Introduction
Bladder cancer is the fourth most common malignant
disease worldwide, accounting for ~6% of all cancer cases (1). Bladder cancers (75%) are
non-muscle-invasive and can be treated by transurethral resection
of the bladder tumor combined with intravesical chemotherapeutic
agents instillation. However, the prognosis of patients with muscle
invasive bladder cancer (25%) remains poor despite the many
advances in treatments made over the past few decades. To improve
survival rate and extend life span, patients with muscle invasive
bladder cancer require chemotherapy after surgery (2). Cisplatin (CDDP)-based chemotherapy is
widely used for treatment of muscle invasive bladder cancer
(3). However, cancer cell
resistance to CDDP is a major obstacle to the effective treatment
of bladder cancer, and the underlying mechanism of the resistance
is unclear.
The molecular mechanisms of CDDP include binding of
the drug to DNA and non-DNA targets to form a variety of
monoadducts and cross links, which contribute to the cytotoxicity
of CDDP by blocking DNA replication and stimulating signals for
apoptosis (4). Despite the
excellent anticancer effect, CDDP results in severe side effects
such as nephrotoxicity, ototoxicity, hepatotoxicity, peripheral
neuropathy and asthenia after prolonged clinical exposure (5). In addition, intrinsic resistance
and/or the resistance developed by cancer cells to CDDP result in
failure in the therapy of bladder cancer (6). Due to the side effects and drug
resistance, wide use of CDDP is restricted. Thus, the important
task of bladder cancer therapy is to identify a suitable method to
improve the sensitivity of cancer cells to CDDP to enhance its
efficacy. Previous results have demonstrated that the combination
of gene therapy and chemotherapeutic agents are crucial in the
treatment of cancer (7).
Leucine-rich repeats and immunoglobulin-like domains
1 (LRIG1) is a transmembrane leucine-rich repeat and immunoglobulin
(Ig)-like domain-containing protein, whose encoding gene is located
at chromosome 3p14.3, a region that is frequently deleted in
various human cancers (8,9). The expression of LRIG1 is
downregulated in several tumors, such as breast tumor, renal cell
and lung carcinoma, and bladder cancer (10,11).
Further studies showed that LRIG1 is one of the natural ligands of
EGFR and acts as a negative regulator of the ErbB family of
receptor tyrosine kinases (12).
EGFR is a widely distributed protein tyrosine kinase that is
overexpressed in many types of tumor cells, including colon,
bladder, lung and prostatic carcinoma, and there is an association
between the upregulation of EGFR and poor clinical prognosis
(13,14). EGFR can be activated by epidermal
growth factor (EGF) and transforming growth factor-α (TGF-α), while
activated EGFR (phosphorylated EGFR, pEGFR) stimulates several
different signal transduction pathways, such as the
phosphoinositide-3 kinase (PI3K)/Akt pathway, the
phospholipase-Cγ/protein kinase C pathway and the
Ras/mitogen-activated protein kinase pathway. These signal
transduction pathways activated by pEGFR are important in cell
differentiation, proliferation, migration, adhesion and apoptosis
(15). It has been reported that
the chemoresistance of several types of tumors including bladder
cancer are associated with EGFR and pEGFR overexpression (16,17).
These findings indicated that LRIG1 may enhance the sensitivity of
cancer cells to chemotherapeutic agents.
In the present study, we upregulated the expression
of LRIG1 by adenovirus vector and determined the effects of LRIG1
on chemosensitivity in the T24 bladder cancer cell line and
investigated the possible mechanisms.
Materials and methods
Cell line and cell culture
The human T24 bladder cancer cell line and human
QBI-293A embryonic kidney cell line were purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA). The
cells were maintained at 37°C in a humidified atmosphere of 5%
CO2 in RPMI-1640 medium supplemented with 10% (v/v)
fetal bovine serum (FBS) (Gibco-BRL, Gaithersburg, MD, USA). The
medium was replaced every 3 days.
