Introduction
Colorectal cancer is one of the most serious
complications of ulcerative colitis (UC), and the risk of
UC-associated neoplasia increases as the size of the affected
region and the duration of the disease increase. The incidence of
UC-associated dysplasia and cancer is higher than that of sporadic
colorectal cancer. UC-associated cancer usually occurs at a younger
age, has a higher proportion of multiple lesions and shows a poorer
survival rate than sporadic colorectal cancer (1). 5-Aminosalicylates (5-ASAs) are widely
used to treat patients with UC, and experimental data suggest that
5-ASAs reduce the risk of UC-associated dysplasia and cancer;
however, observational studies investigating the effect of 5-ASAs
on UC-associated dysplasia and cancer have revealed conflicting
results (2–4). Therefore, the need for more effective
chemo-prevention of UC-associated dysplasia and cancer is well
acknowledged (5).
Heat shock protein (HSP) 70 is a stress-inducible
protein with a strong cytoprotective effect against numerous
stresses (6). HSP induction
inhibited inducible nitric oxide synthase (iNOS), which is related
to oxidative stress and subsequent transversion mutation of DNA,
and prevented dysplastic lesions in an inflammatory model of colon
cancer (7–9). Geranylgeranylacetone (GGA), an
isoprenoid compound, is an anti-ulcer drug developed in Japan. GGA
has been demonstrated to protect a variety of cells and tissues
from damage via induction of HSP70 (6). It has recently been reported that GGA
protects mice from experimental colitis, such as dextran sulfate
sodium (DSS)-induced colitis and trinitrobenzene sulfonic acid
(TNBS)-induced colitis (10,11).
However, it is unknown whether GGA exhibits a preventive effect on
UC-associated neoplasia. The present study investigated the
preventive effects of GGA on colitis-related mouse colon
carcinogenesis.
Materials and methods
Animals
Six-week-old female BALB/c mice (Clea Japan, Tokyo,
Japan) weighing 20–25 g were used in the present study. The animals
were maintained in an animal colony with controlled temperature
(23°C) and light (12/12-h light and dark cycle) at the Osaka
Medical College (OMC; Osaka, Japan), and were permitted free access
to standard mouse chow pellets (MM-3; Funabashi, Chiba, Japan) and
tap water.
Protocol for induction of colorectal
tumors and experimental procedures
Colorectal tumors and experimental colitis were
induced as previously described (12). In brief, the mice received
1,2-dimethylhydrazine (DMH; Wako Pure Chemical Industries, Osaka,
Japan) at a dose of 20 mg/kg body weight subcutaneously three times
within a week. Starting 1 week after the DMH injection, chronic
colitis was induced by the administration of 2 cycles of DSS
(molecular weight, 5,000; Meitou Sangyou, Osaka, Japan) (each
cycle, 3% DSS for 7 days and then distilled water for 14 days). The
mice were sacrificed 28 days after the completion of the 2
cycles.
The mice were divided into six groups (A–F)
according to the diet they received during the experiment. Group A
received a standard diet and served as a disease control; group B
received a diet mixed with 0.25% of GGA (kindly provided by Eisai
Co., Tokyo, Japan); group C received a diet mixed with 0.5% GGA;
group D received a diet mixed with 1.0% GGA; group E received a
diet mixed with 2.0% GGA; group F received a diet containing no
agents, including DSS and served as a normal control.
The entire colorectum from the colocecal junction to
the anal verge was excised and rinsed in phosphate-buffered saline
(PBS). The specimen was opened longitudinally and was fixed on a
cork board in 10% formalin. Then, the specimen was stained with
0.2% methylene blue, and the number of colonic tumors was counted
under a stereomicroscope (13,14).
Histopathological examination was performed on paraffin-embedded
sections after hematoxylin and eosin staining. Colonic mucosal
dysplasia and cancer were diagnosed according to the criteria
described by Ridell et al (15).
Evaluation of the severity of clinical
colitis
The disease activity index (DAI) was determined in
all the animals during the first DSS administration cycle by
scoring the body weight, the stool hemoccult reactivity or the
presence of gross blood and stool consistency, in accordance with
the method described by Murthy et al (16). This method of scoring is a
comprehensive functional measure that correlates well with the
degree of inflammation. The individuals who examined the mice and
determined the DAIs were blinded as to the experimental group to
which the animal belonged.
