted in
accordance with the Guide for the Care and Use Laboratory Animals
of Ruijin Hospital, Shanghai Jiaotong University School of Medicine
(Shanghai, China).
Cell culture
GES-1, SNU-1, SNU-16, MKN-45, KATOIII, BGC-823, AGS,
SGC-7901, NCI-N87, MGC-803, MKN-28 and HUVEC human gastric cancer
cell lines were cultured in RPMI-1640 medium (Gibco-BRL, Carlsbad,
CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL)
at 37°C with 5% CO2 and saturated humidity.
Western blot analysis
Total protein was extracted by using the Mammalian
Protein Extraction reagent (Pierce, Rockford, IL, USA) mixed with
protease inhibitor cocktail (Sigma, St. Louis, MO, USA). Samples
with an equal amount of total protein (100 μg) were fractionated by
10% SDS-PAGE and transferred to PVDF (Bio-Rad, Hercules, CA, USA),
membranes. The PVDF membranes were blocked by TBST buffer with 5%
skim milk for 2 h, and incubated at 4°C with primary antibodies
overnight, TP and TS antibodies (1:1,000; both from Abcam,
Cambridge, MA, USA). Fluorescent secondary antibodies (1:15,000;
Li-COR Biosciences, Lincoln, NE, USA) and Infrared Imaging System
(Li-COR Biosciences) were used to visualize the protein bands of
interest.
CCK-8 cell proliferation assay
The logarithmic growth of SGC-7901 and AGS cells was
seeded at 1×103 cells/well in a 96-well plate and
incubated overnight with different concentrations of 5-Fu (Shanghai
Xudong Haipu Pharmaceutical Co., Ltd., Shanghai, China) (5, 10, 20,
40, 80, 160, 320, and 640 μg/l) which were added and cultured for 5
days. The drug-containing culture medium was replaced every 24 h.
The OD450 values were detected every 24 h using Cell
Counting kit-8 (Dojindo, Kumamoto, Japan) and the inhibitory rate
was calculated. IC25 and IC50 values of 5-Fu
for the SGC-7901 and AGS cells were calculated according to a 48-h
inhibitory rate for the conventional in vitro and metronomic
doses, respectively. Based on the results, different doses of 5-Fu
(standard dose, 20 μg/l once; metronomic chemotherapy dose, 5 μg/l
per day) were used to treat the gastric cancer cells for 5 days,
and the effects of 5-Fu metronomic chemotherapy on the
proliferation of gastric cancer cells in vitro were
observed. Each concentration was repeated three times.
Analysis of apoptosis by flow
cytometry
The cell density was adjusted to 1×105
cells/well in a 24-well plate, and standard and metronomic doses of
5-Fu (20 or 5 μg/l) were administered. The cells were collected
after 24 hand-stained with FITC-conjugated Annexin V (BD
Biosciences, Bedford, MA, USA). Propidium iodide (BD Biosciences)
was subsequently added, and a flow cytometric analysis was
performed using FACS (Becton-Dickinson, Bedford, MA, USA).
HUVEC tube formation assay
Matrigel (100 μl) was added to each well in a
96-well plate, until the glue completely solidified, and 5×10
HUVECs were then added to each well. Standard and metronomic doses
of 5-Fu (20 or 5 μg/l) were used to treat the SGC-7901 and AGS cell
culture supernatants (200 μl were added, respectively). The cells
were incubated at 37°C with 5% CO2 for 8 h, and
non-adherent cells were eluted with PBS. Under high magnification,
three randomly-selected images were assessed for the following: the
number of tubular forms, total length, and the number of connection
points. Each concentration was repeated three times.
Angiogenic factors detection by
enzyme-linked immunosorbent assay
The SGC-7901 and AGS cells were seeded in a 24-well
plate (1×104 cells/well). After cell adherence, the
suspension was centrifuged, and the supernatant was discarded.
Standard and metronomic doses of 5-Fu (20 or 5 μg/l) were added,
and the cells were cultured at 37°C with 5% CO2 for 8 h.
The cell culture supernatant was then collected for VEGF (MAB293;
R&D Systems Inc., Minneapolis, MN, USA) and PDGF (900-K04;
PeproTech, Rocky Hill, NJ, USA) detection using an ELISA kit.
Establishment of gastric cancer
xenografts and tissue collection
Male Balb/c nude mice, 4–6 weeks of age, with a body
weight of 15–20 g, were provided by the Research Center of
Experimental Medicine, Shanghai Jiaotong University School of
Medicine Affiliated Ruijin Hospital. Prior to performing the
experiment, the animals were placed in separate cages for 1 week to
adapt to the new environment. The SGC-7901 cell suspension was
adjusted to a cell density of 1×107/ml, and the nude
mice were subcutaneously inoculated with a 100-μl suspension.
Administration of the therapy was initiated when the subcutaneous
nodules were ~2 mm in diameter. The nude mice were randomly divided
into the following groups: i) control group, intraperitoneally
injected with normal saline (NS); ii) 5-Fu conventional dose group
(5-Fu MTD group), intraperitoneally injected with 50 mg/kg, twice
per week for 2 weeks, with an 1-week discontinuation for 6 weeks
(18); iii) 5-Fu metronomic group
(5-Fu LDM group), intraperitoneally injected with 15 mg/kg, twice a
week for 6 weeks; iv) capecitabine (Roche Company, Shanghai, China)
conventional dose (capecitabine MTD group), IG 500 mg/kg, twice per
week for 2 weeks, with a 1-week discontinuation for 6 weeks
(19); and v) capecitabine
metronomic group (capecitabine LDM group), intragastric
administration at 200 mg/kg, twice a week for 6 weeks. From the
beginning of drug administration, a Vernier caliper was used to
measure the tumor length/diameter (L) and short track (W) every 7
days to calculate the tumor volume (V) according to the following
formula: V = (W + L)/(2 × W × L × 0.5236). A tumor growth curve was
drawn. The nude mice were executed after 6 weeks of treatment, and
the tumor tissue and peripheral blood samples were collected.
