Introduction
Oral cancer consists of malignant lesions in the
oral cavity and is one of the most common types of cancer worldwide
(1,2). Although oral cancer occurs
predominantly in individuals aged 50 years and above, there has
been a 5–6% increase per year in the number of patients under the
age of 45 (3,4). The pathological etiologies of oral
cancer are closely associated with mutations in the genes that
regulate cell growth, proliferation and apoptosis induced by
chronic exposure to carcinogens such as tobacco, alcohol and betel
quid (5). Despite improvements and
innovations in both diagnostic techniques and therapeutics, surgery
often results in loss of function and disfigurement. Furthermore,
radiotherapy and the overall survival rate of patients with oral
cancer have not improved (6).
Therefore, there is an urgent need for the development of
clinically relevant, highly effective chemotherapeutic agents with
minimal side-effects.
Berberine is a natural isoquinoline alkaloid that is
isolated from Oriental herbal plants of the genus Coptis. It
has been widely investigated due to its biological and
pharmacological activities. Recently, the anticancer activity of
berberine has been reported in various types of cancer, including
brain, lung, esophageal, gastric, colon, skin and liver. This
activity is believed to be related to its anti-neoplastic
properties leading to the inhibition of cell proliferation, the
induction of apoptosis, cell cycle arrest and the inhibition of
cell migration (7,8). In a demonstration of the
chemotherapeutic promise of berberine, Liu et al reported
that it exhibited significant cytotoxicity in hepatoma cells, yet
showed negligible cytotoxicity to normal cells (9). Furthermore, Hwang et al
reported that berberine-induced cancer cell apoptosis was mediated
by a mitochondrial-dependent intrinsic apoptotic signaling pathway
through the activation of caspases and the decreased expression of
Bcl-2 and Bcl-xL (10). However,
although its potential as a chemotherapeutic agent has been shown,
the molecular mechanisms of berberine-induced apoptosis in oral
cancer cells are still unknown.
Therefore, the aim of this study was to determine
whether berberine has the potential to function as a
chemotherapeutic agent by acting on KB oral cancer cells and, at
the same time, by not affecting normal cells that originate from
the oral cavity. Furthermore, we aimed to evaluate the potential
apoptotic effect of berberine and to elucidate the
berberine-induced apoptotic signaling pathway in KB cells.
Materials and methods
Materials
Anti-FasL, anti-caspase-8, anti-β-actin, anti-Bax,
anti-Bad, anti-MMP-2 and anti-MMP-9 were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Anti-cleaved caspase-3,
anti-cleaved poly(ADP-ribose) polymerase (PARP), anti-cleaved
caspase-9, anti-Bcl-2, anti-Bcl-xL, anti-Bad, anti-Apaf-1,
phospho-Erk1/2, total-Erk1/2, phospho-p38, total-p38, phospho-JNK
and total JNK were purchased from Cell Signaling (Danvers, MA,
USA). ERK chemical inhibitor (PD98059) and p38 chemical inhibitor
were purchased from EMD Chemicals (Gibbstown, NJ, USA).
Cell culture
Normal human oral keratinocytes (NHOKs) were
purchased from ScienCell Research Laboratories (Carlsbad, CA, USA).
The NHOKs were maintained in Dulbecco’s modified Eagle’s medium
(DMEM) containing 10% fetal bovine serum (FBS). The human oral
squamous cell carcinoma cell line, KB, was obtained from the
American Type Culture Collection (ATCC) and cultured according to
the cell culture instructions provided. Briefly, the KB cells were
grown in MEM (Gibco, Grand Island, NY, USA) containing 10% FBS
(Invitrogen, Carlsbad, CA, USA) at 37°C in an atmosphere containing
5% CO2.
Cell viability assay
Both KB oral cancer cells and NHOKs were seeded at a
density of 5×105 cells/well in 96-well plates, and
allowed to attach to the well overnight. After incubation, the
cultured cells were treated with various concentrations of
berberine in triplicate and incubated at 37°C in a 5% humidified
CO2 incubator for 24 h. MTT was then added to each well
and incubation was continued for a further 4 h at 37°C. In order to
dissolve the resulting formazan, the cells were resuspended in 200
μl dimethyl sulfoxide (DMSO), and the optical density (OD) of the
solution was determined using a spectrometer at an incident
wavelength of 570 nm. The experiments were repeated three times,
independently. The mean OD ± SD for each group of replicates was
calculated. The entire procedure was repeated three times. The
inhibitory rate of cell growth was calculated using the equation: %
Growth inhibition = [(1 − OD extract treated)/(OD negative
control)] × 100.
Cell survival assay
Cell survival was measured, as previously described
(11), using calcein-AM to stain
the live cells and ethidium bromide homodimer 1 to stain the dead
cells. These reagents were obtained from Molecular Probes (Eugene,
OR, USA). For the cell survival assay, KB cells and HNOKs were
plated in a chamber slide, incubated with berberine for 24 h, and
stained with green calcein-AM and ethidium bromide homodimer 1
according to the manufacturer’s protocol. The cells were then
observed and photographed by inverted phase-contrast microscopy
(Eclipse TE2000; Nikon Instruments, Melville, NY, USA).