Immunohistochemistry and immunoblot
analyses
Expression of HSP70 and 8-hydroxy-2′-deoxyguanosine
(8-OHdG) in the intestinal mucosa was assessed by the labeled
streptavidin-biotin method with the LSAB kit (Dako, Carpinteria,
CA, USA) with microwave accentuation. Each segment was fixed in 10%
formalin, embedded in paraffin wax and cut into tissue sections of
4-mm thickness. Tissue sections were mounted on microscope slides,
deparaffinized in xylene (3×3 min) and dehydrated with 100%
ethanol. After washes with PBS, sections were placed in 10 mmol/l
citrate buffer (pH 6.0) and heated to 80°C for 10 min in a
microwave oven. After washes with PBS, endogenous peroxidase
activity was blocked with 0.3% hydrogen peroxide in 10% methanol
for 30 min, and blocking reagent was added for 15 min. The sections
were incubated at 4°C overnight in the primary antibody (anti-HSP70
IgG; Stressgen, Ann Arbor, MI, USA) (anti-8-OHdG IgG; NOF Co.,
Tokyo, Japan). After washes with PBS, the sections were incubated
with a biotinylated immunoglobulin antibody (Dako) at room
temperature for 30 min. The sections were then washed in PBS and
visualized with streptavidin-biotin horseradish peroxidase and
3,3′-diaminobenzidine (both from Dako). Finally, the sections were
counterstained with hematoxylin, dehydrated and coverslipped with
permanent mounting medium for examination under a light microscope
(13).
Lysates of colonic mucosa containing 20 μg/20 μl of
protein were separated by electrophoresis through a
NuPAGE® 4–12% Bis-Tris gel (1.0 mm) (Life Technologies,
Carlsbad, CA, USA), and transferred onto polyvinylidene difluoride
membranes (Pall Corporation, Port Washington, NY, USA). Membranes
were incubated in 5% skim milk in Tris-buffered saline with
Tween-20 (TBST) at room temperature for 10 min and stored at 4°C
overnight. After 3 washes in TBST, the membranes were incubated
with the mouse anti-HSP70 antibody and mouse anti-β-actin antibody
(Sigma Chemicals, St. Louis, MO, USA) for 1 h at room temperature
in 5% skim milk. Membranes were washed 3 times in TBST and
incubated with peroxidase-conjugated secondary antibodies (Santa
Cruz Biotechnology, Dallas, TX, USA). Blots were washed 3 times
with TBST and developed with an enhanced chemiluminescence system
(ECL Prime Western Blotting Detection System; GE Healthcare,
Buckinghamshire, UK) and Fujifilm Imaging System Application Note
LAS-3000 (Fujifilm, Tokyo, Japan).
Analysis of iNOS mRNA expression by
reverse transcription PCR
To evaluate iNOS mRNA expression in background
mucosa, a small sample of intestinal tissue was removed from the
lesion-free mouse colon under a stereomicroscope, frozen in liquid
nitrogen, and stored at −80°C until RNA isolation. Total RNA was
extracted from tissue samples with the total RNEasy Mini kit
(Qiagen GmbH, Hilden, Germany). Reverse transcription and
polymerase chain reaction were performed with the high-fidelity
prime script RT-PCR kit (Takara Bio, Inc., Shiga, Japan) according
to the manufacturer’s instructions. The sequences of sense and
antisense primers for the mouse iNOS were,
5′-TGTCAGTGGCTTCCAGCTCC-3′ and 5′-TAGTCTT CCACCTGCTCCTC-3′, giving
rise to a 450-bp PCR product. For mouse glyceraldehyde-3-phosphate
dehydrogenase (G3DPH), a constitutively expressed gene, the
sequence was, 5′-TGAAGGTCGGTGTGAACGGATTTGC-3′
forthesenseprimerand5′-CATGTAGGCCATGAGGTCCACC AC-3′ for the
antisense primer, giving rise to a 983-bp PCR product. An aliquot
of the reverse transcription reaction product for iNOS expression
served as a template in 35 cycles of PCR with 10 sec of
denaturation at 98°C, 5 sec of annealing at 55°C and 0.5 min of
extension at 72°C on the thermal cycler. A portion of the PCR
mixture was electrophoresed on 1.5% agarose gel in Tris-EDTA-acetic
acid buffer, and the gel was stained with ethidium bromide and
photographed.