Main reagents
CDDP was purchased from the Qilu Pharmaceutical Co.,
Ltd. (Shandong, China). FBS, RPMI-1640, Lipofectamine 2000 and
TRIzol™ reagents were purchased from Life Technologies Inc.
(Carlsbad, CA, USA). MTT and dimethyl sulfoxide (DMSO) were
obtained from Sigma Chemical Inc. (St. Louis, MO, USA). Polyclonal
rabbit anti-LRIG1 antibody was purchased from Agrisera, Sweden.
Polyconal rabbit anti-EGFR antibody, anti-phospho-EGFR
(anti-pEGFR), anti-Bcl-2, anti-Bax and anti-caspase-3 were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The recombinant adenovirus-encoding human LRIG1 (Ad-LRIG1)
and recombinant adenovirus (Ad-GFP) carrying the green fluorescent
protein were constructed in our laboratory (18).
Cell transfection and viral
infection
The Ad-LRIG1 and control Ad-GFP adenoviruses were
prepared as previously described (18). Titers of purified adenoviruses were
measured using the gene transfer unit (GTU) method by calculating
the number of reporter gene GFP-expressing QBI-293A cells within 18
h after adenoviral infection under fluorescence microscopy. To
evaluate the optimal multiplicity of infection (MOI) for a maximal
infection and transgene expression, the T24 human bladder cancer
cells were infected with Ad-LRIG1 and Ad-GFP at various MOIs (0, 1,
10, 25, 50, 100 and 200) for 24 h, respectively. The adenoviral
infection efficiency was assessed based on GFP expression. LRIG1
gene expression in T24 cells was then examined using RT-PCR and
western blot analysis.
RT-PCR analysis
Total RNA was extracted from Ad-LRIG1 or Ad-GFP
infected and uninfected T24 cells using TRIzol reagent according to
the manufacturer’s instructions. Total RNA (3 μg) was reverse
transcribed using M-MuLV reverse transcriptase (Promega, Madison,
WI, USA). cDNA was amplified by PCR using Taq DNA Polymerase
(Promega). The primers used were: LRIG1, sense:
5′-ATCATCACCCAGCCAGAAAC-3′ and antisense: 5′-CTACCGTGGTCCCATCCTT-3′
(product size, 892 bp); GAPDH, sense: 5′-ACGGATTTGGTCGTATTGGG-3′
and antisense: 5′-TGATTTTGGAGGGATCTCGC-3′ (product size, 230 bp).
The reaction conditions were as follows: denaturation for 30 sec at
94°C, 1 min at 58°C, annealing 1 min at 72°C, 31 cycles and a final
extension at 72°C for 10 min. The PCR products were separated in
1.5% agarose gel electrophoresis with ethidium bromide
staining.
Western blot analysis
The cells (n=1×106) were washed twice in
ice-cold phosphate-buffered saline (PBS). Total proteins were
extracted from Ad-LRIG1 or Ad-GFP infected and uninfected T24
cells, respectively, using Mammalian Protein Extraction Reagent
(Pierce, Rockford, IL, USA) according to the manufacturer’s
instructions. The protein concentrations were measured using a
Lowry protein assay (Bio-Rad, Hercules, CA, USA). Cell lysates (30
μg of each sample) were diluted with 2X SDS sample buffer (125 mM
Tris, 2.2 M glycerol, 1.42 M β-mercaptoethanol, 160 mM SDS, 10 mg/l
bromophenol blue, pH 0.8), boiled for 10 min and analyzed by
SDS-PAGE (8%), and then transferred to a membrane (Millipore) and
incubated with 5% non-fat dry milk overnight. The membrane was
washed with PBS-0.1% Tween-20 (Sigma) and incubated with antibodies
such as LRIG1 (rabbit anti-human IgG, 1:300 dilution), EGFR (rabbit
anti-human IgG, 1:300 dilution), pEGFR (rabbit anti-human IgG,
1:300 dilution), Bax (rabbit anti-human IgG, 1:200 dilution), Bcl-2
(rabbit anti-human IgG, 1:200 dilution), caspase-3 (rabbit
anti-human IgG, 1:600 dilution) and β-actin (rabbit anti-human IgG,
1:600 dilution) for 1 h at 37°C. The membrane was then washed three
times with TBST and incubated with a peroxidase horseradish
peroxidase-conjugated secondary antibodies in blocking solution for
90 min at 37°C. After three washes with TBST, the blots were
detected by ECL western blotting detection system (Amersham,
Aylesbury, UK). Band intensities shown by western blotting were
semi-quantified by densitometry.
