Introduction
As one of the most common malignancies worldwide,
gastric cancer is a complex disease and is frequently characterized
by genetic and epigenetic alterations (1,2). The
interaction of genetic predispositions with environmental factors
is one major pathogenesis of this cancer (3). Metastasis, the most critical aspect of
tumorigenesis, is considered as a leading cause of cancer-related
mortality (3,4). Thus, important goals of cancer
research are the identification of key genes and signaling pathways
involved in metastasis and tumor progression, which may facilitate
the prediction of those events and hereby identify therapeutic
targets.
It is generally accepted that the tumor environment
determines the behavior of malignant cells. In the tumor
environment, extracellular matrix (ECM) proteoglycans are essential
components and represent a complex structural entity that supports
and interacts with epithelial structures, playing crucial
pathological roles (5). Asporin
(ASPN), a novel member of the small leucine-rich repeat
proteoglycan (SLRP) family, contains a unique aspartate-rich N
terminus and is involved in the stromal modification of ECM
(6). The expression levels of ASPN
and other related matrix proteoglycans, for instance decorin and
biglycan, have been assessed in gastric pathology, and are
implicated in the oncogenesis and development of human gastric
carcinoma (7–10). Particularly, Satoyoshi et al
recently concluded that ASPN activates the coordinated invasion of
scirrhous gastric cancer and cancer-associated fibroblasts by
activation of the CD44-Rac1 pathway (7). In addition, by applying quantitative
mass spectrometry, innovative proteomic technologies or microarray
analysis, ASPN was proven to be upregulated in a series of cancers,
including pancreatic cancer (11),
pancreatic ductal adenocarcinoma (12), prostate cancer (13) and breast carcinoma (14). In osteoarthritis, Kizawa et
al found a direct interaction between ASPN and TGF-β. An ASPN
allele with a 14-aspartic acid repeat in the N-terminal region, was
designated as D14. The frequency of the D14 allele was found to
increase with disease severity, and the effect of ASPN on TGF-β
activity was allele-specific (15).
However, the exact roles of ASPN responsible for tumor cell
proliferation and migration and the specific molecular mechanisms
have not been fully elucidaded.
Epidermal growth factor (EGF) and its receptor
(EGFR) are closely associated with tumorigenesis. Overexpression or
activation of these has been observed in a variety of tumor types
and cell lines, including gastric cancer (16,17).
Binding of EGF to EGFR may accelerate cell proliferation and
migration, and trigger epithelial cell signaling in gastric cancer
(18). The MAPK/ERK pathway is a
classic downstream signaling pathway of EGFR signaling (19). The Bcl-2 gene family could be
regulated by the MAPK/ERK pathway. The homeostatic balance between
pro-apoptotic and anti-apoptotic homodimers (such as Bax and Bcl-2)
can reflect cellular apoptosis or proliferation (20). CD44, a cell surface molecule, is
associated with EGFR and is involved in diverse cell-cell and
cell-matrix interactions, resulting in the metastasis of mammary
carcinoma cells (21). In addition,
matrix metalloproteinases (MMPs), particularly the gelatinase
subfamily MMP-2, has been considered to participate in tumor
progression and metastasis in many types of cancers (22).
Therefore, based on these previous findings, the
present study aimed to further investigate the expression and the
specific cellular function of ASPN in human primary gastric
adenocarcinomas, to extend the knowledge of this matrix
proteoglycan in the development and progression of cancer.
Materials and methods
Clinical materials
Pairs of primary gastric carcinoma tissues and their
corresponding non-neoplastic gastric mucosal tissues (≥5 cm) were
acquired from 46 gastric cancer patients who underwent curative
surgery at the First Affiliated Hospital of Yangtze University.
Written informed consent was obtained from all the participants
according to a protocol approved by the Yangtze University of
Medicine Institutional Review Board. The characteristics of the
patients were obtained from medical records and are summarized in
Table I. Portions of the tissues
were processed for immunohistochemistry, quantitative real-time PCR
and western blot analyses. All samples were frozen immediately in
liquid nitrogen and stored at −80°C until required.
 | Table IClinical characteristics of the
gastric cancer patients. |
Table I
Clinical characteristics of the
gastric cancer patients.
|
Characteristics | No. of patients (%)
(N=46) |
|---|
| Gender |
| Male | 27 (58.70) |
| Female | 19 (41.30) |
| Age (years) |
| >60 | 24 (52.17) |
| ≤60 | 22 (47.83) |
| Tumor size
(cm) |
| <6 | 25 (54.35) |
| ≥6 | 21 (45.65) |
| Type |
| Tubular
adenocarcinoma | 17 (36.96) |
| Mucosal
adenocarcinoma | 16 (34.78) |
| Signet-ring cell
carcinoma | 13 (28.26) |
| Clinical stage |
| I and II | 19 (41.30) |
| III and IV | 27 (58.70) |
Immunohistochemical analysis
Gastric tissues were formaldehyde-fixed and
paraffin-embedded. Sections (5-μm) were deparaffinized in
Citrisolve and rehydrated through graded ethanol.
Immunohistochemical staining was performed using an EnVision kit
(Dako, Carpinteria, CA, USA) according to the manufacturer’s
protocol. The sections were incubated with a primary antibody
against ASPN (Sigma-Aldrich, St. Louis, MO, USA) or the negative
control (normal rabbit IgG; Cell Signaling Technology, Danvers, MA,
USA). Analysis of the immunohistochemical staining was performed
independently by two pathologists. Five random fields from each
section were photographed and analyzed with Image-Pro Plus 6.0
software.