Statistical analysis
All the results are expressed as means ± SD.
Comparisons were carried out with a one-way analysis of variance
(ANOVA) or the Kruskal-Wallis test followed by Fisher’s PLSD test.
Statistical significance was defined as p<0.05.
Results
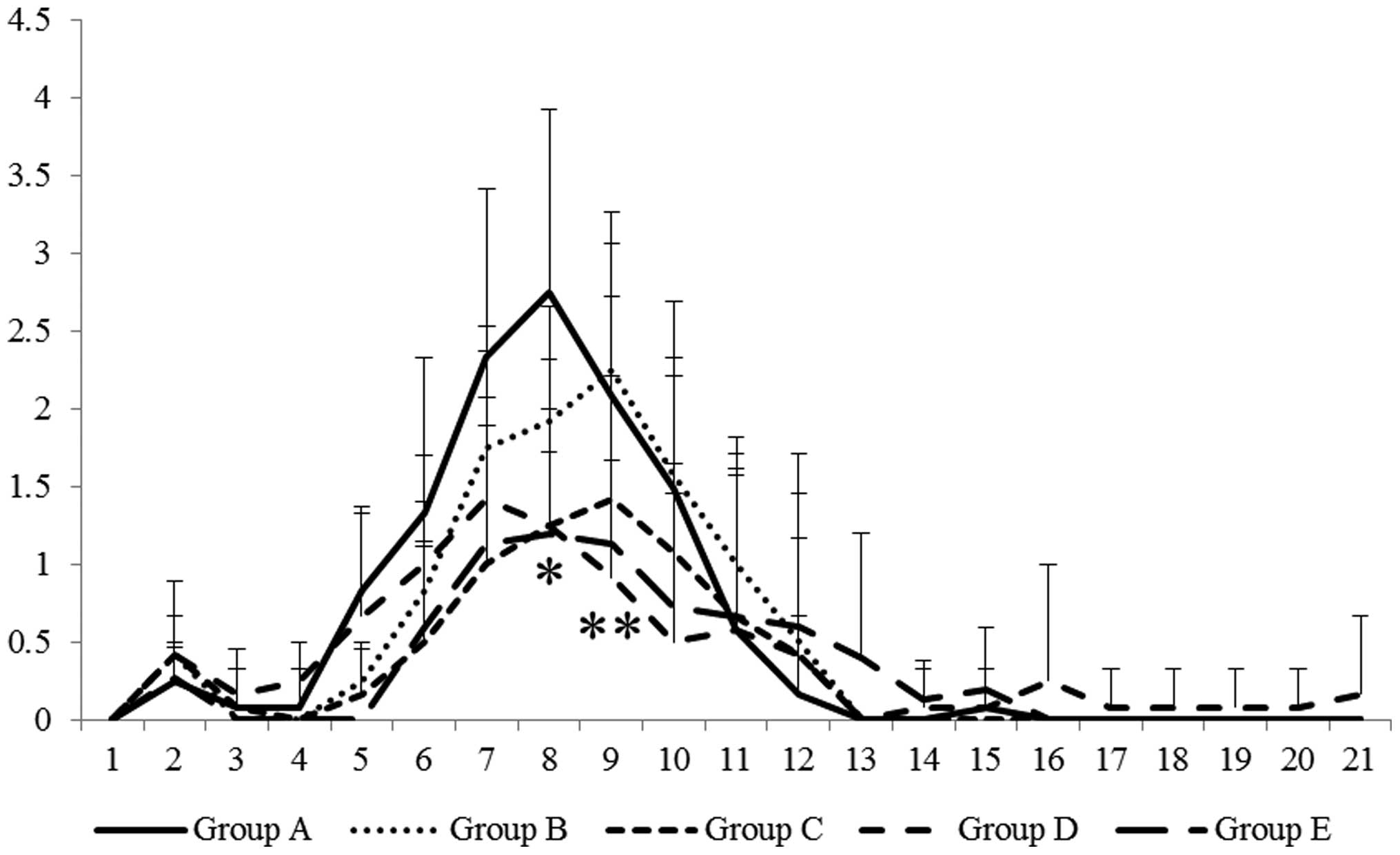
Changes in DAI
As previously described (12), the DAI gradually increased until day
8, and usually reverted to normal by day 21. Clinical symptoms of
colitis, including bloody stool, diarrhea and loss of body weight,
progressed until day 8. Although there was no significant
difference between the groups, body weight loss was
dose-dependently attenuated by GGA treatment. Accordingly, the
administration of GGA significantly suppressed the DAI in a
dose-dependent manner (Fig. 1).