DNA fragmentation assay
KB oral cancer cells were collected after treatment
with berberine (0, 0.1 and 1 μg/ml) and incubation for 24 h, and
rinsed three times in phosphate-buffered saline (PBS) at 4°C. The
cells were then treated with 100 μl of a cell lysate buffer (1%
NP-40, 20 mM EDTA, 50 mM Tris-HCl, pH 7.5) and incubated at 4°C for
10 min, followed by centrifugation at 12,000 × g for 30 min. RNase
A was added to the supernatant and incubated at 37°C for 1 h.
Proteinase K was then added to the supernatant, which was then
incubated at 37°C for 8 h. An equal volume of isopropanol was added
and followed by incubation at −80°C for 24 h to precipitate the
genomic DNA. The supernatant was removed after centrifugation at
12,000 × g for 15 min at 4°C. The supernatant was allowed to dry
naturally and was dissolved in TE buffer, followed by
electrophoresis on 1.5% agarose gel. A gel imaging system was used
for observation and images were captured.
Histology
KB cells were collected after treatment with
berberine (0, 0.1 and 1 μg/ml) and incubation for 24 h, and rinsed
three times in PBS at 4°C. The cells were then fixed with
pre-chilled 4% paraformaldehyde for 30 min at 4°C. Hematoxylin and
eosin staining was performed in order to observe the morphological
changes in the cells. The cells were observed and photographed by
inverted phase-contrast microscopy.
DAPI staining
KB oral cancer cells that had been treated with 0,
0.1 and 1 μg/ml berberine and incubated for 24 h were fixed with 4%
paraformaldehyde before washing with PBS. The washed cells were
stained with 1 mg/ml [4′,6-diamidino-2-phenylindole dihydrochloride
(DAPI); Roche Diagnostics] for 20 min. The nuclear condensation was
observed by fluorescence microscopy (Eclipse TE200; Nikon
Instruments).
Caspase-3/-7 activity assay
The apoptotic activity of executioner caspase-3/-7
was determined using the cell-permeable fluorogenic substrate,
PhiPhiLux-G1D2 (OncoImmunin Inc.,
Gaithersburg, MD, USA), according to the manufacturer’s
instructions.
Flow cytometric analysis
The extent of apoptosis and necrosis was determined
using Annexin V-fluorescein isothiocyanate (FITC) and propidium
iodide (PI), respectively. The cells were washed twice in PBS and
resuspended in a binding buffer (BD Biosciences, San Diego, CA,
USA). Annexin V-FITC and PI (BD Biosciences) were added to the
cells, which were then incubated in the dark for 15 min. The
mixture was then resuspended in 400 ml of binding buffer. The cells
were analyzed using a fluorescence-activated cell sorting Calibur
flow cytometer (Becton-Dickinson, San Jose, CA, USA). Data analysis
was performed using standard Cell Quest software
(Becton-Dickinson).
Immunoblotting
The cell and tissue lysates were prepared using
modified radioimmunoprecipitation assay buffer (1 M Tris-HCl, 150
mM NaCl, 1% Triton X-100, 2 mM EDTA) with a protease inhibitor
(Sigma, USA) and phosphatase inhibitor cocktail (Sigma-Aldrich, St.
Louis, MO, USA). The total protein concentrations of the cell
lysates were determined by bicinchoninic acid protein assays
(Pierce, Rockford, IL, USA). Equal amounts of the protein were
resolved using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to a nitrocellulose membrane for
immunoblotting analyses. After blocking with 5% bovine serum
albumin (BSA) in Tris-buffered saline with 0.1% Tween-20 (TBS-T) at
room temperature for 1 h, the membranes were incubated sequentially
in TBS-T containing 5% BSA and primary antibodies of the
corresponding proteins, at 4°C overnight, in accordance with the
optimized protocol supplied by the manufacturer. After rinsing with
TBS-T, the membranes were incubated with the HRP-conjugated
secondary antibody at room temperature for 1 h. The
immunoreactivity was visualized using an ECL system (Pierce).
Gelatin zymography
An equal volume of cell culture supernatant was
mixed with non-reducing sample buffer [4% SDS, 0.15 M Tris (pH 6.8)
and 20% (vol/vol) glycerol containing 0.05% (wt/vol) bromophenol
blue] and resolved on a 10% polyacrylamide gel containing
copolymerized 0.2% (1 mg/ml) swine skin gelatin (Sigma). After
electrophoresis of the conditioned medium supernatant samples, gels
were washed twice, for 15 min each time, with 2.5% Triton X-100.
Digestion was carried out by incubating the gel in gelatinase
buffer [50 mM Tris-HCl (pH 7.6), 10 mM CaCl2, 50 mM NaCl
and 0.05% Brij-35] at 37°C for 24 h. The gel was stained with 0.1%
Coomassie Brilliant Blue R-250 (GE Healthcare, Piscataway, NJ,
USA), and the locations of gelatinolytic activity were revealed as
clear bands on a background of uniform light blue staining. After
development, gel images were captured and the clear bands were
analyzed using ‘ImageJ’ image analysis software (www.imagej.nih.gov).
Migration assay
To perform the migration assays, KB cells were
cultured onto culture inserts (2×0.22 cm2; Ibidi,
Regensburg, Germany) at 1×104 cells/well. Wounds were
introduced by removing the culture inserts after 24 h of
incubation. Wound widths were measured using images obtained using
an inverted microscope.