Cell lines and culture
The human normal gastric epithelial cell line GES
and, human gastric cancer cell lines SGC-7901 and BGC-823 were
purchased from the Institute of Biochemistry and Cell Biology of
the Chinese Academy of Science. Human gastric cancer cell lines
AGS, MKN45 and N87 were obtained from the China Center for Type
Culture Collection. All of the cell lines were maintained in
RPMI-1640 (Hyclone, Logan, UT, USA) medium, and the media were
supplemented with 10% FBS (Hyclone), 100 U/ml penicillin, 100 μg/ml
streptomycin sulphate and 1 μM sodium pyruvate. The cells were
cultured at 37°C in a humidified incubator with 5% CO2.
The cells were passaged with trypsin-EDTA (0.05% trypsin and 0.53
μM tetrasodium EDTA).
Plasmid construction and cell
transfection
The ASPN silencing plasmid shRNA was constructed by
Shanghai GenePharma Co., Ltd. (Shanghai, China) targeting the gene
aspn (Gene Bank accession no. NM_017680), and was cloned
into the mammalian expression vector GV248 with AgeI and
EcoRI restriction enzyme sites. Four siRNAs targeting the
indicated genes were designed as follows: ASPN-RNAi (26538), CGG
TGA AAT ACT GGG AAA T; ASPN-RNAi (26539), TCT TGA TAA TAA TGG GAT
A; ASPN-RNAi (26540), CTC TTG ATA ATA ATG GGA T; and ASPN-RNAi
(26541), AAC CAA CAT TCC ATT TGA T.
According to the manufacturer’s specifications,
human gastric cancer cells were transfected with siRNA-ASPN-GV248
or siRNA-NC-GV248 with Lipofectamine 2000 transfection reagent
(Invitrogen, Gaithersburg, MD, USA) in 6-well plates. The plasmid
expressing EGFP was used to evaluate the transfection efficacy.
Quantitative real-time PCR analyses
Total RNA from the cells or the frozen tissues was
extracted with TRIzol reagent (Takara, Dalian, China) according to
the manufacturer’s instructions. The RNA quality (A260/A280 ratio)
and quantity were determined using a standard spectrophotometer.
One microgram of RNA was reversely transcribed using the RevertAid™
First Strand cDNA Synthesis kit (#1622; Fermentas, Vilnius,
Lithuania) at 42°C for 45 min, followed by 70°C for 5 min.
Quantitative real-time PCR was performed using QPK-201 SYBR-Green
Master Mix (Toyobo, Osaka, Japan) on a 7300 Real-time PCR system
(Applied Biosystems, Foster City, CA, USA). The target
cDNA-specific primers of ASPN were utilized as described previously
(14). GAPDH was used as the
normalization control, and duplicate experiments were performed for
ASPN mRNA measurement. The mRNA expression level was determined
using the 2(−Δ ΔCt) method.
Cell viability assay
AGS cells were seeded onto 96-well plates for 24 h
after siRNA-ASPN-GV248 or siRNA-NC-GV248 transfection at a density
of 3,000 cells/well (day 0). Cell viability was assessed on day 24,
48 and 72 h, using the Cell Counting Kit-8 assay (CCK-8; Dojindo
Laboratories, Tokyo, Japan). Ten microliters of reagent was added
to each well, incubated at 37°C for 1 h, and the absorbance was
detected at 450 nm according to the manufacturer’s instructions.
With 5 replicates in each experiment, independent experiments were
repeated 3 times.
Colony formation assay
AGS cells transfected with siRNA-ASPN-GV248 or the
GV248 empty vector were selected with G418-resistance
(Sigma-Aldrich) for 2 weeks. The stably transfected cells were
cultured in 6-well plates (500/well) until colonies formed
consisting of more than 50 cells. Then the cells were fixed in
cold-methanol and stained with crystal violet solution. The
colonies were counted with Quantity One software (Bio-Rad,
Hercules, CA, USA) and photographed. The experiment was performed
in triplicate wells in 3 independent experiments.
Transwell assay
Transwell assays were performed after the expression
of ASPN was downregulated to observe the direct effect of the
apsn gene on the migration of gastric cancer cells. Briefly,
Transwell inserts with 6.5-mm diameter polycarbonate 8-μm
microporous membranes (Corning, Inc., Corning, NY, USA) were placed
over the bottom chambers that were filled with culture medium
containing 20% FBS. The AGS cells that transfected for 24 h with
siRNA-ASPN-GV248 or siRNA-NC-GV248 were suspended in RPMI-1640
medium containing 1% FBS and added to the upper chamber. After
another 20-h incubation, cells on the upper surface of the well
were removed with a cotton swab, and the migrated cells were fixed
in cold methanol and stained with crystal violet (23). The number of transmigrated cells in
5 random fields from each well was counted and photographed. Each
experiment was performed in triplicate, and migration was expressed
as the mean ± SD of total cells counted per field.
Wound-healing assay
Wound-healing assays were performed as described
previously (23). Briefly, after
transfecting with siRNA-ASPN-GV248 or siRNA-NC-GV248 for 24 h, the
cells were seeded in 6-well plates (2.0×106 cells/well).
Once a confluent cell monolayer formed, it was scraped using a
yellow sterile pipette tip to generate a scratch wound. Then, the
cells were washed with PBS to remove the cellular debris. The
migration of the cells into the scratch was observed and
photographed at time-points of 0, 10 and 20 h. Wound areas were
calculated to determine the migrated distance. Experiments were
performed in triplicate.
Protein extraction and
immunoblotting
Total protein was prepared from the human tissue
specimens and treated cells with RIPA lysis buffer. The protein
concentration was determined using the protein assay reagent (BCA;
Beyotime, Shanghai, China). The protein extracts were boiled with
5X loading buffer. Equal amounts of protein (40 μg) were separated
on polyacrylamide gel and electro-transferred, transferred to a
nitrocellulose membrane, and then blocked with 5% non-fat dry milk
in TBS containing 0.1% Tween-20. The following primary antibodies
were used for the assays: anti-ASPN (Sigma-Aldrich), anti-Bcl-2,
anti-Bax, anti-ERK and anti-p-ERK (Cell Signaling Technology),
anti-EGFR, anti-p-EGFR and anti-CD44 (Peprotech, Inc., Rocky Hill,
NJ, USA), anti-MMP-2 (Abcam, Cambridge, UK) and anti-GAPDH
(Bioworld Technology Inc., St. Louis. Park, MN, USA). The blots
were visualized with a HRP-conjugated antibody followed by
chemiluminescence reagent (Millipore, Billerica, MA, USA) detection
on photographic film. The target protein was normalized to GAPDH
through a comparison of the gray scale values, and the analysis was
performed with Quantity One software.