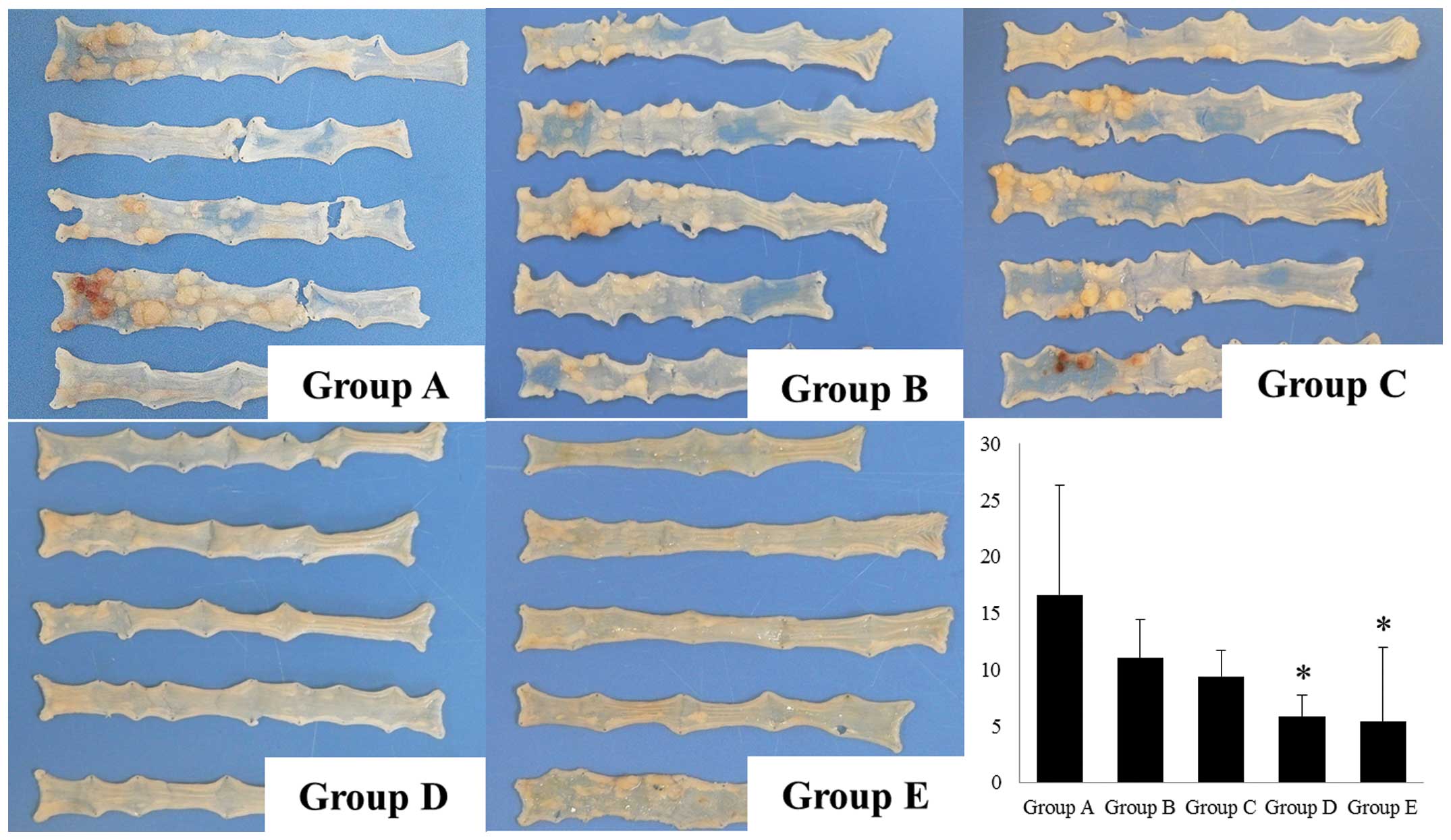
Colorectal length and number of
tumors
The colorectal length in groups A–F was 8.3±0.4,
8.2±0.4, 8.7±1.1, 9.7±0.2, 9.4±0.7 and 9.6±0.3 cm, respectively.