Cell growth inhibitory rate assay with
CCK-8
The sensitivity of cells to CDDP was assayed with
the Cell Counting Kit-8 (CCK-8) kit (Dojindo Molecular
Technologies, Gaithersburg, MD, USA). Briefly, T24 cells infected
with Ad-LRIG1 or Ad-GFP and uninfected T24 cells were seeded in
96-well culture plates (1×104/well) and incubated for 24
h at 37°C and then treated with CDDP at concentrations of 0, 1.25,
2.5, 5, 10, 20 and 40 μg/ml for 24 h to obtain a dose-response
curve. Cell viability of the three groups was then analyzed by
CCK-8 kit according to the manufacturer’s instructions. The values
of each well were measured by a microplate reader at 450 nm. The
inhibitory rate (%) was calculated according to the formula: (1 -
experimental OD value/control OD value) ×100%. The experiments were
carried out in triplicate. Dose-dependent response curves were
plotted on the basis of the data derived from the CCK-8 assay. The
half maximal inhibitory concentration (IC50) was
determined graphically from the concentration response curves.
Annexin V-APC/7-AAD double labeling for
FCM-assessed apoptosis
The Annexin V-APC (Bender MedSystems, Vienna,
Austria) was used to detect apoptosis according to the
manufacturer’s instructions. Synchronization was achieved by serum
starving cells for 24 h. The cells were then collected by
trypsinization and washed twice with cold PBS (0.1 M, pH 7.2). The
cells were centrifuged at 2,000 rpm for 5 min, the supernatant was
discarded and the pellet was re-suspended in 1X binding buffer at a
density of 1×106 cells/ml. Each suspension (100 μl) was
transferred into individually labeled tubes and incubated with 5 μl
of APC-conjugated Annexin V and 5 μl of 7-AAD (KeyGene Co.,
Nanjing, China) for 15 min at room temperature in the dark. PBS
binding buffer (500 μl) was added to each sample tube without
washing and analyzed within 1 h by FACS using CellQuest Research
Software (BD Biosciences, San Jose, CA USA). Each group was
measured three times.
Analysis of chemosensitizing effects
To assess the chemosensitizing effects of Ad-LRIG1,
the following groups were studied: CDDP+T24 (T24/CDDP),
Ad-GFP+CDDP+T24 (Ad-GFP/CDDP) and Ad-LRIG1+CDDP+T24
(Ad-LRIG1/CDDP). T24 cells (1×106/well) were cultured in
6-well culture plates (marked A–F, respectively) for 24 h, then 25
MOI Ad-LRIG1 was added into wells A and B, 25 MOI Ad-GFP was added
into wells C and D, and equivalent PBS was added into wells E and F
as controls. After 24 h, 30 μg/ml CDDP was added into each well.
After 24 h, all the groups were harvested and washed twice with
ice-cold PBS, the apoptotic rate was examined by flow cytometry (BD
Biosciences) with the Annexin V-PE/7-AAD apoptosis detection kit
(Wuhan, China) according to the manufacturer’s instructions.
Briefly, the cells (1×106) were incubated with 5 μl
Annexin V-PE and 5 μl 7-AAD in 100 μl 1X Annexin V-binding buffer
at room temperature. Following incubation for 15 min, 400 μl of 1X
binding buffer was added, and the apoptotic cells were analyzed by
FACS using CellQuest version 3.3 software. Each experiment was
repeated three times.