Statistical analysis
Quantitative data are presented as means ± SD.
GraphPad Prism version 5.0 for Windows was used for all statistical
analysis, and the Student’s t-test was used to compare the values
of the test and control samples. P<0.05 was considered to
indicate a statistically significant result.
Results
Abnormally expressed ASPN in gastric
cancer
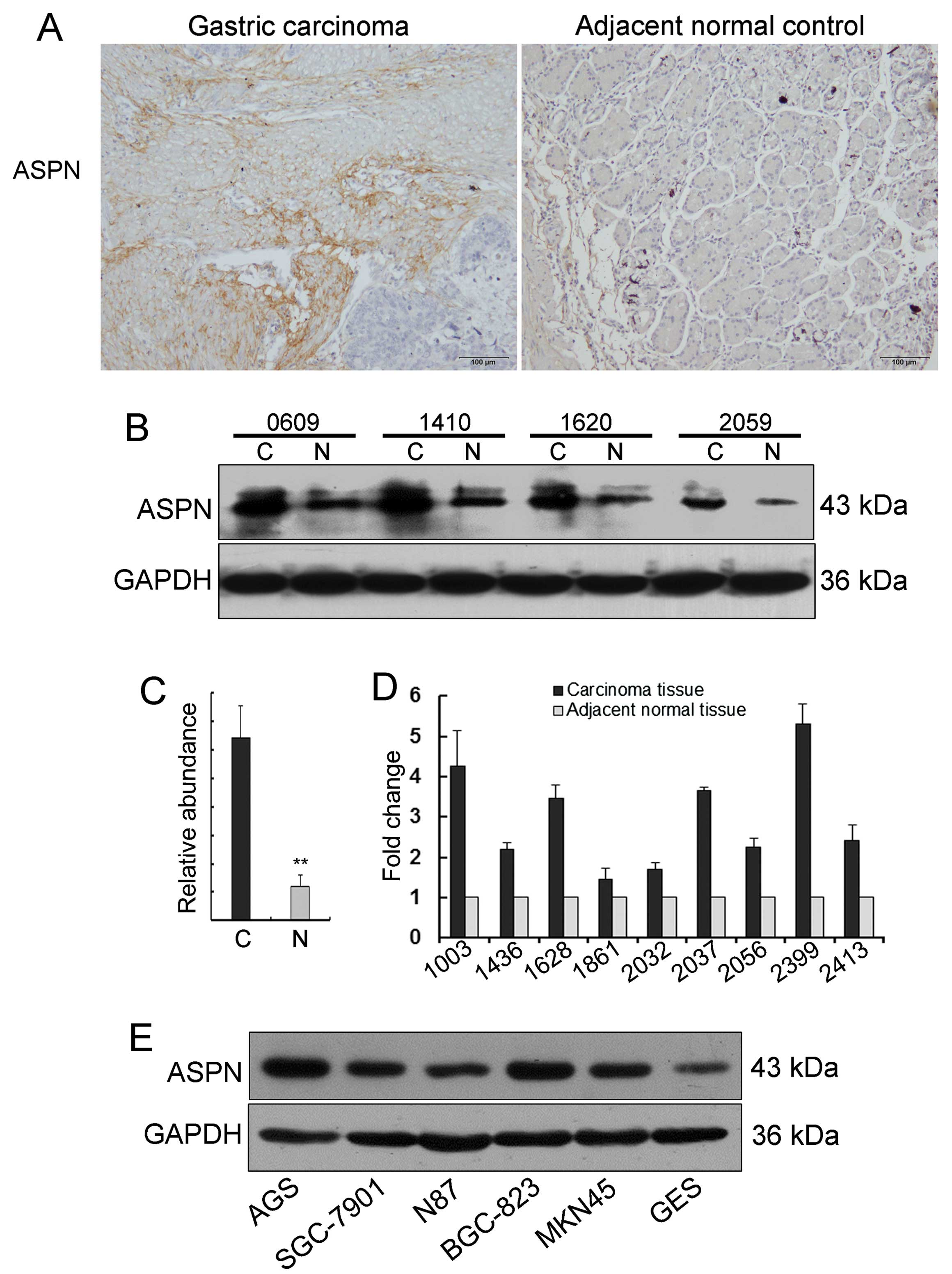
To evaluate the expression of ASPN in gastric
cancer, 46 pairs of primary gastric carcinoma tumors and matched
adjacent non-tumor tissues were analyzed by immunohistochemical
staining. The sections showed markedly increased ASPN staining
(31/46) in cancer relative to the matching non-cancerous gastric
mucosa samples from the same patients (Fig. 1A). Additionally, the aberrant ASPN
expression was confirmed by western blot analysis in 10-paired
gastric carcinoma tissues. This analysis also revealed that ASPN
protein levels were significantly increased in the primary tumor
tissues when compared to the tumor adjacent tissues (Fig. 1B and C). Furthermore, ASPN mRNA
expression was investigated in gastric cancer with quantitative
real-time PCR. There was an insignificant trend toward ASPN mRNA
upregulation in the gastric cancer tissues relative to the matched
adjacent non-tumor tissues (Fig.
1D). Taken together, these results suggest that ASPN is
aberrantly expressed in gastric cancer and the proteoglycan may
function as an oncogene in gastric carcinogenesis.