Immunohistochemical staining (IHC)
The nude mouse transplantation tumor was fixed with
10% formaldehyde. After HE staining for tumor confirmation, the
immunohistochemical staining was performed on 4-μm sections
following the EnVision two-step procedure of Dako REAL™ Envision™
Detection system (Dako, Denmark). The slides were incubated with
the primary antibodies for Ki-67 (1:50; Dako), CD34 (1:150; Abcam)
and VEGF (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), respectively. The HRP-labeled antibody to rabbit and mouse
immunoglobulin was used as secondary antibodies. The slides were
visualized by diaminobenzidine under a microscope. For selection of
three high-power fields to count for microvascular density (MVD),
the staining result criteria were as follows: a tumor with
yellowish-brown granules in the cell nucleus was positive for Ki-67
staining; a tumor with brownish-yellow granules was positive for
VEGF staining; and a tumor with brown particles in the cell plasma
was positive for CD34 staining (20).
Measurement of circulating endothelial
progenitors (CEPs) by flow cytometry
The peripheral blood cells in the nude mice were
treated with an EDTA anticoagulant (BD Biosciences). Following
erythrocyte lysis, the dead cells, platelets, and cell debris were
washed away. FCS500 MC (BD Biosciences) was used to detect cell
suspension. The monoclonal antibodies CD117, Flk-1 (VEGFR-2), and
Sca-1 (eBioscience, San Diego, CA, USA) were used to identify CEP
subsets. From each specimen, we obtained at least 5×104
cells. With the CEP counts, we collected enough cells in the window
(>50, typically 100–200) to determine the percentage of stained
cells.
Statistical analysis
The SPSS software (v13.0; SPSS Inc., Chicago, IL,
USA) was used for statistical analysis. Data are presented as mean
± standard deviation, and differences between the groups were
compared using ANOVA and the Mann-Whitney U test. P<0.05 was
considered significant.
Results
Effects of metronomic chemotherapy on the
proliferation of gastric cancer cells in vitro
The antitumor effects of capecitabine partly depend
on TP enzymes, which inside the cells convert 5-Fu precursor to
5-Fu and plays a role for the target enzyme of TS. Therefore, the
AGS and SGC-7901 gastric cancer cell lines, which have a high
expression of TP and a low expression of TS were screened by
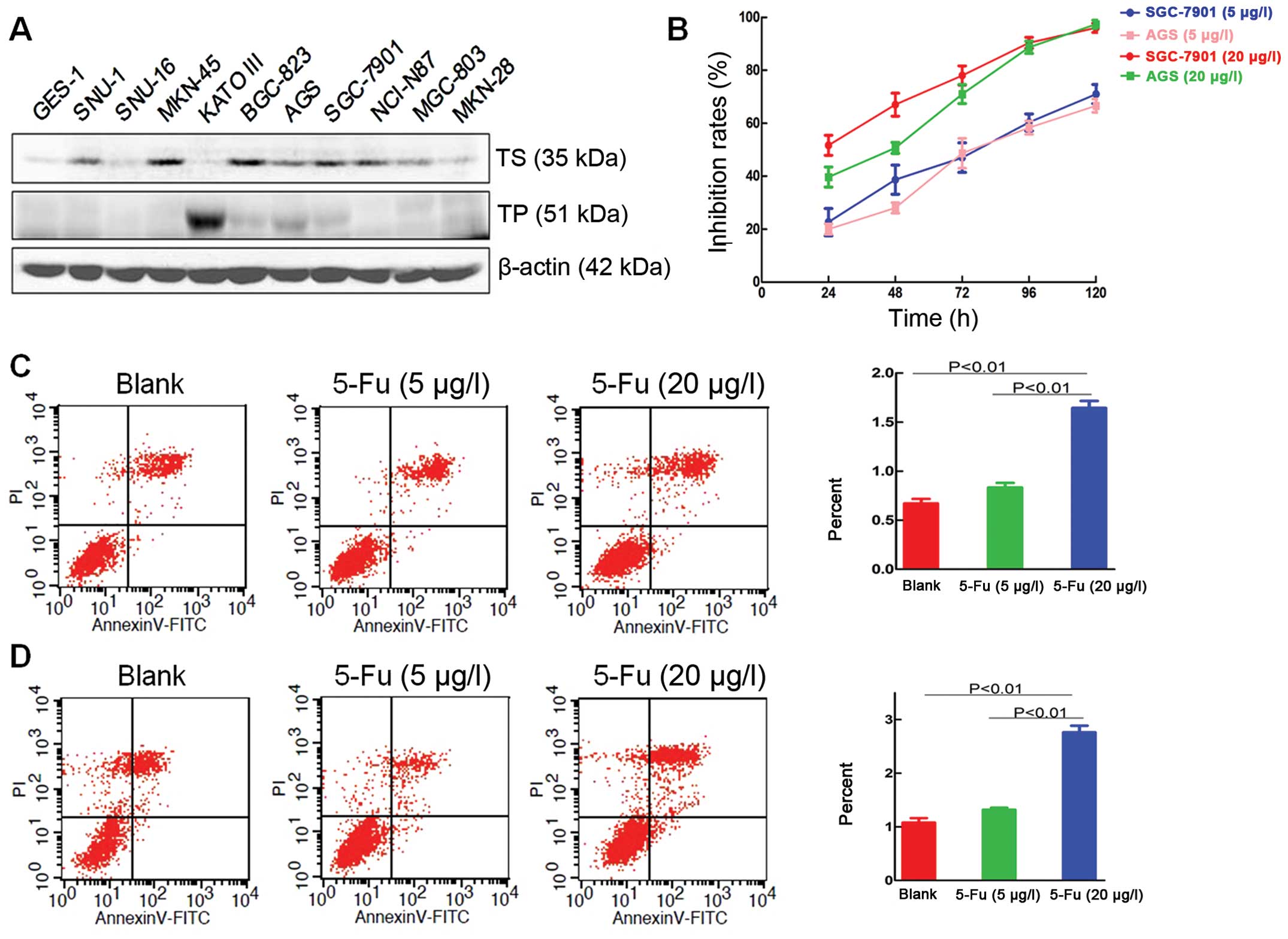
western blot analysis for subsequent experiments (Fig. 1A). The proliferation inhibitory
rates of 5-Fu at 48 h, as well as the IC25 and
IC50 of 5-Fu, for SGC-7901 and AGS were calculated
(Table I). The in vitro
concentrations of the conventional and metronomic doses were
confirmed (20 and 5 μg/l, respectively).
 | Table IIC25 and IC50
of 5-Fu for gastric cancer cells. |
Table I
IC25 and IC50
of 5-Fu for gastric cancer cells.
| Gastric cancer
cells | IC25
(μg/l) | IC50
(μg/l) |
|---|
| SGC-7901 | 6.71±0.83 | 18.59±1.27 |
| AGS | 5.37±0.73 | 16.45±1.22 |
The cell proliferation results showed that the 5-Fu
metronomic dose significantly inhibited proliferation of the
SGC-7901 and AGS gastric cancer cell lines in vitro, as
compared with the control group, and after 5 days, the inhibitory
rates in the SGC-7901 and AGS groups were 71±4.0 and 66.6±2%,