The colorectal length was significantly shortened in groups A–E
compared to the normal control (group F) 70 days after the start of
DSS administration. The administration of GGA significantly reduced
the decrease in colon length (groups D and E) compared to the
disease control (group A). Although the incidence of colonic
neoplasia (number of mice with neoplasms) in groups A–E was 100%,
the multiplicity (number of tumors/mouse) in groups A–E was
16.6±9.7, 11.0±3.4, 9.4±2.3, 5.8±1.9 and 5.4±6.6, respectively
(Fig. 2). The administration of GGA
significantly suppressed the number of tumors/mouse in a
dose-dependent manner (Fig. 2).
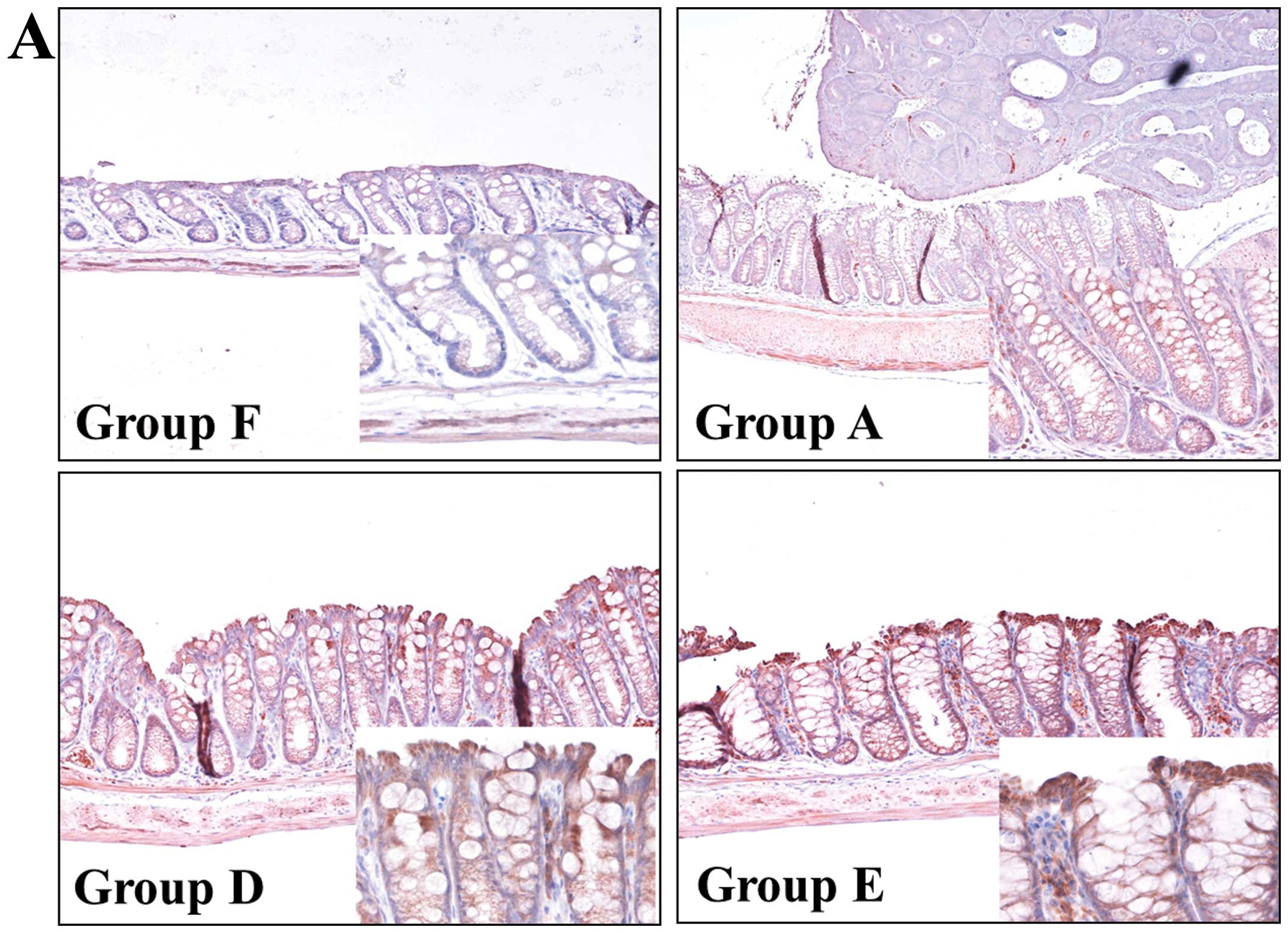
HSP70 expression
Since it has been reported that GGA induces HSP70,
which has a protective effect against inflammation, and the DAI was
significantly suppressed by GGA treatment in the present study, we
examined the expression of HSP70 in the background mucosa (i.e.