ASPN has varied expression in gastric
cancer epithelial cell lines
We further examined ASPN protein levels in different
gastric cancer epithelial cell lines, including AGS, MKN28,
SGC-7901, N87, BGC-823, MKN45, and the human immortalized gastric
mucosa epithelial cell GES. Among these cell lines, as shown in
Fig. 1E, the poorly differentiated
adenocarcinoma cell lines AGS, BGC-823 and MKN45 exhibited
relatively high levels of ASPN expression; the moderately
differentiated adenocarcinoma cell line SGC-7901 and the
well-differentiated adenocarcinoma cell line N87 revealed low
levels of ASPN; and the human immortalized gastric mucosa
epithelial cell line GES showed apparently low levels of ASPN
(2). Collectively, the results
suggest that ASPN expression is varied in an inverse trend
following the differentiation degree of gastric cancer epithelial
cell lines, at least to some extent.
ASPN is required for the proliferation of
gastric cancer cells
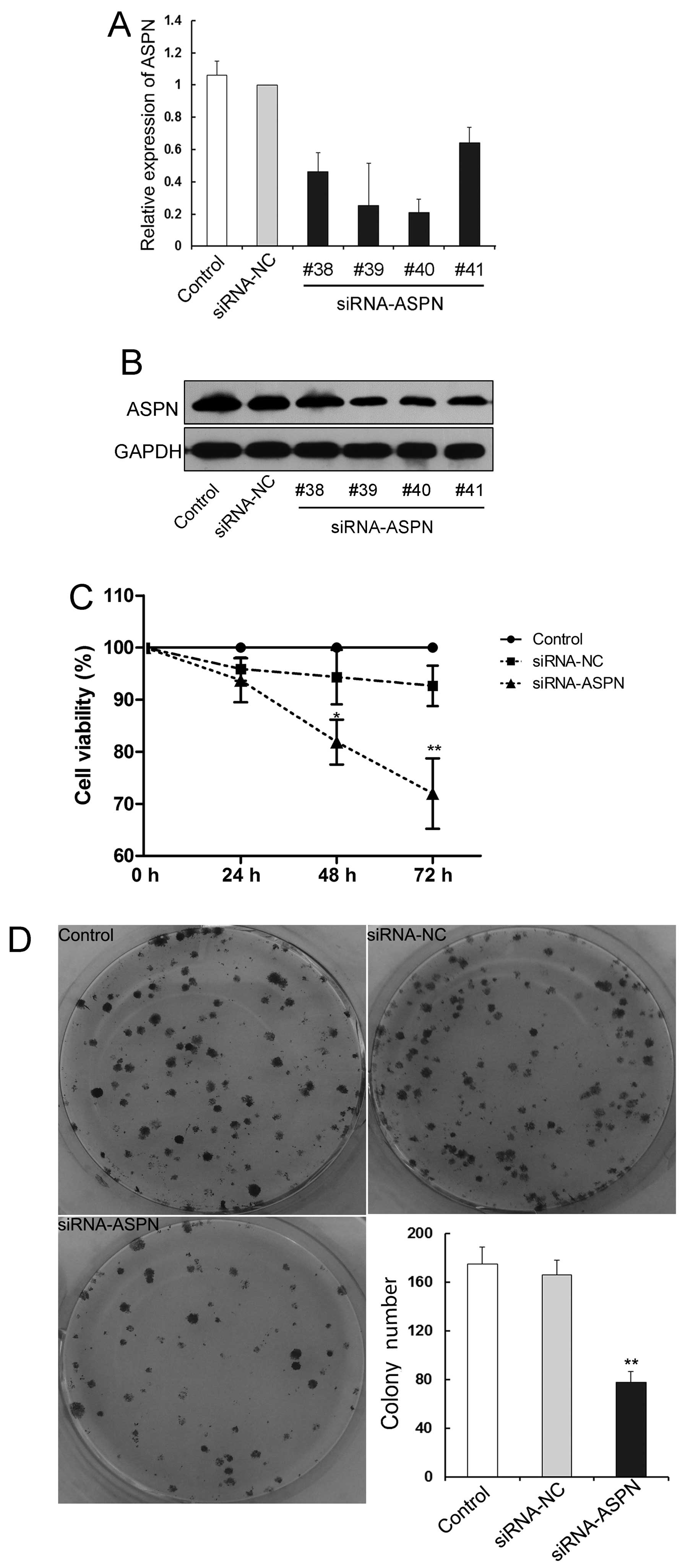
To understand the potential function of ASPN in
gastric cancer, the most highly ASPN-expressing AGS cells were
transfected with siRNA-ASPN-GV248 or siRNA-NC-GV248 vector. The
transfection efficiency was identified and confirmed by
quantitative real-time PCR and western blotting. Among the 4 siRNAs
targeting for the indicated gene, siRNA-ASPN (26540, #40) induced
the highest level of downregulation (Fig. 2A and B). The effect of ASPN on the
cell proliferation in AGS cells was confirmed by CCK-8 assay, which
showed significant reduction in cell viability in the
siRNA-ASPN-transfected cells at the time-points of 48 and 72 h, but
not at 24 h, compared with the control vector transfectants
(Fig. 2C). In addition, similar
results were revealed in the colony formation assay. The cells
stably transfected with siRNA-ASPN displayed a markedly decreased
number of surviving colonies forming on the plates (Fig. 2D). Collectively, these results
indicate that aberrant expression of ASPN may be critical for the
proliferation and survival of gastric tumor cells.