Hoechst staining assay
T24, T24/Ad-LRIG1 and T24/Ad-GFP cells
(1×106, respectively) were cultured in 6-well culture
plates for 24 h, and then treated with CDDP for an additional 24 h.
Hoechst 33342 (BD Biosciences) was then added to the culture medium
of living cells, and changes in nuclear morphology were detected by
fluorescence microscopy (Nikon, Tokyo, Japan) with a filter for
Hoechst 33342 (365 nm). The percentages of the Hoechst-positive
nuclei/optical field (≥50 fields) were counted.
Caspase-3 activity assay
Caspase-3 activity was measured with a caspase-3
cellular activity assay kit (Nanjing KeyGen Biotech, Co., Ltd.
China) according to the manufacturer’s instructions. Briefly, T24,
T24/Ad-LRIG1 and T24/Ad-GFP cells (1×106, respectively)
were cultured in 6-well culture plates for 24 h. The cells were
treated with CDDP for another 24 h and harvested, resuspended in 50
μl of lysis buffer and incubated on ice. After 30 min, the cell
debris was pelleted and the lysates (50 μl) were transferred to
96-well plates. The lysates were added to 50 μl 2.0X reaction
buffer together with 5 μl caspase-3 substrate and incubated for 4 h
at 37°C, 5% CO2 incubator. The activities were
quantified spectrophotometrically at a wavelength of 405 nm.
Statistical analysis
Data were presented as the means ± standard
deviation (SD). Statistical analyses were performed using SPSS
version 13 (SPSS, Inc., Chicago, IL, USA). Statistical significance
between two groups was determined by the paired or unpaired
Student’s t-test. Comparisons between multiple groups were
performed by one-way analysis of variance. P<0.05 was considered
to indicate a statistically significant result.
Results
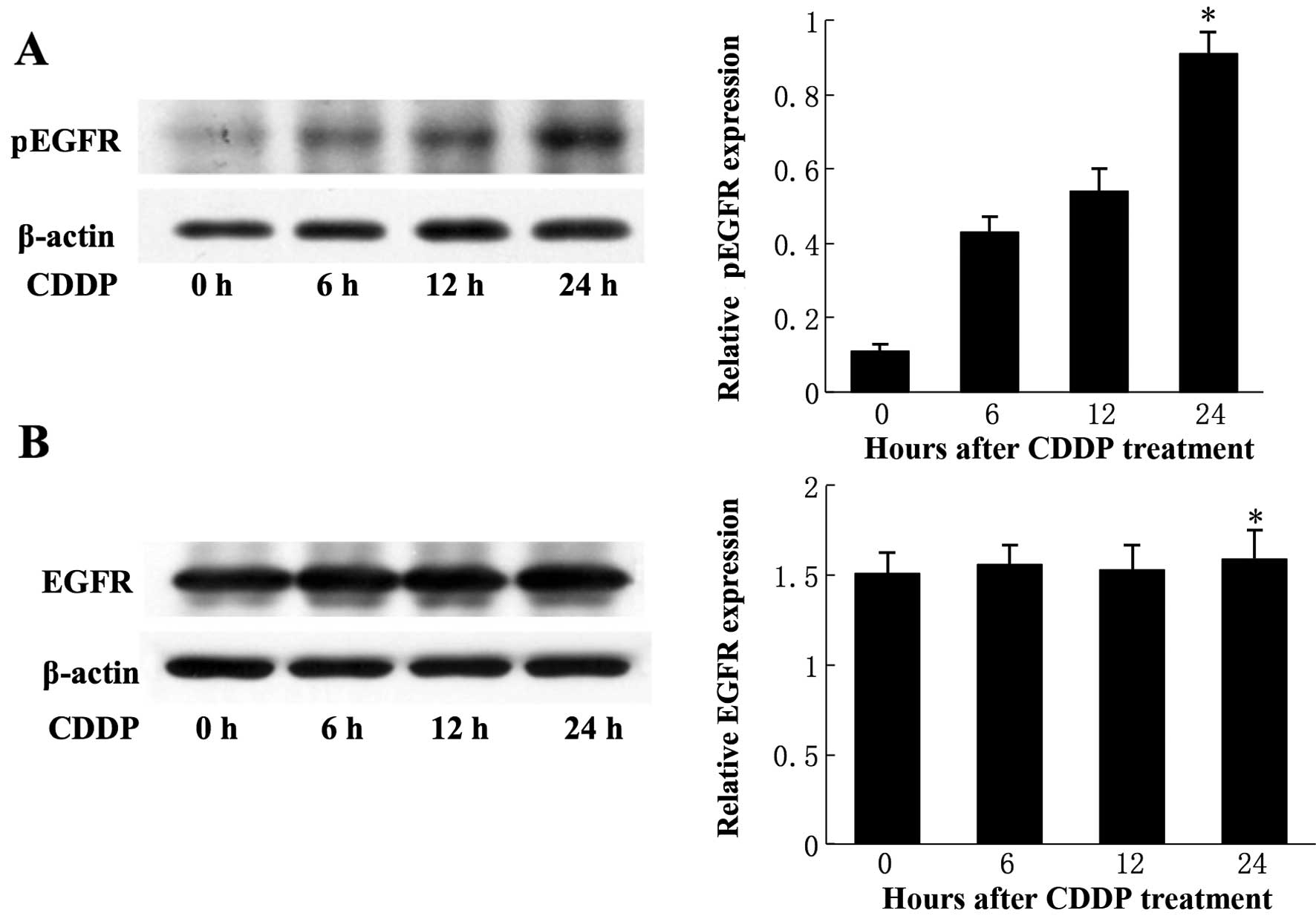
CDDP induces phosphorylation and
activation of EGFR in T24 cells
CDDP (30 μg/ml) induced the activation of EGFR in
T24 cells in a time-dependent manner (Fig. 1A). The level of phosphorylated EGFR
(pEGFR) in T24 cells increased with time and reached a peak of
~5-fold compared to the baseline level (P<0.05) at 24 h.
However, there was no significant difference in the total level of
EGFR expression in T24 cells at different time points (P>0.05)
(Fig. 1B). Taken together, these