Statistical analysis
Data are reported as the mean ± SD of three
individual experiments performed in triplicate. Statistical
analysis was carried out using a Student’s t-test, and a p-value
<0.05 was considered to indicate a statistically significant
result.
Results
Berberine induces the cell death of KB
oral cancer through apoptosis
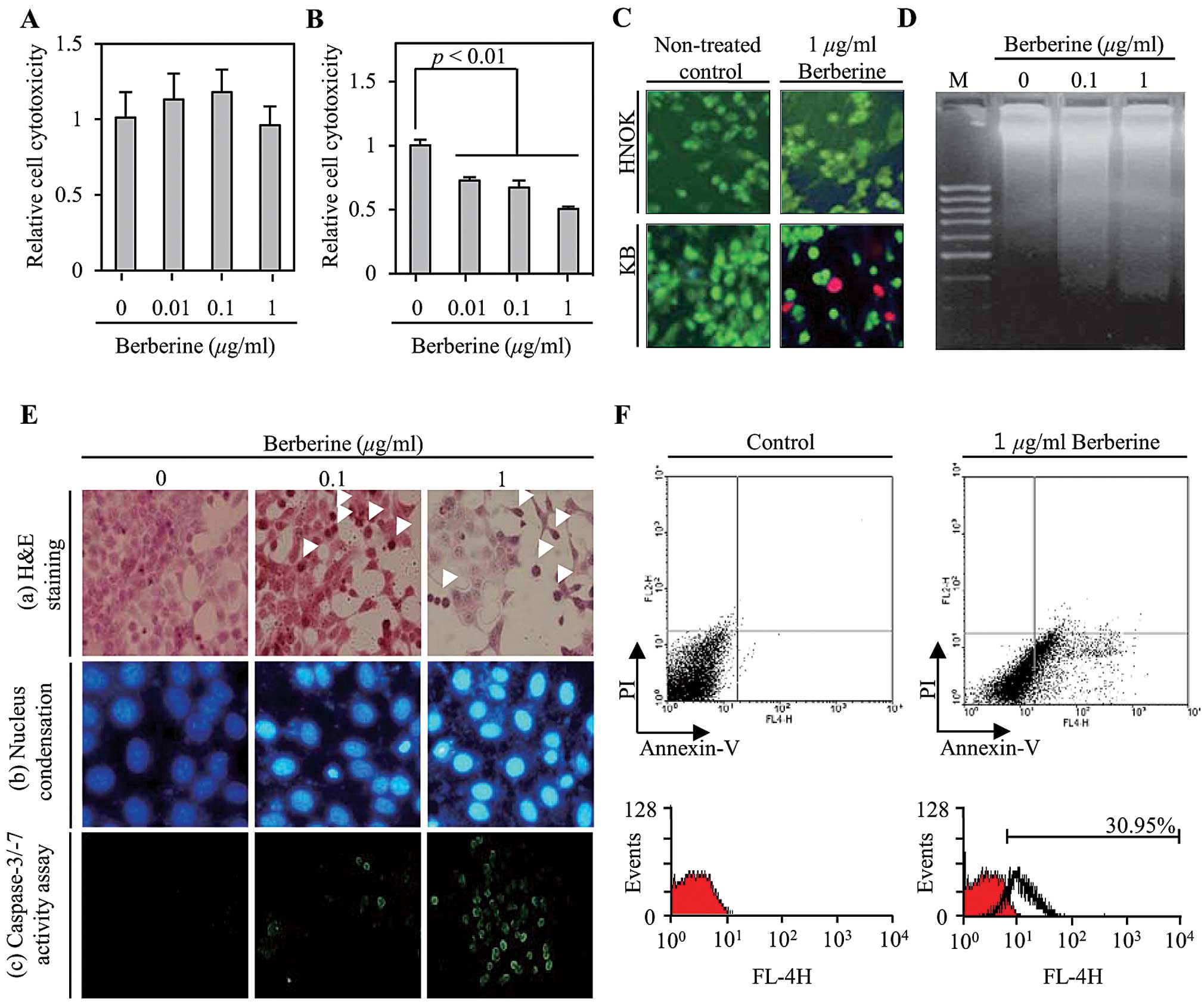
To measure the cytotoxicity of berberine in KB oral
cancer cells and NHKOs, both cells were treated with various
concentrations (0.01, 0.1 and 1 μg/ml) of berberine for 24 h. An
MTT assay was then performed to assess the cell viability. As shown
in Fig. 1A, berberine did not
affect the survival of the primary NHKOs used as normal cells.