ASPN silencing suppresses the migration
of gastric cancer cells
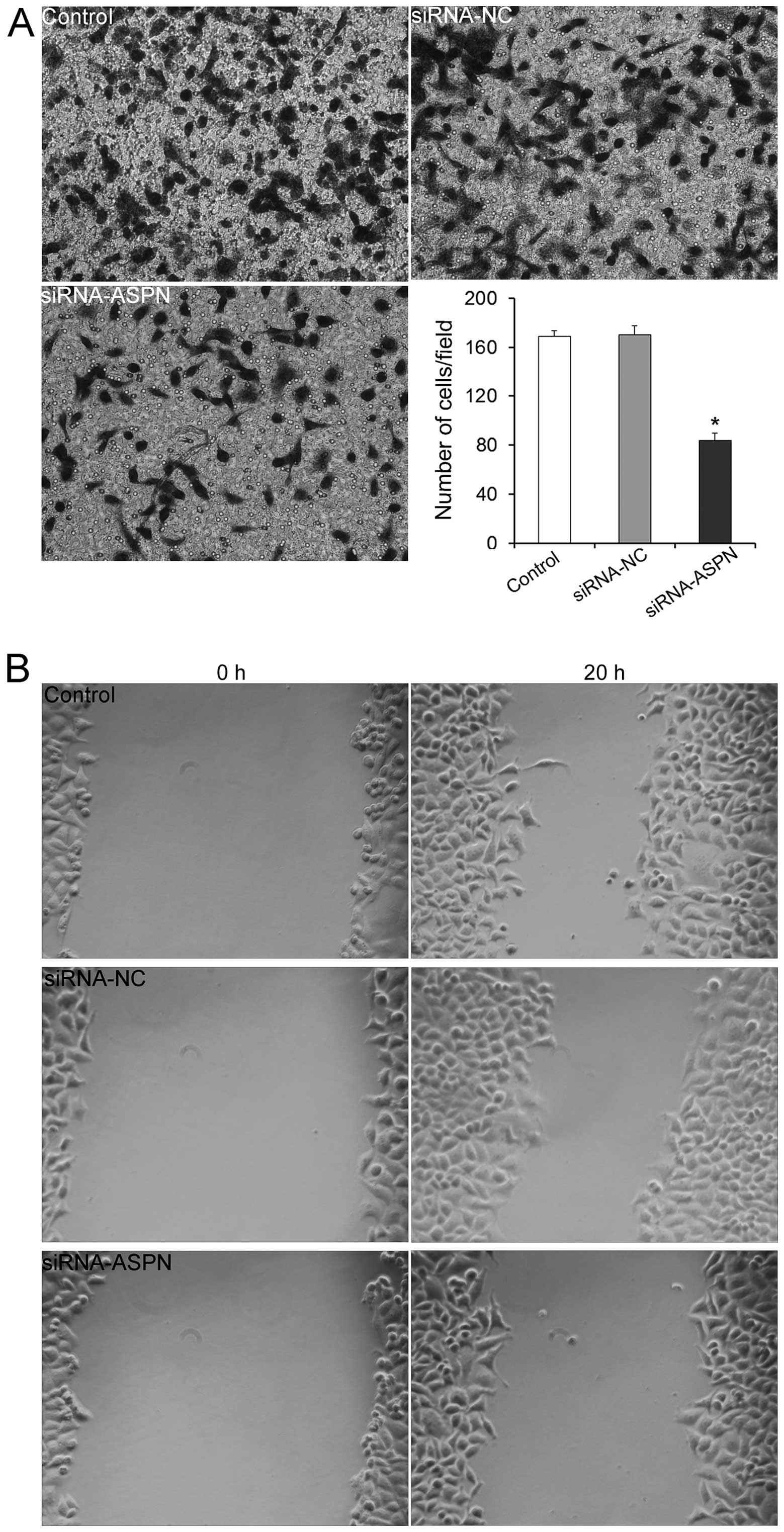
Increased cell motility is one of the properties
shared by cancer cells. Thus, Transwell and wound-healing assays
were performed to further determine whether the downregulation of
ASPN influences the migratory ability of gastric cancer cells. The
results of the Transwell assay indicated that the numbers of cells
in the siRNA-ASPN-GV248 (#40) group that migrated to the lower
surfaces were significantly reduced at the time-points of 20 h, in
comparison to these numbers in the siRNA-NC and untreated control
groups (Fig. 3A). Similarly, the
wound-healing assay showed that ASPN silencing apparently induced
less migrating cells than did the control siRNA transfectants
(Fig. 3B). These results indicated
that ASPN downregulation reduced the migratory ability of gastric
cancer cells. Conversely, it could be concluded that ASPN
expression levels were correlated with the gastric cancer migration
levels.
ASPN is critical for the activation of
the EGFR/ERK signaling pathway
To further substantiate the proliferation and
migration activity of ASPN, apoptosis-related proteins (Bcl-2 and
Bax) and migration-related proteins (CD44 and MMP-2) were analyzed
after silencing of the expression of ASPN. As expected, the western
blot analysis results revealed that the downregulation of ASPN
blocked the expression of the anti-apoptotic molecule Bcl-2 and
increased the pro-apoptotic molecule Bad, keeping in line with the
results of the cell viability and colony formation assays.
Meanwhile, ASPN silencing also reduced the levels of the
migration-related proteins CD44 and MMP-2, confirming the results
of the Transwell and wound-healing assays, at the protein level
(Fig. 4A). The EGFR/ERK pathway,
with many interactions occurring, has been well characterized as a
key element of a complex signaling network involved in cell
survival, migration and angiogenesis (24,25).
To evaluate the potential influence of ASPN on EGFR/ERK signaling,
total and general phosphorylation of expression of the related
molecules were measured. As shown in Fig. 4B, ectopic ASPN significantly
influenced the activation of the phosphorylation status of ERK and
EGFR, but not the total molecules. Thus, these findings indicate
the possibility that ASPN is involved in gastric cancer cell growth
and migration via EGFR-ERK signaling, at least in part.