respectively. At 5 days, the 5-Fu conventional dose had inhibitory
rates that were close to 100% in the SGC-7901 and AGS groups, and
almost all the cells were dead (Fig.
1B).
Effect of metronomic chemotherapy on the
apoptosis of gastric cancer cells in vitro
Compared with the SGC-7901 control group
(0.67±0.08%), the apoptotic index for the 5-Fu metronomic group was
0.83±0.08% (P=0.087), and the apoptotic index for the 5-Fu
conventional dose group was 1.64±0.13% (P<0.01, Fig. 1C).
In the control AGS cells, the apoptotic index was
1.08±0.15%, and in the 5-Fu metronomic group, the apoptotic index
was 1.32±0.07% (P=0.105). The apoptotic index was 2.76±0.21% for
the 5-Fu conventional dose group (P<0.01; Fig. 1D). These results indicated that a
conventional dose of 5-Fu promoted the apoptosis of gastric cancer
cells in vitro. However, metronomic chemotherapy had no
effect on the apoptosis of gastric cancer cells in vitro.
Different doses of 5-FU induced the apoptosis of gastric cancer
cells in different ways. The standard doses showed a direct
cytotoxic effect on gastric cancer cells, while metronomic
chemotherapy inhibited the proliferation of gastric cancer cells
in vitro without any effect on cell apoptosis.
Effect of metronomic chemotherapy on the
HUVEC tube formation ability in vitro
Image-Pro Plus Professional software was used to
measure the number of small tubes, total length, and number of
connection points between HUVEC tubes. Compared with those in the
control group, the number and total length of the small tubes, as
well as the number of tubular junctions, in the 5-Fu metronomic and
conventional dose groups were significantly reduced (P<0.01).
Compared with the 5-Fu conventional dose group, the number and
total length of the small tubes, as well as the number of tubular
junction numbers in the 5-Fu metronomic group were significantly
reduced (P<0.01; Fig. 2A and B,
Table II). This indicated that
5-Fu significantly inhibited HUVEC tube formation ability in
vitro, and the inhibitory effect of 5-Fu metronomic was
evident.
 | Table IIHUVEC tubule formation ability in
vitro. |
Table II
HUVEC tubule formation ability in
vitro.
| SGC-7901 | AGS |
|---|
|
|
|
|---|
| Variables | Blank | 5-Fu (5 μg/l) | 5-Fu (20 μg/l) | Blank | 5-Fu (5 μg/l) | 5-Fu (20 μg/l) |
|---|
| No. of tubules | 45.7±2.5 | 5.0±1.0 | 14.3±1.5 | 47.0±3.6 | 4.3±0.6 | 16.3±2.5 |
| Tubule length
(mm) | 496.9±8.1 | 63.4±10.1 | 177.2±20.7 | 511.8±23.3 | 69.5±6.8 | 210.5±12.4 |
| No. of
intersections | 42.3±1.2 | 7.7±2.1 | 17.7±2.5 | 48.7±3.8 | 7.3±0.6 | 18.7±2.5 |
Effect of metronomic chemotherapy on the
secretory angiogenesis-related factors of gastric cancer cells
The ELISA results showed that the VEGF concentration
in the SGC-7901 cell culture supernatants in the control group was
605.07±10.75 pg/ml, while the VEGF concentrations were 411.53±28.79
pg/ml (P<0.01) and 956.43±25.21 pg/ml (P<0.01) in the 5-Fu
metronomic and conventional dose groups, respectively. The VEGF
concentration in the AGS cell culture supernatants in the control
group was 914.93±35.02 pg/ml, while the VEGF concentrations were
594.60±34.99 pg/ml (P<0.01) and 1300.23±70.29 pg/ml (P<0.01;
Fig. 2C) in the 5-Fu metronomic and
conventional dose groups, respectively.