However, berberine decreased the cell survival rate of the KB oral
cancer cells both significantly and in a dose-dependent manner
(Fig. 1B). Of particular note, the
viability of the KB cells that were treated with 1 μg/ml berberine
was decreased by 50% compared to the control. To confirm the
berberine-induced cell cytotoxicity in KB cells, microscopy was
used to visualize the live and dead cells stained with calcein-AM
(green fluorescence) and ethidium homodimer 1 (red fluorescence),
respectively. As shown in Fig. 1C,
the primary HNOKs incubated with 1 μg/ml berberine for 24 h were
stained green due to the cleavage of the membrane permeable
calcein-AM by the cytosolic esterase in living cells. Dead cells
were stained red by ethidium bromide homodimer 1 and were observed
to be a significant presence in the KB oral cancer cells that had
been treated with 1 μg/ml berberine. Next, in order to determine
whether or not berberine-induced KB oral cancer cell death is
related to apoptosis, we measured the extent of representative
apoptotic phenomena such as DNA fragmentation, the formation of
apoptotic bodies and DNA condensation. As shown in Fig. 1D, DNA fragmentation was observed to
a significant degree in the KB oral cancer cells that were treated
with 0.1 and 1 μg/ml berberine when compared to the non-treated
control. Furthermore, berberine reduced the cell number and altered
the typical morphology of KB oral cancer cells in a dose-dependent
manner (Fig. 1E-a). Moreover, the
number of KB oral cancer cells that had a condensed nucleus was
significantly increased by berberine treatment as shown in Fig. 1E-b. To further verify the occurrence
of berberine-induced apoptosis in the KB oral cancer cell, we
performed a caspase-3/-7 activity assay using PhiPhiLux substrate
analyzed using flow cytometry with Annexin V and PI as the stains.
As shown in Fig. 1E-c, the
activities of caspase-3/-7 increased in a dose-dependent manner in
the KB cells that had been treated with berberine. Furthermore,
apoptotic population was significantly increased by 30.95% compared
to the non-treated control (Fig.
1F). Taken together, these results suggest that berberine
induces cell death through apoptosis signaling pathways in KB oral
cancer cells.
Berberine-induced cell death of KB oral
cancer is mediated by both extrinsic death receptor-dependent and
intrinsic mitochondrial-dependent apoptotic signaling pathways
Next, to determine exactly which apoptotic signaling
pathways are involved in berberine-induced apoptosis in KB oral
cancer cells, we performed western blot analysis using antibodies
associated with either the extrinsic death receptor-dependent
apoptotic signaling pathway or the intrinsic
mitochondrial-dependent apoptotic signaling pathway. As shown in
Fig. 2A, FasL (molecular weight 28
kDa), which triggers apoptosis through binding with Fas receptor
(FasR) and is known as a member of the death receptor family, was
significantly upregulated in a dose-dependent manner in the KB oral
cancer cells that were treated with berberine. Furthermore, cleaved
caspase-8 (molecular weight 18 kDa), which is a representative
death receptor-dependent pro-apoptotic factor, was upregulated by
FasL expression. At the same time, berberine decreased the
expression of mitochondrial-dependent anti-apoptotic factors such
as Bcl-2 (molecular weight 26 kDa) and Bcl-xL (molecular weight 16
kDa) in the KB oral cancer cells. In contrast,
mitochondrial-dependent pro-apoptotic factors including Bax
(molecular weight 21 kDa), Bad (molecular weight 23 kDa), Apaf-1
(molecular weight 130 kDa) and cleaved caspase-9 (molecular weight
37 kDa) were significantly upregulated following berberine
treatment (Fig. 2B). Finally, to
induce apoptosis, caspase-3 and PARP were cleaved and activated by
upregulation of both extrinsic death receptor-dependent and
intrinsic mitochondrial-dependent apoptotic factors in the
berberine-treated KB cells (Fig.
2C). Furthermore, to verify whether berberine-induced apoptosis
of KB cells is associated with the activation of caspases in both
the extrinsic death receptor-dependent and intrinsic
mitochondrial-dependent apoptotic signaling pathways, we observed
the activation of caspase-3 and PARP in the KB cells that had been
treated with 1 μg/ml berberine in either the presence or absence of
a cell-permeable pan-caspase inhibitor Z-VAD-FMK (50 μM). As shown
in Fig. 2D, in the absence of
Z-VAD-FMK, 1 μg/ml berberine induced the activation of caspase-3
and PARP in KB cells. Whereas, the activation of caspase-3 and PARP
was significantly suppressed in the KB oral cancer cells treated
with 1 μg/ml berberine in addition to Z-VAD-FMK. The observations
are consistent with berberine-induced apoptosis of KB cells being
mediated by the activation of caspases in both the extrinsic death
receptor-dependent and intrinsic mitochondrial-dependent apoptotic
signaling pathways.
Berberine-induced apoptosis of KB oral
cancer cells is regulated by expression of death receptor ligand
FasL through the p38-MAPK signaling pathway
To understand the signaling pathways involved in the
berberine-induced apoptosis of KB oral cancer cells, we examined
the activation of mitogen-activated protein kinase (MAPK) subgroups
such as ERK1/2, p38 and JNK in response to berberine. As shown in
Fig. 3A, the administration of
berberine induced the phosphorylation of ERK1/2 (molecular weight
42 and 44 kDa for p42 and p44, respectively) and p38 (molecular
weight 38 kDa) MAPK at the earliest time point studied (5 min), and
this effect reached a plateau until 60 min post-treatment. However,
the phosphorylation of JNK was not observed in the
berberine-treated KB oral cancer cells at any of the time points.