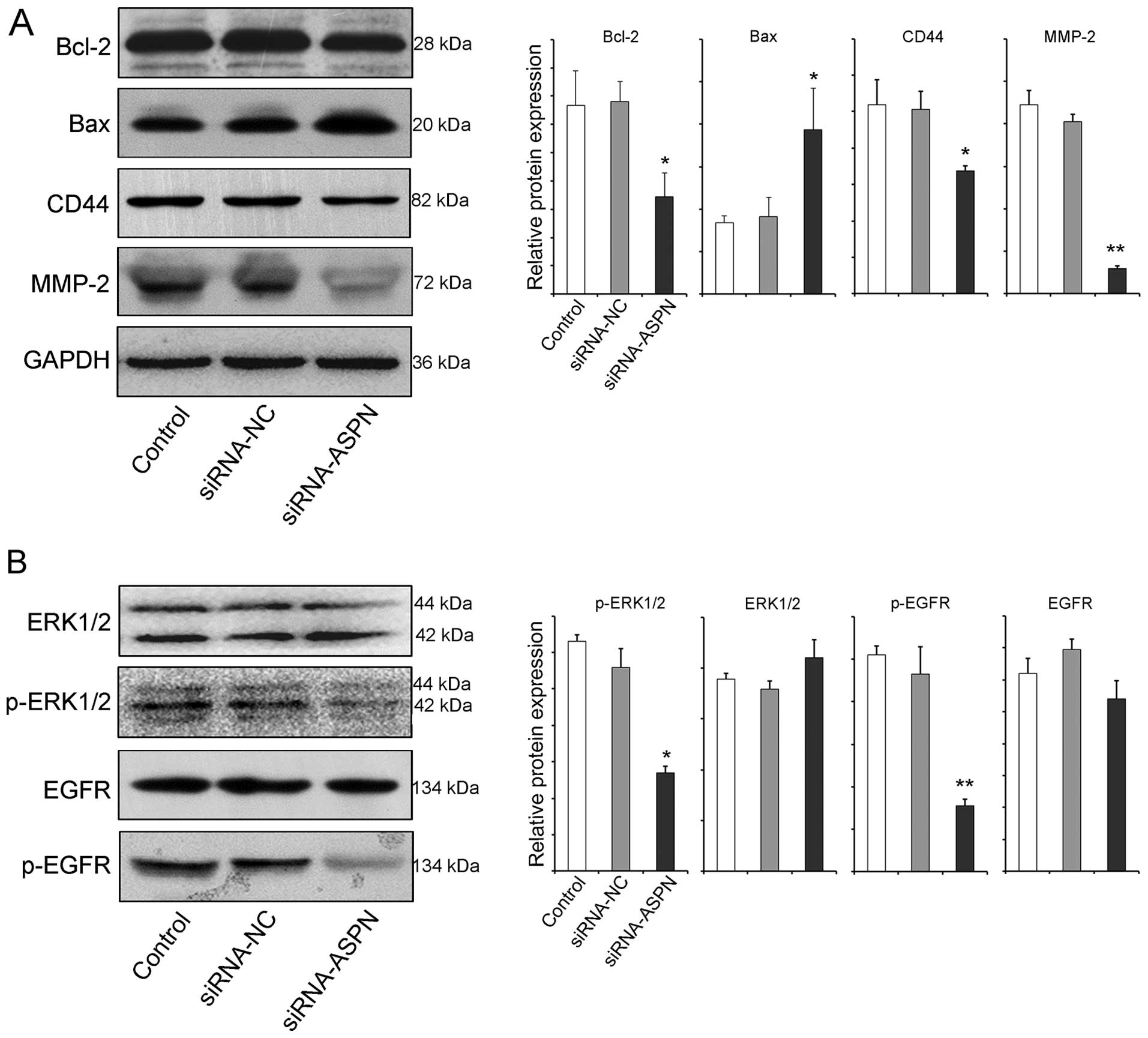 | Figure 4ASPN silencing reduces the activity of
the EGFR/ERK pathway. Protein indicators related to Bcl-2, Bax,
CD44, MMP-2 (A), and ERK1/2, p-ERK1/2, EGFR, p-EGFR (B) were
detected by western blotting after AGS cells were transfected with
siRNA-ASPN-GV248 (#40) or siRNA-NC-GV248 for 72 h.
*P<0.05, **P<0.01. p- indicates the
phosphorylated form. ASPN, asporin; EGFR, epidermal growth factor
receptor; MMP, matrix metalloproteinase. |
Discussion
The tumor environment is one major factor that
determines the behavior of malignant cells, and the ECM
proteoglycans are essential components of the structural entity
(23). Thus, we hypothesized that
aberrant expression of proteoglycans is a more frequent event and
plays many physiological and pathological roles in the development
and progression of tumors and merits further investigation. It is
clear that SLRPs are biologically active components of ECM
(26). ASPN, a new member of the
SLRPs, is expressed abundantly in several malignant tumors,
suggesting its involvement in the tumorigenesis and progression of
cancer (6). Our present results,
consistent with previous studies, revealed a significantly elevated
ASPN expression in carcinoma tissues relative to the matched
adjacent non-tumor tissues (11–14).
In addition, ASPN had varied expression in an inverse trend
according to the differentiation degree of gastric cancer
epithelial cell lines, hinting its oncogenic property, to some
extent.
ASPN may have numerous ways to contribute to tumor
progression. However, owing to the limited studies to date, the
association between stromal ASPN expression and clinicopathological
tumor characteristics remains largely uncharacterized. Even though
Satoyoshi et al (7) reported
that ASPN promotes the coordinated invasion of cancer-associated
fibroblasts and gastric cancer both in vitro and in
vivo, further investigation is required to clarify both the
general role and exact mechanisms of action of ASPN in human
neoplasia.
On the basis of the literature, we performed cell
proliferation assays to evaluate the potential role of ASPN in
gastric cancer epithelial cell proliferation. Our findings from
both the CCK-8 and colony formation assays showed that ASPN
blockade abrogates the proliferation of AGS cells. At the protein
level, ASPN silencing also induced a decreased level of
anti-apoptotic Bcl-2 and an increased level of pro-apoptotic Bad,
indicating that ASPN may play an oncogenic role in gastric tumor
cell survival and proliferation.