The PDGF concentration in the SGC-7901 cell culture
supernatants in the control group was 5.73±0.20 ng/ml, while the
VEGF concentrations were 3.35±0.28 ng/ml (P<0.01) and 4.47±0.23
ng/ml (P<0.01) in the 5-Fu metronomic and conventional dose
groups, respectively (Fig. 2C). The
PDGF concentration in the AGS cell culture supernatants in the
control group was 4.50±0.18 ng/ml, while the PDGF concentrations
were 3.64±0.28 ng/ml (P<0.01) and 4.16±0.20 ng/ml (P=0.11)
(P<0.05, Fig. 2D) in the 5-Fu
metronomic and conventional dose groups, respectively.
These results indicated that 5-Fu metronomic
chemotherapy significantly inhibited the gastric secretions of VEGF
and PDGF in vitro and that conventional doses of 5-Fu had an
anti-VEGF-secretion effect on gastric cancer cells.
Effect of metronomic chemotherapy on
gastric cancer xenografts
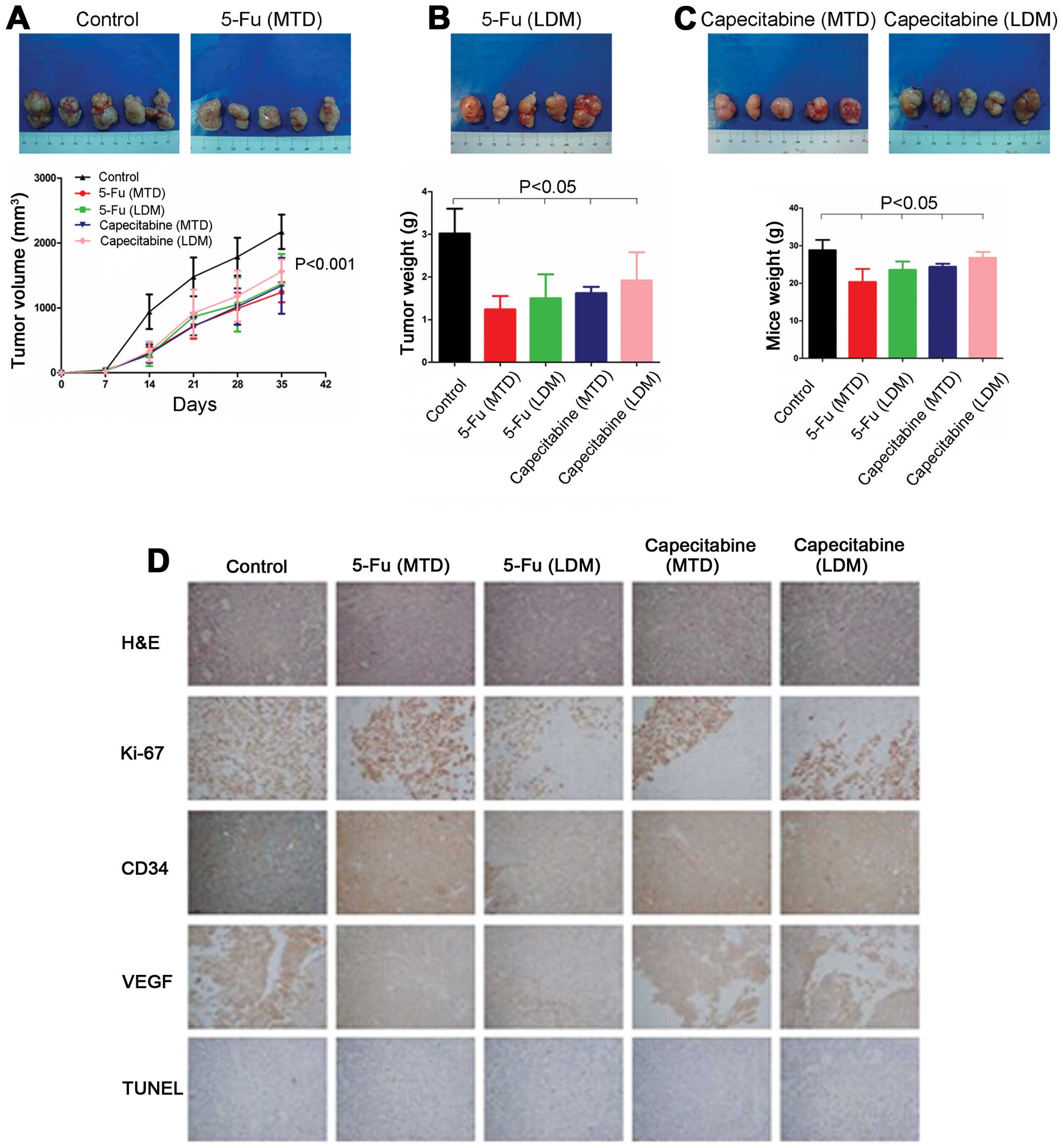
Compared with the control group, the tumor growth
rates in gastric cancer xenografts were significantly decreased.