lesion-free colon) by immunohistochemistry and immunoblotting. As
shown in Fig. 3A,
immunohistochemistry revealed weak positivity for HSP70 in the
colon of the normal (group F) and disease control (group A) mice.
The administration of GGA enhanced the cytoplasmic HSP70 expression
in the epithelium of the background mucosa. Western blot analysis
also showed that mucosal HSP70 expression was increased in the
GGA-treated mice (groups D and E) (Fig.
3B).
iNOS and 8-OHdG expression
It is well known that iNOS can cause oxidative
stress and leads to 8-OHdG accumulation. Since GGA has been
reported to increase mucosal HSP70 without increasing the 8-OHdG
production, we analyzed iNOS and 8-OHdG expression by
reverse-transcription PCR and immunohistochemistry. As shown in
Fig. 4A, iNOS mRNA expression in
the background mucosa was upregulated in groups A and B. However,
iNOS mRNA expression was decreased in groups C–E.
Immunohistochemistry revealed that 8-OHdG was strongly expressed in
the background mucosa of group A, and the administration of GGA
resulted in markedly decreased 8-OHdG expression (Fig. 4B). These results suggest that GGA
suppressed colitis-induced mouse colon carcinogenesis via
suppression of oxidative DNA damage.
Discussion
It is well known that HSPs are induced by various
stressors and protect cells from such stressors (6). In particular, HSP70 has been reported
to provide protective effects against gastrointestinal diseases,
such as gastric ulcer, experimental colitis, and nonsteroidal
anti-inflammatory drug (NSAID)-induced enteritis in rodents
(10,11,17,18).
Regarding NSAID-induced intestinal mucosal injuries, we recently
demonstrated via video capsule endoscopy that GGA markedly
inhibited the development of lesions in human subjects (19). Since GGA increases mucosal HSP70
expression without increasing the oxidative damage followed by
8-OHdG accumulation (20), GGA is
expected to be therapeutically beneficial for gastrointestinal
tract diseases as a non-toxic HSP inducer. However, to date, it
remains unknown whether GGA exhibits a preventive effect in
UC-associated neoplasia. To our knowledge, the present study
demonstrated for the first time that the administration of GGA
exerts a suppressive effect on the development of neoplasia in a
murine model of colitis.
Cyclooxygenase (COX)-2, which is an inducible COX
and upregulated in response to various stimuli, such as
interleukin-1 and tumor necrosis factor, is progressively
overexpressed during the stepwise sequence from adenoma to cancer
(21). Therefore, NSAIDs are often
administered to patients with polyposis to inhibit COX activity and
induce apoptosis, which suppresses 8-OHdG formation (22). However, for patients with UC, it is
thought that NSAIDs may trigger UC relapse, so they should not be
administered to patients with UC (23). Indeed, we previously administered a
COX-2 inhibitor to mice with DSS-induced colitis and demonstrated
that the presence of a COX-2 inhibitor during colitis exacerbated
the colitis and did not show a preventive effect against
colitis-induced tumorigenesis (13). Nishida et al evaluated the
protective effect of GGA against noxious agents in rat gastric
mucosa and demonstrated that GGA dose-dependently increased COX-2
expression and prostaglandin E2 production (24). In the present study, the
administration of GGA suppressed iNOS expression and
dose-dependently attenuated DSS-induced colitis. Therefore, we
consider that these characteristics of GGA may be suitable for
patients with UC to prevent colitis-associated cancer
development.