This suggests that berberine-induced activation of the p38 and
ERK1/2 MAPK pathways is the principal pathway involved in the
apoptosis mediated by berberine in KB cells. To determine which of
these pathways is more principally involved, we inhibited the ERK
and p38 MAPK signaling pathways using chemical inhibitors PD98059
and SB203580, respectively. As shown in Fig. 3B, the berberine-induced upregulation
of FasL was significantly decreased in the presence of the p38
MAPK-specific chemical inhibitor. In contrast, the ERK1/2
MAPK-specific chemical inhibitor synergistically increased the
expression of FasL in the berberine-treated KB cells. Moreover, the
observed amounts of both cleaved caspase-3 and cleaved PARP were
decreased by the presence of the p38 MAPK-specific chemical
inhibitor as well as FasL. Collectively, these findings demonstrate
that berberine-induced apoptosis of KB oral cancer cells is
regulated by FasL expression via the p38 MAPK signaling
pathway.
Migration of KB oral cancer cells is
suppressed by the inhibition of MMP-2/-9 via inhibition of the p38
MAPK signaling pathway
Although recent studies have shown that berberine
inhibits the migration of various types of cancer cells, the
precise effect of berberine on migration and its underlying
mechanisms are not fully understood. Therefore, we observed the
effect that berberine treatment has on the migration of KB oral
cancer cells. As shown in Fig. 4A,
the migration of KB cells was significantly decreased in the
presence of 1 μg/ml berberine compared to the non-treated control.
Therefore, changes in migration-associated factors such as matrix
metalloproteinases (MMPs) were examined by carrying out an MMP
activation assay using gelatin zymography in conditioned media
harvested from KB cells that had been treated with 1 μg/ml
berberine for 24 h. As shown in Fig.
4B, the MMP activation was decreased by berberine treatment in
a dose-dependent manner. Furthermore, the expression levels of
MMP-2 (molecular weight 63 kDa) and MMP-9 (molecular weight 92
kDa), representative MMPs associated with the migration of cancer
cells, were downregulated following berberine treatment (Fig. 4C). In addition, the expression of
both MMP-2 and MMP-9 was significantly decreased in the KB oral
cancer cells that had been co-stimulated with berberine and the p38
MAPK-specific chemical inhibitor compared to those that had been
treated with berberine only (Fig.
4D). ERK1/2 MAPK-specific chemical inhibitor synergistically
enhanced the expression of both MMP-2 and MMP-9 in the KB cells
treated with berberine. Taken together, these results suggest that
berberine may suppress the migration of KB oral cancer cells
through p38-mediated expressional regulation of MMPs.
Discussion
Oral cancer is the sixth most common cancer
worldwide (1). The 5-year survival
rate of patients with oral cancer is less than 50% and this has not
improved significantly over the past few decades (12). Oral cancer is defined clinically as
cancerous tissue formed in the oral cavity or oropharynx, and it
can arise as a primary lesion originating in any of the oral
tissues including the soft palate, roof or floor of the mouth, the
gums, the tongue, and in other areas of the oral cavity. The
clinical symptoms of oral cancer are leukoplakia, erythroplakia,
non-healing wounds, sores and tender lesions characterized by
painful chewing or swallowing. On the other hand, despite
improvements in radiotherapy and chemotherapy for oral cancer,
serious side-effects are still encountered. Therefore, an ideal
chemotherapeutic agent that effectively suppresses tumorigenesis,
leads to minimal side-effects, is inexpensive and easily available
is required for clinical oral cancer therapy.
Considerable preclinical and clinical studies have
suggested that various plant-derived natural products with a long
history of use in Oriental herbal medicine are promising
therapeutic agents against a range of cancers. Furthermore,
phytochemicals isolated from Oriental herbal plants have shown
potential for the prevention of cancer with minimal side-effects,
good safety and high efficacy (13). Among the many phytochemicals
obtained from Oriental herbal plants, berberine, an isoquinoline
derivative alkaloid isolated from the rhizome, roots and stem bark
of a number of Oriental herbal plants, such as Rhizoma
coptidis and Cortex phellodendri, has been used as an
anti-inflammatory agent (14,15),
anti-bacterial compound (16) and
antioxidant (17,18). In particular, the anticancer
activities of berberine in models of various organs, such as the
liver (19), esophagus (20), colon (21), ovaries (22), bladder (23) and breast (24), have been studied. However, the
anticancer activity of berberine in oral cancer is not completely
understood. Therefore, the present study examined berberine-induced
apoptosis and its cellular mechanisms in KB oral cancer cells.
Ideally, chemotherapeutic agents for cancer therapy
should not affect adjacent healthy tissues in order to minimize
side-effects. In studies of berberine-induced cancer cell-specific
cytotoxicity, berberine inhibited the invasion of human cells
without eliciting cytotoxicity in healthy cells (9,25).
Therefore, we assessed berberine-induced cell cytotoxicity using an
MTT assay on primary HNOKs originating from the oral cavity. As
shown in Fig. 1A, berberine did not
affect the survival of HNOKs at the defined treatment
concentration. In contrast, the survival rate of KB oral cancer
cells was decreased significantly by berberine in a dose-dependent
manner (Fig. 1B). Furthermore, a
cell survival assay was performed using image analysis to confirm
the results of berberine-induced cell cytotoxicity on both HNOKs
and KB cells. According to the data in Fig. 1C, a large number of the KB cells
that had been treated with berberine were killed as evidenced by
red ethidium bromide homodimer 1 staining. These results suggest
that berberine could be used as a potent chemotherapeutic agent
with cancer cell-specific effects and minimal side-effects.