The 5-Fu and capecitabine metronomic groups demonstrated no
significant differences, as compared with the conventional dose
groups. After drug treatment for 6 weeks, the tumor volume in the
control group was 2172.81±264.44 mm3, which was
significant compared with the 5-Fu conventional dose group
(1239.16±154.25 mm3; P<0.01) and 5-Fu metronomic
group (1369.74±459.73 mm3; P<0.01). However, no
significant difference was found between the 5-Fu conventional dose
and metronomic groups (P=0.531). The capecitabine conventional dose
and metronomic groups demonstrated a significant a significant
difference of 1339.71±430.40 and 1560.24±186.44 mm3,
respectively, as compared with the control group (P<0.01).
However, no significant difference was found between the
capecitabine conventional dose and metronomic groups (P=0.295;
Fig. 3A).
After a 6-week drug treatment, the tumor weight in
the control group was 3.02±0.58 g, which was statistically
different from that of the 5-Fu conventional dose (1.24±0.31 g;
P<0.01) and metronomic groups (1.50±0.56 g; P<0.01). However,
no significant difference was found between the 5-Fu conventional
dose and metronomic groups (P=0.415). The capecitabine conventional
dose and metronomic groups were 1.60±0.16 g (P<0.01) and
1.88±0.60 g (P<0.01), respectively. However, no significant
difference was found between the capecitabine conventional dose and
metronomic groups (P=0.384; Fig.
3B).
The tumor weight in the control group was 28.82±2.70
g, which was statistically different from that of the 5-Fu
conventional dose (20.32±3.49 g; P<0.01) and metronomic groups
(23.54±2.22 g; P<0.01). The tumor weight in the 5-Fu
conventional dose group was significantly decreased, as compared
with the 5-Fu metronomic group (P<0.05). The capecitabine
conventional dose and metronomic groups were 24.38±0.81 g
(P<0.01) and 27.60±1.09 g, respectively (P=0.41). The
capecitabine conventional dose group was statistically different,
as compared with the capecitabine metronomic group (P<0.05;
Fig. 3C), indicating that
metronomic chemotherapy had a lower toxicity than conventional dose
chemotherapy in the tumor-bearing nude mice.
Effect of metronomic chemotherapy on the
proliferation and apoptosis of gastric cancer xenografts
IHC revealed that the Ki-67 expression was
significantly lower in the 5-Fu conventional dose and metronomic
groups than the control group (57.67±2.52, 29.67±2.08 vs. 85±4.00%,
respectively; P<0.01). The 5-Fu metronomic group was
significantly lower than the 5-Fu conventional dose group
(P<0.01). The Ki-67 expression in the capecitabine conventional
dose and metronomic groups was also significantly lower than that
of the control group (39.67±2.52, 30.67±4.73 vs. 85±4.00%,
respectively; P<0.01). The capecitabine metronomic group was
significantly lower than the capecitabine conventional dose group
(P<0.01; Fig. 3D).
TUNEL staining showed that the 5-Fu and capecitabine
conventional dose groups were significantly increased. However,
tumor apoptosis in the 5-Fu and capecitabine metronomic groups
demonstrated no significant increase (Fig. 3D), indicating that conventional
doses of 5-Fu and capecitabine promoted apoptosis in gastric cancer
cells but that Fu and capecitabine metronomic chemotherapy had no
obvious apoptotic effect.
Effect of metronomic chemotherapy on the
expression of MVD and VEGF in gastric cancer xenografts in nude
mice
The IHC results showed that the MVD in the control
group was 5.7±1.2, while the MVDs in 5-Fu conventional dose and
metronomic groups were 4.7±1.5 (P=0.365) and 3.0±1.0 (P<0.05),
respectively. The MVDs in the capecitabine conventional dose and
metronomic groups were 4.7±1.2 (P=0.365) and 2.7±1.5 (P<0.05,
Fig. 3D), respectively, indicating
that 5-Fu and capecitabine metronomic doses decreased the MVD in
the gastric cancer xenografts, but conventional 5-Fu and
capecitabine metronomic chemotherapy had no obvious effect on the
MVD in gastric cancer xenografts.
The VEGF expression in the 5-Fu and capecitabine
conventional dose groups was higher than that of the control group.
However, VEGF expression in the 5-Fu and capecitabine metronomic
groups was lower than that of the control group, indicating that
5-Fu and capecitabine metronomic chemotherapy reduced VEGF
expression in the gastric cancer xenografts. By contrast, 5-Fu and
capecitabine conventional doses increased the VEGF expression
(Fig. 3D).
Effect of metronomic chemotherapy on the
CEPs in gastric cancer xenografts
The flow cytometry results showed that the CEP
percentage in the tumor-bearing nude mice in the control group was
32.12±4.11% and that the CEP percentages in the 5-Fu conventional
dose and metronomic groups were 29.18±1.61% (P=0.526) and
18.94±1.45% (P<0.05), respectively. The capecitabine
conventional dose and metronomic groups were 27.08±4.89% (P=0.286)
and 20.15±2.47% (P<0.05; Fig.