According to the National Center for Advancing
Translational Sciences, the term ‘drug repurposing’ generally
refers to the study of a compound or biologic to treat one disease
or condition to see whether it is safe and effective for treating
other diseases (http://www.ncats.nih.gov/). Since developing a new
drug takes an enormous amount of time, and repurposing
(repositioning) candidates have often been through several stages
of clinical development and have well-known safety and
pharmacokinetic profiles, drug repurposing (repositioning) may
enable a faster development time and reduce risks (25). Since it is well known that GGA has
no significant adverse effects and is an inexpensive drug, GGA is
considered to be one of the most suitable agents for drug
repurposing (repositioning), and its use is being attempted in
other models of disease in Japan (26,27).
In conclusion, although it remains unknown whether
the usual dose for patients with gastric ulcer will be approved for
patients with UC, the results of the present study suggest that GGA
could be a useful therapeutic agent for the prevention of
UC-associated cancer. Further studies to evaluate the effect of GGA
on the human colonic mucosa and to clarify the optimal dose for
colonic inflammation are required.
References
|
1
|
Watanabe T, Konishi T, Kishimoto J, Kotake
K, Muto T and Sugihara K: Japanese Society for Cancer of the Colon
and Rectum: Ulcerative colitis-associated colorectal cancer shows a
poorer survival than sporadic colorectal cancer: a nationwide
Japanese study. Inflamm Bowel Dis. 3:802–808. 2011. View Article : Google Scholar
|
|
2
|
Lyakhovich A and Gasche C: Systematic
review: molecular chemoprevention of colorectal malignancy by
mesalazine. Aliment Pharmacol Ther. 31:202–209. 2010.
|
|
3
|
Herfarth H: The role of chemoprevention of
colorectal cancer with 5-aminosalicylates in ulcerative colitis.
Dig Dis. 30(Suppl 2): 55–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lopez A and Peyrin-Biroulet L:
5-Aminosalicylic acid and chemoprevention: does it work? Dig Dis.
31:248–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ekbom A, Helmick C, Zack M and Adami HO:
Ulcerative colitis and colorectal cancer. A population-based study.
N Engl J Med. 323:1228–1233. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mizushima T: RETRACTED: protective role of
HSP70 against various gastrointestinal diseases. Curr Opin
Pharmacol. 19:1–5. 2014. View Article : Google Scholar
|
|
7
|
Hauser GJ, Dayao EK, Wasserloos K, Pitt BR
and Wong HR: HSP induction inhibits iNOS mRNA expression and
attenuates hypotension in endotoxin-challenged rats. Am J Physiol.
271:H2529–H2535. 1996.PubMed/NCBI
|
|
8
|
Chan JY, Ou CC, Wang LL and Chan SH: Heat
shock protein 70 confers cardiovascular protection during
endotoxemia via inhibition of nuclear factor-κB activation and
inducible nitric oxide synthase expression in the rostral
ventrolateral medulla. Circulation. 110:3560–3566. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tao Y, Hart J, Lichtenstein L, Joseph LJ,
Ciancio MJ, Hu S, Chang EB and Bissonnette M: Inducible heat shock
protein 70 prevents multifocal flat dysplastic lesions and invasive
tumors in an inflammatory model of colon cancer. Carcinogenesis.
30:175–182. 2009. View Article : Google Scholar :
|
|
10
|
Ohkawara T, Nishihira J, Takeda H,
Miyashita K, Kato K, Kato M, Sugiyama T and Asaka M:
Geranylgeranylacetone protects mice from dextran sulfate
sodium-induced colitis. Scand J Gastroenterol. 40:1049–1057. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohkawara T, Nishihira J, Takeda H,
Katsurada T, Kato K, Yoshiki T, Sugiyama T and Asaka M: Protective
effect of geranylgeranylacetone on trinitrobenzene sulfonic
acid-induced colitis in mice. Int J Mol Med. 17:229–234.