results demonstrated that CDDP induced the activation of EGFR in
T24 cells and suggested that CDDP specifically activates EGFR but
has no effect on EGFR expression.
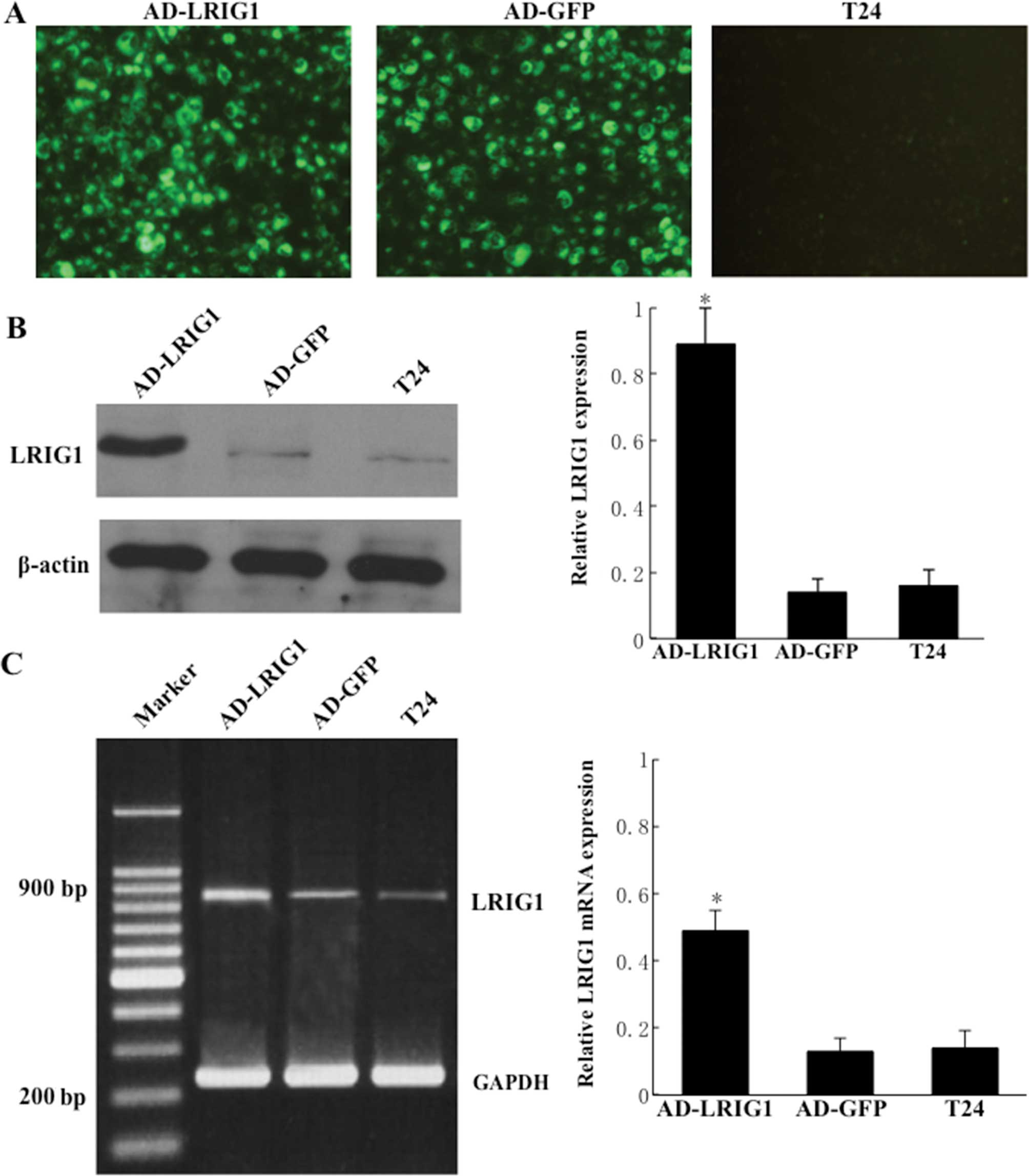
Adenovirus-mediated overexpression of
LRIG1 in T24 cells
To determine the optimal MOI for a maximal gene
transfer with minimal adenovirus itself-induced cytotoxicity, the
T24 cells were infected with Ad-LRIG1 or Ad-GFP at different MOIs
and observed under fluorescence microscopy (488 nm; Nikon). Over
80% of GFP expression was found in the Ad-LRIG1- or Ad-GFP-infected
T24 cells at a MOI of ≥25. However, green fluorescence was not
identified in uninfected T24 cells (Fig. 2A). Furthermore, adenovirus-mediated
exogenous LRIG1 gene was significantly expressed in 25 MOI
Ad-LRIG1-infected T24 cells but not in Ad-GFP-infected and
uninfected T24 control cells (Fig. 2B
and C). Additionally, there was a negligible
adenovirus-elicited cytotoxic effect in 25 MOI blank
Ad-GFP-infected T24 cells. These results showed that 25 MOI serves
as an optimal dose for adenovirus-mediated LRIG1 transgene
expression in T24 cells.