4A), respectively, suggesting that the 5-Fu and capecitabine
metronomic groups had reduced CEP percentages in the peripheral
blood endothelial progenitor cells and that the 5-Fu and
capecitabine conventional doses had no significant effect.
Effect of metronomic chemotherapy on
angiogenesis-related factors in the peripheral blood of gastric
cancer-bearing nude mice
The ELISA test showed that the VEGF level in the
peripheral blood of nude mice in the control group was 75.63±1.24
pg/ml. The VEGF levels in the 5-Fu conventional dose and metronomic
groups were 87.00±0.92 pg/ml (P<0.01) and 68.32±4.58 pg/ml
(P<0.05), respectively. The capecitabine conventional dose and
metronomic groups were 94.61±4.21 pg/ml (P<0.01) and 65.39±4.44
pg/ml (P<0.01; Fig. 4B),
respectively.
The PDGF level in the peripheral blood of the nude
mice in the control group was 38.20±1.43 pg/ml. The PDGF levels in
the 5-Fu conventional dose and metronomic groups were 38.34±1.28
pg/ml (P=0.931) and 31.03±1.46 pg/ml (P<0.01), respectively. The
capecitabine conventional dose and metronomic groups were
47.00±3.34 pg/ml (P<0.01) and 28.46±1.28 pg/ml (P<0.01;
Fig. 4C), respectively.
These results suggested that 5-Fu and capecitabine
metronomic chemotherapy reduced the peripheral blood levels of VEGF
and PDGF in the gastric cancer cells of tumor-bearing nude mice.
However, 5-Fu conventional dose chemotherapy decreased the VEGF
level, while capecitabine conventional dose chemotherapy increased
the levels of VEGF and PDGF.
Discussion
5-FU-based chemotherapy is a first-line treatment
for a variety of malignant tumors including gastric cancer
(21,22). Capecitabine is a precursor of 5-Fu
drugs that is converted by the TP enzyme in tumor cells and has
antitumor effects. As an oral agent, 5-Fu is more suitable for
metronomic chemotherapy with a frequent low-dose pattern that has
obvious advantages over intravenous drugs (23). Metronomic chemotherapy has low
toxicity, good compliance, and better efficacy and represents a new
trend in tumor chemotherapy (24,25).
In the present study, 5-Fu and capecitabine were administered in
vitro, and the antitumor effects of 5-FU-based metronomic
chemotherapy were observed and compared with conventional dose
chemotherapy to verify the antitumor efficacy of metronomic
chemotherapy.
The present study showed that metronomic 5-FU-based
chemotherapy significantly inhibited the proliferation of gastric
cancer cells in vitro and in vivo. The inhibitory
effect was not weaker than that of conventional-dose chemotherapy.
The present results show that metronomic 5-FU-based chemotherapy
inhibited the expression of secretory angiogenesis-related factors
and vascular endothelial cell small tube formation, rather than
having a cell toxicity effect that directly induced apoptosis and
cell death, as compared with conventional dose chemotherapy. These
results were similar to our preliminary study on colon cancer
(9) with metronomic chemotherapy in
other cancer types. Hackl et al (26) confirmed that irinotecan metronomic
chemotherapy suspended transplanted colorectal cancer tumor growth
and extended the survival of tumor-bearing nude mice. In animal
experiments, Mainetti et al (27) found that CTX and adriamycin
metronomic applications inhibited transplanted breast cancer growth
and significantly reduced the VEGF level in a transplant tumor
model. Ng et al (28) found
that paclitaxel metronomic applications inhibited transplanted
breast cancer growth by reducing the peripheral blood CEPs in
tumor-bearing mice.
By inhibiting the proliferation and growth of
tumors, we found that the body weight for the metronomic
chemotherapy group was significantly higher than that of the
standard chemotherapy group, indirectly indicating that metronomic
chemotherapy had advantages regarding attenuating toxicity. For the
first-line treatment of patients with gastric cancer, the PFS was
4–6 months. Existing drugs had limited efficacy, although the
toxicities of combined chemotherapy made it less tolerable
(29). Along with the emergence of
new drugs such as taxanes and trastuzumab, first-line gastric
cancer treatment prolonged the PFS to some extent, although
toxicity remained the limiting factor (30,31).
Therefore, the use of metronomic chemotherapy for maintenance
treatment has also been considered for gastric cancer, because of
its low toxicity. Wu et al (32) reported that capecitabine metronomic
chemotherapy suppressed the tumor growth of gastric cancer and had
low toxicity. Fedele et al (33) treated metastatic breast cancer
patients with oral Xeloda, showing a clinical benefit rate of 62%,
and grade 3–4 chemotherapy side effects were uncommon. In addition
to cytotoxic effects, the inhibitory mechanism was also
investigated. Instead of inhibiting tumor growth through
cytotoxicity, metronomic chemotherapy attenuated the toxicity of
conventional chemotherapy through the targeted inhibition of tumor
angiogenesis.
Moreover, the level of the angiogenesis-related
factor VEGF was increased in the conventional chemotherapy group to
a certain extent, indicating that high-dose interval treatment with
conventional chemotherapy may lead to the intermittent
proliferation of remnant tumor cells. Bertolini et al
(3) found that the CEP number and
VEGF level were decreased with conventional chemotherapy but
rebounded between chemotherapy cycles, suggesting that such
high-dose intermittent treatment may promote tumor angiogenesis.