Furthermore, we performed migration assays to
investigate whether ASPN influences the migratory ability of
gastric cancer cells. To exclude the possibility that cell
proliferation influenced cell migration, migration assays were
carried out within 24 h of siRNA transfection, when siRNA-ASPN did
not induce a significant reduction in cell viability. Our results
revealed that siRNA-mediated knockdown of ASPN in AGS cells
significantly decreased the migration of gastric cancer cells. CD44
together with other oncoproteins, such as MMP-2 and MMP-9, have
been reported to be associated with gastric cancer metastasis
(27). Khurana et al
recently documented that loss of CD44 in vivo results in
decreased proliferation of gastric epithelium and the
ERK→CD44→STAT3 signaling pathway regulates normal and
atrophy-induced gastric stem/progenitor-cell proliferation
(28). Additionally, lines of
evidence suggest that MMPs participate in cell growth, angiogenesis
and epithelial-mesenchymal transition (3). Particularly MMP-2, regulated by tissue
inhibitor of MMP-2, was proven to play an important role in the
progression of gastric cancer (29). Particularly, the activation of the
MMP-2 protein is positively related to the metastasis of gastric
cancer AGS cells (30). In this
context, our data showed that the expression levels of CD44 and
MMP-2 were decreased significantly when ASPN was silenced, which
further suggests that ASPN is related to the migration of gastric
cancer cells.
An ASPN allele with a 14-aspartic acid repeat in the
N-terminal region, was designated as D14. The frequency of the D14
allele was found to increase with disease severity, and the effect
of ASPN on TGF-β activity was allele-specific (15). Song et al also reported that
the D14 allele is associated with lumbar disc degeneration
(31). Likewise, through its
leucine-rich repeat motif, ASPN inhibits the activation of bone
morphogenetic protein receptor, resulting in the inactivation of
differentiation of periodontal ligament cells (32). Nevertheless, the biological
mechanisms of ASPN involved in the carcinogenesis and metastasis of
tumors are not yet fully clarified.
Decorin, with a similar structure to ASPN, is a
multifunctional molecule of the ECM (33). Previous evidence suggests that
decorin, as a key component of the tumor stroma, interacts with
various growth factors such as EGF and TGF-β and is necessary for
cell migration (34,35). Furthermore, it may regulate the
action of several tyrosine-kinase receptors, including the EGFR,
ERK and insulin-like growth factor receptor I (36,37).
Thereupon, whether similar mechanisms of ASPN play a role in tumors
warrants attention. In the present study, ectopic ASPN
significantly blocked the activation of the phosphorylation status
of ERK and EGFR, but not the total molecules.
Because of the causal involvement in gastric cancer
differentiation and progression, EGFR and its ligands have recently
received considerable attention (17). MAPK/ERK, one classic downstream
signal of the EGFR pathway, could regulate CD44 to modulate tumor
aggressiveness (38). The most
recent study showed that prostate cancer cell migration and
invasion were inhibited by reducing MMP-2/-9 expression via the
ROS/ERK pathways (39). Based on
this evidence and our present results, it seems reasonable to
conclude that ASPN regulates gastric cancer metastasis through
activation of EGFR and the ERK-CD44/MMP-2 pathway.
In summary, our study implies that ASPN, a
proteoglycan that is aberrant expressed in gastric carcinoma
tissues and cell lines, possesses characteristics of an oncogene to
a certain extent in gastric carcinogenesis. The cell and molecular
biological analyses of ASPN suggest that the proteoglycan
contributes to gastric cancer growth and metastasis by regulating
the EGFR signaling pathway. Thus, our research provides additional
support of previous studies that report that ASPN represents a
viable therapeutic regimen with which to treat gastric cancer aimed
at manipulating the cancer micro-environment.
References
|
1
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Z, Miao L, Xin X, et al:
Underexpressed CNDP2 participates in gastric cancer growth
inhibition through activating the MAPK signaling pathway. Mol Med.
20:17–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sampieri CL, León-Córdoba K and
Remes-Troche JM: Matrix metalloproteinases and their tissue
inhibitors in gastric cancer as molecular markers. J Cancer Res
Ther. 9:356–363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Canavese G, Candelaresi G, Castellano I
and Mano MP: Expression of proteoglycan versican in in situ breast
lesions: relations between stromal changes, histotype, and
invasion. Pathol Res Pract. 207:97–103. 2011. View Article : Google Scholar
|
|
6
|
Lorenzo P, Aspberg A, Onnerfjord P, et al:
Identification and characterization of asporin. A novel member of
the leucine-rich repeat protein family closely related to decorin
and biglycan. J Biol Chem. 276:12201–12211. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Satoyoshi R, Kuriyama S, Aiba N, et al:
Asporin activates coordinated invasion of scirrhous gastric cancer
and cancer-associated fibroblasts. Oncogene. 34:650–660. 2015.
View Article : Google Scholar
|
|
8
|
Theocharis AD, Vynios DH,
Papageorgakopoulou N, et al: Altered content composition and
structure of glycosaminoglycans and proteoglycans in gastric
carcinoma. Int J Biochem Cell Biol. 35:376–390. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu L, Duan YT, Li JF, et al: Biglycan
enhances gastric cancer invasion by activating FAK signaling
pathway. Oncotarget. 5:1885–1896. 2014.PubMed/NCBI
|
|
10
|
Wang B, Li GX, Zhang SG, et al: Biglycan
expression correlates with aggressiveness and poor prognosis of
gastric cancer. Exp Biol Med (Maywood). 236:1247–1253. 2011.