Furthermore, these results showed that the
anticancer effect of berberine in KB oral cancer cells is closely
associated to apoptotic cell death, as shown by the extent of DNA
fragmentation, formation of apoptotic bodies and nuclear
condensation. Similarly, berberine-induced inhibition of cell
proliferation, morphological alteration and nuclear condensation
were also observed in the human lung cancer cell lines, A549 and
H1299 (25). Therefore, a
caspase-3/-7 activity assay was performed using the cell-permeable
fluorogenic substrate PhiPhiLux staining. As shown in Fig. 1E-c, activated caspase-3/-7 was
observed in the berberine-treated KB cells. Caspase-3, a
representative pro-apoptotic factor, was activated in the end
stages of both the extrinsic death receptor-mediated and the
mitochondrial-dependent apoptotic signaling pathways (Fig 2C). Furthermore, the apoptotic
population stained using Annexin-5 (that binds to the surface of
apoptotic cells) increased in a time-dependent manner in the KB
oral cancer cells that had been treated with berberine (Fig. 1F). Therefore, berberine-induced
cancer-specific cell death is mediated via the apoptotic signaling
pathway.
According to a recent report, berberine-induced cell
death is mediated via the intrinsic mitochondrial-dependent
apoptotic signaling pathway in a range of cancers, including liver
(26,27) and breast cancer (28). To verify the apoptotic signaling
pathway in berberine-treated KB, the activation and/or expression
of apoptotic factors associated with apoptosis was assessed by
immunoblotting. As shown in Fig. 2,
death receptor ligand FasL, which initiates the extrinsic death
receptor-mediated apoptotic signaling pathway, was upregulated
significantly by berberine treatment in the KB oral cancer cells.
Pro-caspase-8 was then activated via interaction with
Fas-interacting protein FADD. In addition, activated caspase-8
induced the cleavage of pro-caspase-3 to activate poly(ADP ribose)
polymerase (PARP). Finally, activated PARP induced DNA
fragmentation via single-strand DNA breaking in the nucleus of
cancer cells. Furthermore, Bcl-2 and Bcl-xL, anti-apoptotic factors
associated with the intrinsic mitochondrial-dependent apoptotic
signaling pathway, were downregulated significantly by berberine
treatment in the KB oral cancer cells. Both the downregulation of
the anti-apoptotic factors and upregulation of both pro-apoptotic
factors and tumor suppression factors triggered the release of
cytochrome c from the mitochondria. In addition, apoptotic
protease activating factor-1 (Apaf-1) was upregulated significantly
by berberine in the KB oral cancer cells. Both the released
cytochrome c and upregulated Apaf-1 induced the activation
of caspase-9 leading to the activation of caspase-3 and PARP.
Recent studies of berberine-induced apoptosis in oral cancer
reported that berberine induced apoptosis by promoting the
expression of caspase-8, -9 and -3 in the human tongue squamous
carcinoma cell line, SCC-4 (29).
Ho et al suggested that berberine-induced apoptosis was
mediated by both apoptotic signaling pathways (29). Similarly, Lin et al reported
that berberine induced apoptosis in human HSC-3 oral cancer cells
via activation of both the death receptor-mediated and the
mitochondrial pathways (30).
Therefore, these findings suggest that berberine-induced KB cell
death is mediated by both the extrinsic death receptor-mediated
pathway and the intrinsic mitochondrial-dependent apoptotic
signaling pathway. Upregulated FasL, however, not only triggers the
extrinsic death receptor-mediated apoptotic signaling pathway, but
also initiates the intrinsic mitochondria-dependent apoptotic
pathway via the cleavage of Bid to tBid by activated caspase-8.
Although the cleavage of Bid to tBid was not observed in the
present study, Bax, a downstream, pro-apoptotic factor of tBid, was
upregulated significantly by berberine treatment (Fig. 2B). This suggests that activated
caspase-8 in the extrinsic apoptosis signaling pathway can affect
the intrinsic mitochondrial-dependent apoptotic signaling pathway
in KB oral cancer cells.
Although there have been a large number of in
vitro studies on the cellular function of MAPKs, the biological
connection between berberine-induced apoptosis and the MAPK
signaling pathway is largely unknown. Recently, Zheng et al
(31), reported that
berberine-induced apoptosis of human lung adenocarcinoma was
mediated by activation of the p38 MAPK signaling pathway.
Similarly, our results showed that berberine induced the
phosphorylation of both ERK1/2 and p38 MAPK in the KB oral cancer
cells (Fig. 3). Furthermore,
berberine-induced apoptosis was mediated by the expression of death
receptor ligand FasL, which induces apoptosis in various cancer
cells, through the activation of the p38 MAPK signaling pathway,
yet not the ERK1/2 MAPK signaling pathway. Even though we did not
observe a connection at the cellular level between the activation
of the p38 MAPK signaling pathway and the expression of p53 in the
present study, Zheng et al (31), reported that berberine upregulates
the tumor suppressor p53 through the activation of the p38 MAPK
signaling pathway and reduces the cancer cell proliferation and
induces cell cycle arrest. Therefore, our data suggest that
berberine may induce cell cycle arrest and the inhibition of cell
proliferation in KB oral cancer cells.