Although the present study did not observe that the MVD was more
intense with conventional chemotherapy than that of the control and
metronomic chemotherapy groups, elevated VEGF levels in the
peripheral blood and serum were demonstrated with no significant
inhibition of peripheral blood CEPs. A 6-week drug treatment may
not necessarily reflect an obvious difference in MVD, but
significant differences in VEGF and PDGF provided clues for further
investigation.
A variety of anti-VEGF drugs such as bevacizumab and
aflibercept have demonstrated clear clinical efficacy (34,35).
However, bevacizumab did not demonstrate efficacy in the treatment
of gastric cancer (10).
Ramucizumab demonstrated positive results in second-line clinical
investigations (11). These seem to
contradict the clinical study results on at least two points:
differences in treatment lines and mechanisms. The blockade of
VEGFR or VEGF may lead to different treatment efficacies. The
present study demonstrates the effect of metronomic chemotherapy on
gastric cancer and focused not on tumor shrinkage, but on low-dose
high-frequency tumor treatment. Metronomic chemotherapy played
roles if the tumor load was relatively small (e.g., maintenance
treatment after first-line PR treatment). However, it was not
suitable for relatively large tumor loads. Second, the inhibition
of tumor cell VEGF secretion demonstrated early molecular events by
blocking the upstream tumor angiogenic effect, thus avoiding a
standard-dose chemotherapy-induced rebound increase in VEGF
secretion. This finding was similar to that of sorafenib treatment
for the liver, as reported by Zhang et al (36). Those authors found that
standard-dose chemotherapy and sorafenib treatment may lead to
increased VEGF secretion from liver cancer cells (36); from the point of tumor angiogenesis,
the addition of conventional dose chemotherapy induced a
drug-resistant mechanism.
As for drug economics, as compared with conventional
dose chemotherapy, metronomic chemotherapy has decreased indirect
costs and advantages given its high-potency ratio, including its
low dose, high frequency and low toxicity and cumulative dose.
The current OS of patients with advanced gastric
cancer was lower than that of breast and colorectal cancer.
However, for the treatment of advanced gastric cancer, the status
and value of metronomic chemotherapy has yet to be verified. As for
advanced gastric cancer treatment, issues that remain to be
addressed include, when to use metronomic chemotherapy,
specifically, maintenance therapy; which patients; whether serum
and tissue markers should be explored; the main roles of
capecitabine (i.e., 5-Fu precursor plays a role in metronomic
chemotherapy) in gastric cancer; and whether other oral medications
(e.g., S-1) have similar effects.
Acknowledgements
The present study was supported by the National
Science Foundation of China (81372645), the Shanghai Natural
Science Foundation from the Municipal Government (13ZR1425900), the
Shanghai Jiaotong University School of Medicine Science and
Technology Foundation (13XJ10035), the Fong Shu Fook Tong
Foundation and the National Key Clinical Discipline (Oncology).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Shah MA and Kelsen DP: Gastric cancer: a
primer on the epidemiology and biology of the disease and an
overview of the medical management of advanced disease. J Natl
Compr Canc Netw. 8:437–447. 2010.PubMed/NCBI
|
|
3
|
Bertolini F, Paul S, Mancuso P, et al:
Maximum tolerable dose and low-dose metronomic chemotherapy have
opposite effects on the mobilization and viability of circulating
endothelial progenitor cells. Cancer Res. 63:4342–4346.
2003.PubMed/NCBI
|
|
4
|
Hanahan D, Bergers G and Bergsland E: Less
is more, regularly: metronomic dosing of cytotoxic drugs can target
tumor angiogenesis in mice. J Clin Invest. 105:1045–1047. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamat AA, Kim TJ, Landen CN Jr, et al:
Metronomic chemotherapy enhances the efficacy of antivascular
therapy in ovarian cancer. Cancer Res. 67:281–288. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Man S, Bocci G, Francia G, et al:
Antitumor effects in mice of low-dose (metronomic) cyclophosphamide
administered continuously through the drinking water. Cancer Res.
62:2731–2735. 2002.PubMed/NCBI
|
|
7
|
Orlando L, Cardillo A, Ghisini R, et al:
Trastuzumab in combination with metronomic cyclophosphamide and
methotrexate in patients with HER-2 positive metastatic breast
cancer. BMC Cancer. 6:2252006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fakhrejahani E and Toi M: Antiangiogenesis
therapy for breast cancer: an update and perspectives from clinical
trials. Jpn J Clin Oncol. 44:197–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi H, Jiang J, Ji J, et al:
Anti-angiogenesis participates in antitumor effects of metronomic
capecitabine on colon cancer. Cancer Lett. 349:128–135. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fuchs CS, Tomasek J, Yong CJ, et al:
Ramucirumab monotherapy for previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (REGARD): an
international, randomised, multicentre, placebo-controlled, phase 3
trial. Lancet. 383:31–39. 2014. View Article : Google Scholar
|
|
11
|
Ohtsu A, Shah MA, Van Cutsem E, et al:
Bevacizumab in combination with chemotherapy as first-line therapy
in advanced gastric cancer: a randomized, double-blind,
placebo-controlled phase III study. J Clin Oncol. 29:3968–3976.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang BW, Kim JG, Kwon OK, Chung HY and Yu
W: Non-platinum-based chemotherapy for treatment of advanced
gastric cancer: 5-fluorouracil, taxanes, and irinotecan. World J
Gastroenterol. 20:5396–5402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cunningham D, Starling N, Rao S, et al:
Capecitabine and oxaliplatin for advanced esophagogastric cancer. N
Engl J Med. 358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ryu MH and Kang YK: ML17032 trial:
capecitabine/cisplatin versus 5-fluorouracil/cisplatin as
first-line therapy in advanced gastric cancer. Expert Rev
Anticancer Ther. 9:1745–1751. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bang YJ, Kim YW, Yang HK, et al: Adjuvant
capecitabine and oxaliplatin for gastric cancer after D2
gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled
trial. Lancet. 379:315–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee J, Lim do H, Kim S, et al: Phase III
trial comparing capecitabine plus cisplatin versus capecitabine
plus cisplatin with concurrent capecitabine radiotherapy in
completely resected gastric cancer with D2 lymph node dissection:
the ARTIST trial. J Clin Oncol. 30:268–273. 2012. View Article : Google Scholar
|
|
17
|
Shi M, Lou B, Ji J, et al: Synergistic
antitumor effects of dasatinib and oxaliplatin in gastric cancer
cells. Cancer Chemother Pharmacol. 72:35–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang WK, Park EK, Lee HS, et al: A
biologically active angiogenesis inhibitor, human serum
albumin-TIMP-2 fusion protein, secreted from Saccharomyces
cerevisiae. Protein Expr Purif. 53:331–338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Q, Kang X, Yang B, Wang J and Yang
F: Antiangiogenic effect of capecitabine combined with ginsenoside
Rg3 on breast cancer in mice. Cancer Biother Radiopharm.