View Article : Google Scholar
|
|
11
|
Ansari D, Aronsson L, Sasor A, et al: The
role of quantitative mass spectrometry in the discovery of
pancreatic cancer biomarkers for translational science. J Transl
Med. 12:872014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Turtoi A, Musmeci D, Wang Y, et al:
Identification of novel accessible proteins bearing diagnostic and
therapeutic potential in human pancreatic ductal adenocarcinoma. J
Proteome Res. 10:4302–4313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klee EW, Bondar OP, Goodmanson MK, et al:
Candidate serum biomarkers for prostate adenocarcinoma identified
by mRNA differences in prostate tissue and verified with protein
measurements in tissue and blood. Clin Chem. 58:599–609. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Turashvili G, Bouchal J, Baumforth K, et
al: Novel markers for differentiation of lobular and ductal
invasive breast carcinomas by laser microdissection and microarray
analysis. BMC Cancer. 7:552007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kizawa H, Kou I, Iida A, et al: An
aspartic acid repeat polymorphism in asporin inhibits
chondrogenesis and increases susceptibility to osteoarthritis. Nat
Genet. 37:138–144. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kopp R, Rothbauer E, Ruge M, et al:
Clinical implications of the EGF receptor/ligand system for tumor
progression and survival in gastrointestinal carcinomas: evidence
for new therapeutic options. Recent Results Cancer Res.
162:115–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moon WS, Tarnawski AS, Chai J, et al:
Reduced expression of epidermal growth factor receptor related
protein in gastric cancer. Gut. 54:201–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ashktorab H, Daremipouran M, Wilson M, et
al: Transactivation of the EGFR by AP-1 is induced by Helicobacter
pylori in gastric cancer. Am J Gastroenterol. 102:2135–2146. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hwang YP, Yun HJ, Choi JH, et al:
Suppression of EGF-induced tumor cell migration and matrix
metalloproteinase-9 expression by capsaicin via the inhibition of
EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling.
Mol Nutr Food Res. 55:594–605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murphy KM, Ranganathan V, Farnsworth ML,
et al: Bcl-2 inhibits Bax translocation from cytosol to
mitochondria during drug-induced apoptosis of human tumor cells.
Cell Death Differ. 7:102–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wobus M, Rangwala R, Sheyn I, et al: CD44
associates with EGFR and erbB2 in metastasizing mammary carcinoma
cells. Appl Immunohistochem Mol Morphol. 10:34–39. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gurgel DC, Valença-Junior JT, Dornelas CA,
et al: Immuno expression of metalloproteinases 2 and 14 and TIMP-2
inhibitor in main types of primary gastric carcinomas and lymph
node metastasis. Pathol Oncol Res. 21:73–81. 2015. View Article : Google Scholar
|
|
23
|
Zhang Z, Zhang J, Miao L, et al:
Interleukin-11 promotes the progress of gastric carcinoma via
abnormally expressed versican. Int J Biol Sci. 8:383–393. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmidt M and Lichtner RB: EGF receptor
targeting in therapy-resistant human tumors. Drug Resist Updat.
5:11–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kolch W: Coordinating ERK/MAPK signalling
through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 6:827–837.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Merline R, Schaefer RM and Schaefer L: The
matricellular functions of small leucine-rich proteoglycans
(SLRPs). J Cell Commun Signal. 3:323–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi M, Zheng D, Sun L, et al: XB130
promotes proliferation and invasion of gastric cancer cells. J
Transl Med. 12:12014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khurana SS, Riehl TE, Moore BD, et al: The
hyaluronic acid receptor CD44 coordinates normal and metaplastic
gastric epithelial progenitor cell proliferation. J Biol Chem.
288:16085–16097. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Łukaszewicz-Zając M, Mroczko B,
Guzińska-Ustymowicz K, et al: Matrix metalloproteinase 2 (MMP-2)
and their tissue inhibitor 2 (TIMP-2) in gastric cancer patients.
Adv Med Sci. 58:235–243. 2013. View Article : Google Scholar
|
|
30
|
Hwang TL, Changchien TT, Wang CC and Wu
CM: Claudin-4 expression in gastric cancer cells enhances the
invasion and is associated with the increased level of matrix
metalloproteinase-2 and -9 expression. Oncol Lett. 8:1367–1371.
2014.PubMed/NCBI
|
|
31
|
Song YQ, Cheung KM, Ho DW, et al:
Association of the asporin D14 allele with lumbar-disc degeneration
in Asians. Am J Hum Genet. 82:744–747. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tomoeda M, Yamada S, Shirai H, et al:
PLAP-1/asporin inhibits activation of BMP receptor via its
leucine-rich repeat motif. Biochem Biophys Res Commun. 371:191–196.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cabello-Verrugio C and Brandan E: A novel
modulatory mechanism of transforming growth factor-beta signaling
through decorin and LRP-1. J Biol Chem. 282:18842–18850. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zafiropoulos A and Tzanakakis GN:
Decorin-mediated effects in cancer cell biology. Connect Tissue
Res. 49:244–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mohan RR, Tovey JC, Gupta R, et al:
Decorin biology, expression, function and therapy in the cornea.
Curr Mol Med. 11:110–128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morcavallo A, Buraschi S, Xu SQ, et al:
Decorin differentially modulates the activity of insulin receptor
isoform A ligands. Matrix Biol. 35:82–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goldoni S, Iozzo RA, Kay P, et al: A
soluble ectodomain of LRIG1 inhibits cancer cell growth by
attenuating basal and ligand-dependent EGFR activity. Oncogene.
26:368–381. 2007. View Article : Google Scholar
|
|
38
|
Judd NP, Winkler AE, Murillo-Sauca O, et
al: ERK1/2 regulation of CD44 modulates oral cancer aggressiveness.
Cancer Res. 72:365–374. 2012. View Article : Google Scholar :
|
|
39
|
Chen Y, Zheng L, Liu J, et al: Shikonin
inhibits prostate cancer cells metastasis by reducing matrix
metalloproteinase-2/-9 expression via AKT/mTOR and ROS/ERK1/2
pathways. Int Immunopharmacol. 21:447–455. 2014. View Article : Google Scholar : PubMed/NCBI
|