One of main characteristics of malignant cancer
cells is invasion and metastasis. Recently, many studies found that
berberine suppressed the migration of various types of cancer cells
such as T24 bladder (32) and human
colon cancer (33), melanoma
(34) and human SCC-4 tongue
squamous cancer cells (35), yet
the cellular physiological mechanisms of berberine involved in the
migration of KB oral cancer cells have yet to be reported. However,
the migration and invasion of cancer cells are closely associated
with expression of MMPs, which are required for remodeling of the
extracellular matrix. MMP-9, a downstream target molecule of the
MAPK subfamily, is particularly associated with cancer cell
invasion. Furthermore, the expression of MMP-2 is associated with
tumor invasion and metastasis. Therefore, the expressional
suppression of MMP-2 and MMP-9 may result in the inhibition of
migration in cancer cells. In the present study, we demonstrated
that berberine suppressed the migration of KB oral cancer cells
through the downregulation of both MMP-2 and MMP-9 (Fig. 4). Recently, Ho et al
(35) reported that berberine suppressed in vitro
migration and invasion of human SCC-4 tongue squamous cancer cells
through the inhibition of MMP-2 and MMP-9 and was regulated by
nuclear factor-κB (NFκB). In contrast, in the KB oral cancer cells
that were treated with berberine, the expression of MMP-2 and MMP-3
was regulated by the p38 MAPK signaling pathway (Fig. 4D).
In summary, our data demonstrated that
berberine-induced apoptosis of KB oral cancer cells was triggered
by the expression of FasL via the activation of the p38 MAPK
signaling pathway and was mediated by both extrinsic death
receptor-dependent and intrinsic mitochondrial-dependent apoptotic
signaling pathways. Furthermore, berberine-induced suppression of
migration in KB cells was mediated by the downregulation of MMP-2
and MMP-7 through the activation of the p38 MAPK signaling pathway.
Indeed, berberine may have great potential for the future treatment
of oral cancer, and its potent anticancer properties highlight its
promise as a chemotherapeutic agent.
References
|
1
|
Sunil PM, Ramachandran CR, Arulmoli, Devi
S and Soma SV: Cytogenetic alterations in oral squamous cell
carcinoma detected by karyotyping (g-banding). OMPJ. 2:89–95.
2011.
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scully C: Oral cancer aetiopathogenesis;
past, present and future aspects. Med Oral Patol Oral Cir Bucal.
16:e306–e311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Llewellyn CD, Johnson NW and
Warnakulasuriya KA: Risk factors for squamous cell carcinoma of the
oral cavity in young people - a comprehensive literature review.
Oral Oncol. 37:401–418. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugerman PB and Savage NW: Current
concepts in oral cancer. Aust Dent J. 44:147–156. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gupta S, Kong W, Peng Y, Miao Q and
Mackillop WJ: Temporal trends in the incidence and survival of
cancers of the upper aerodigestive tract in Ontario and the United
States. Int J Cancer. 125:2159–2165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang J, Feng Y, Tsao S, Wang N, Curtain R
and Wang Y: Berberine and Coptidis rhizoma as novel antineoplastic
agents: a review of traditional use and biomedical investigations.
J Ethnopharmacol. 126:5–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen TC, Lai KC, Yang JS, et al:
Involvement of reactive oxygen species and caspase-dependent
pathway in berberine-induced cell cycle arrest and apoptosis in C6
rat glioma cells. Int J Oncol. 34:1681–1690. 2009.PubMed/NCBI
|
|
9
|
Liu B, Wang G, Yang J, Pan X, Yang Z and
Zang L: Berberine inhibits human hepatoma cell invasion without
cytotoxicity in healthy hepatocytes. PLoS One. 6:e214162011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hwang JM, Kuo HC, Tseng TH, Liu JY and Chu
CY: Berberine induces apoptosis through a mitochondria/caspases
pathway in human hepatoma cells. Arch Toxicol. 80:62–73. 2006.
View Article : Google Scholar
|
|
11
|
Kim JS, Ellman MB, An HS, van Wijnen AJ,
Borgia JA and Im HJ: Insulin-like growth factor 1 synergizes with
bone morphogenetic protein 7-mediated anabolism in bovine
intervertebral disc cells. Arthritis Rheum. 62:3706–3715. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brinkman BM and Wong DT: Disease mechanism
and biomarkers of oral squamous cell carcinoma. Curr Opin Oncol.
18:228–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sundin T, Peffley DM and Hentosh P:
Disruption of an hTERT-mTOR-RAPTOR protein complex by a
phytochemical perillyl alcohol and rapamycin. Mol Cell Biochem.
375:97–104. 2013.PubMed/NCBI
|
|
14
|
Jiang Q, Liu P, Wu X, et al: Berberine
attenuates lipopolysaccha-ride-induced extracelluar matrix
accumulation and inflammation in rat mesangial cells: involvement
of NF-κB signaling pathway. Mol Cell Endocrinol. 331:34–40. 2011.
View Article : Google Scholar
|
|
15
|
Lin K, Liu S, Shen Y and Li Q: Berberine
attenuates cigarette smoke-induced acute lung inflammation.