23:647–653. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Emoto M, Tachibana K, Iwasaki H and
Kawarabayashi T: Antitumor effect of TNP-470, an angiogenesis
inhibitor, combined with ultrasound irradiation for human uterine
sarcoma xenografts evaluated using contrast color Doppler
ultrasound. Cancer Sci. 98:929–935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li DH, Pan ZK, Ye F, An HX and Wu JX:
S-1-based versus 5-FU-based chemotherapy as first-line treatment in
advanced gastric cancer: a meta-analysis of randomized controlled
trials. Tumour Biol. 35:8201–8208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sastre J, Garcia-Saenz JA and Diaz-Rubio
E: Chemotherapy for gastric cancer. World J Gastroenterol.
12:204–213. 2006.PubMed/NCBI
|
|
23
|
Bang YJ: Capecitabine in gastric cancer.
Expert Rev Anticancer Ther. 11:1791–1806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gasparini G: Metronomic scheduling: the
future of chemotherapy? Lancet Oncol. 2:733–740. 2001. View Article : Google Scholar
|
|
25
|
Loven D, Hasnis E, Bertolini F and Shaked
Y: Low-dose metronomic chemotherapy: from past experience to new
paradigms in the treatment of cancer. Drug Discov Today.
18:193–201. 2013. View Article : Google Scholar
|
|
26
|
Hackl C, Man S, Francia G, Milsom C, Xu P
and Kerbel RS: Metronomic oral topotecan prolongs survival and
reduces liver metastasis in improved preclinical orthotopic and
adjuvant therapy colon cancer models. Gut. 62:259–271. 2013.
View Article : Google Scholar :
|
|
27
|
Mainetti LE, Rico MJ, Fernandez-Zenobi MV,
et al: Therapeutic efficacy of metronomic chemotherapy with
cyclophosphamide and doxorubicin on murine mammary adenocarcinomas.
Ann Oncol. 24:2310–2316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ng SS, Sparreboom A, Shaked Y, et al:
Influence of formulation vehicle on metronomic taxane chemotherapy:
albumin-bound versus cremophor EL-based paclitaxel. Clin Cancer
Res. 12:4331–4338. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bilici A: Treatment options in patients
with metastatic gastric cancer: current status and future
perspectives. World J Gastroenterol. 20:3905–3915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gravalos C, Gomez-Martin C, Rivera F, et
al: Phase II study of trastuzumab and cisplatin as first-line
therapy in patients with HER2-positive advanced gastric or
gastroesophageal junction cancer. Clin Transl Oncol. 13:179–184.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo Z, Wang X, Lin R, et al:
Paclitaxel-based regimens as first-line treatment in advanced
gastric cancer. J Chemother. Feb 18–2014.(Epub ahead of print).
View Article : Google Scholar
|
|
32
|
Wu H, Xin Y, Xu C and Xiao Y: Capecitabine
combined with (−)-epigallocatechin-3-gallate inhibits angiogenesis
and tumor growth in nude mice with gastric cancer xenografts. Exp
Ther Med. 3:650–654. 2012.PubMed/NCBI
|
|
33
|
Fedele P, Marino A, Orlando L, et al:
Efficacy and safety of low-dose metronomic chemotherapy with
capecitabine in heavily pretreated patients with metastatic breast
cancer. Eur J Cancer. 48:24–29. 2012. View Article : Google Scholar
|
|
34
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Van Cutsem E, Tabernero J, Lakomy R, et
al: Addition of aflibercept to fluorouracil, leucovorin, and
irinotecan improves survival in a phase III randomized trial in
patients with metastatic colorectal cancer previously treated with
an oxaliplatin-based regimen. J Clin Oncol. 30:3499–3506. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang W, Zhu XD, Sun HC, et al: Depletion
of tumor-associated macrophages enhances the effect of sorafenib in
metastatic liver cancer models by antimetastatic and antiangiogenic
effects. Clin Cancer Res. 16:3420–3430. 2010. View Article : Google Scholar : PubMed/NCBI
|