2006.PubMed/NCBI
|
|
12
|
Inoue T, Murano M, Yoda Y, et al:
R-etodolac induces E-cadherin and suppresses colitis-related mouse
colon tumorigenesis. Oncol Rep. 24:1487–1492. 2010.PubMed/NCBI
|
|
13
|
Inoue T, Murano M, Abe Y, et al:
Therapeutic effect of nimesulide on colorectal carcinogenesis in
experimental murine ulcerative colitis. J Gastroenterol Hepatol.
22:1474–1481. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wedemeyer J and Galli SJ: Decreased
susceptibility of mast cell-deficient
KitW/KitW-v mice to the development of
1,2-dimeth-ylhydrazine-induced intestinal tumors. Lab Invest.
85:388–396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Riddell RH, Goldman H, Ransohof DF, et al:
Dysplasia in inflammatory bowel disease: standardized
classification with provisional clinical application. Human Pathol.
14:931–968. 1983. View Article : Google Scholar
|
|
16
|
Murthy SN, Cooper HS, Shim H, Shah RS,
Ibrahim SA and Sedergran DJ: Treatment of dextran sulfate
sodium-induced murine colitis by intracolonic cyclosporine. Dig Dis
Sci. 38:1722–1734. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suemasu S, Tanaka K, Namba T, et al: A
role for HSP70 in protecting against indomethacin-induced gastric
lesions. J Biol Chem. 284:19705–19715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Asano T, Tanaka K, Yamakawa N, Adachi H,
Sobue G, Goto H, Takeuchi K and Mizushima T: HSP70 confers
protection against indomethacin-induced lesions of the small
intestine. J Pharmacol Exp Ther. 330:458–467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Umegaki E, Kuramoto T, Kojima Y, Nouda S,
Ishida K, Takeuchi T, Inoue T, Tokioka S and Higuchi K:
Geranylgeranylacetone, a gastromucoprotective drug, protects
against NSAID-induced esophageal, gastroduodenal and small
intestinal mucosal injury in healthy subjects: a prospective
randomized study involving a comparison with famotidine. Intern
Med. 53:283–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yanaka A, Zhang S, Sato D, Tauchi M,
Suzuki H, Shibahara T, Matsui H, Nakahara A and Hyodo I:
Geranylgeranylacetone protects the human gastric mucosa from
diclofenac-induced injury via induction of heat shock protein 70.
Digestion. 75:148–155. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan AT, Ogino S and Fuchs CS: Aspirin and
the risk of colorectal cancer in relation to the expression of
COX-2. N Eng J Med. 356:2131–2142. 2007. View Article : Google Scholar
|
|
22
|
Tardieu D, Jaeg JP, Deloly A, Corpet DE,
Cadet J and Petit CR: The COX-2 inhibitor nimesulide suppresses
superoxide and 8-hydroxy-deoxyguanosine formation, and stimulates
apoptosis in mucosa during early colonic inflammation in rats.
Carcinogenesis. 21:973–976. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Forrest K, Symmons D and Foster P:
Systematic review: is ingestion of paracetamol or non-steroidal
anti-inflammatory drugs associated with exacerbations of
inflammatory bowel disease? Aliment Pharmacol Ther. 20:1035–1043.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishida T, Yabe Y, Fu HY, Hayashi Y, Asahi
K, Eguchi H, Tsuji S, Tsujii M, Hayashi N and Kawano S:
Geranylgeranylacetone induces cyclooxygenase-2 expression in
cultured rat gastric epithelial cells through NF-κB. Dig Dis Sci.
52:1890–1896. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ashburn TT and Thor KB: Drug
repositioning: identifying and developing new uses for existing
drugs. Nat Rev Drug Discov. 3:673–683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoshino T, Suzuki K, Matsushima T,
Yamakawa N, Suzuki T and Mizushima T: Suppression of Alzheimer’s
disease-related phenotypes by geranylgeranylacetone in mice. PLoS
One. 8:e763062013. View Article : Google Scholar
|
|
27
|
Namba T, Tanaka K, Hoshino T, Azuma A and
Mizushima T: Suppression of expression of heat shock protein 70 by
gefitinib and its contribution to pulmonary fibrosis. PLoS One.
6:e272962011. View Article : Google Scholar : PubMed/NCBI
|