Inflammation. 36:1079–1086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bandyopadhyay S, Patra PH, Mahanti A, et
al: Potential antibacterial activity of berberine against multi
drug resistant enterovirulent Escherichia coli isolated from yaks
(Poephagus grunniens) with haemorrhagic diarrhoea. Asian Pac J Trop
Med. 6:315–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan Y, Tang Q, Hu BR and Xiang JZ:
Antioxidant properties of berberine on cultured rabbit corpus
cavernosum smooth muscle cells injured by hydrogen peroxide. Acta
Pharmacol Sin. 28:1914–1918. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou JY and Zhou SW: Protective effect of
berberine on anti-oxidant enzymes and positive transcription
elongation factor b expression in diabetic rat liver. Fitoterapia.
82:184–189. 2011. View Article : Google Scholar
|
|
19
|
Tan YL, Goh D and Ong ES: Investigation of
differentially expressed proteins due to the inhibitory effects of
berberine in human liver cancer cell line HepG2. Mol Biosyst.
2:250–258. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iizuka N, Miyamoto K, Okita K, et al:
Inhibitory effect of Coptidis Rhizoma and berberine on the
proliferation of human esophageal cancer cell lines. Cancer Lett.
148:19–25. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukuda K, Hibiya Y, Mutoh M, Koshiji M,
Akao S and Fujiwara H: Inhibition by berberine of cyclooxygenase-2
transcriptional activity in human colon cancer cells. J
Ethnopharmacol. 66:227–233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marverti G, Ligabue A, Lombardi P, et al:
Modulation of the expression of folate cycle enzymes and polyamine
metabolism by berberine in cisplatin-sensitive and -resistant human
ovarian cancer cells. Int J Oncol. 43:1269–1280. 2013.PubMed/NCBI
|
|
23
|
Yan K, Zhang C, Feng J, et al: Induction
of G1 cell cycle arrest and apoptosis by berberine in bladder
cancer cells. Eur J Pharmacol. 661:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma X, Zhou J, Zhang CX, et al: Modulation
of drug-resistant membrane and apoptosis proteins of breast cancer
stem cells by targeting berberine liposomes. Biomaterials.
34:4452–4465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu L, Chen W, Guo W, et al: Berberine
targets AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF and
cytochrome-c/caspase signaling to suppress human cancer cell
growth. PLoS One. 8:e692402013. View Article : Google Scholar
|
|
26
|
Yip NK and Ho WS: Berberine induces
apoptosis via the mitochondrial pathway in liver cancer cells.
Oncol Rep. 30:1107–1112. 2013.PubMed/NCBI
|
|
27
|
Wang N, Feng Y, Zhu M, et al: Berberine
induces autophagic cell death and mitochondrial apoptosis in liver
cancer cells: the cellular mechanism. J Cell Biochem.
111:1426–1436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Patil JB, Kim J and Jayaprakasha GK:
Berberine induces apoptosis in breast cancer cells (MCF-7) through
mitochondrial-dependent pathway. Eur J Pharmacol. 645:70–78. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ho YT, Lu CC, Yang JS, et al: Berberine
induced apoptosis via promoting the expression of caspase-8, -9 and
-3, apoptosis-inducing factor and endonuclease G in SCC-4 human
tongue squamous carcinoma cancer cells. Anticancer Res.
29:4063–4070. 2009.PubMed/NCBI
|
|
30
|
Lin CC, Yang JS, Chen JT, et al: Berberine
induces apoptosis in human HSC-3 oral cancer cells via simultaneous
activation of the death receptor-mediated and mitochondrial
pathway. Anticancer Res. 27:3371–3378. 2007.PubMed/NCBI
|
|
31
|
Zheng F, Tang Q, Wu J, et al: p38α
MAPK-mediated induction and interaction of FOXO3a and p53
contribute to the inhibited-growth and induced-apoptosis of human
lung adenocarcinoma cells by berberine. J Exp Clin Cancer Res.
33:362014. View Article : Google Scholar
|
|
32
|
Yan L, Yan K, Kun W, et al: Berberine
inhibits the migration and invasion of T24 bladder cancer cells via
reducing the expression of heparanase. Tumour Biol. 34:215–221.
2013. View Article : Google Scholar
|
|
33
|
Park JJ, Seo SM, Kim EJ, et al: Berberine
inhibits human colon cancer cell migration via AMP-activated
protein kinase-mediated downregulation of integrin β1 signaling.
Biochem Biophys Res Commun. 426:461–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh T, Vaid M, Katiyar N, Sharma S and
Katiyar SK: Berberine, an isoquinoline alkaloid, inhibits melanoma
cancer cell migration by reducing the expressions of
cyclooxygenase-2, prostaglandin E2 and prostaglandin
E2 receptors. Carcinogenesis. 32:86–92. 2011. View Article : Google Scholar
|
|
35
|
Ho YT, Yang JS, Li TC, et al: Berberine
suppresses in vitro migration and invasion of human SCC-4 tongue
squamous cancer cells through the inhibitions of FAK, IKK, NF-κB,
u-PA and MMP-2 and -9. Cancer Lett. 279:155–162. 2009. View Article : Google Scholar : PubMed/NCBI
|