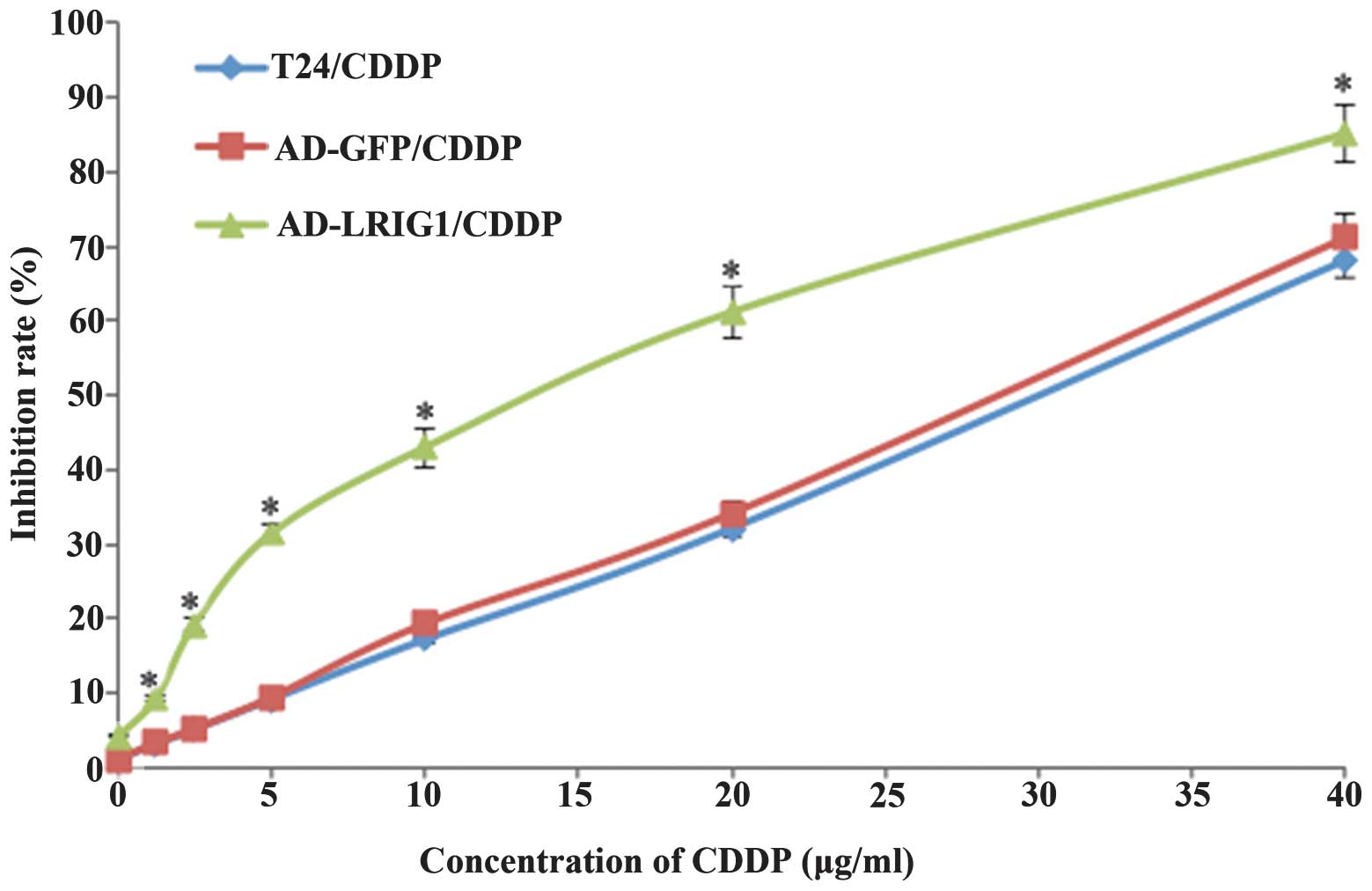
Effect of LRIG1 on the sensitivity to
CDDP in T24 cells
T24 cells infected with Ad-LRIG1 or Ad-GFP and
uninfected T24 cells were treated with CDDP at different
concentrations. After 24 h, the cell viability was reduced with
increasing concentrations of CDDP (Fig.
3). The levels of cytotoxicity were indicated as the
concentration that inhibits the response by 50%, IC50
value. The IC50 values in Ad-LRIGl/CDDP, Ad-GFP/CDDP and
T24/CDDP were (14.09±0.31, 31.55±0.48 and 30.96±0.57),
respectively. Data showed that LRIG1 over-expression was able to
enhance the sensitivity of T24 cells to CDDP (P<0.05). Since the
IC50 value in T24 cells (T24/CDDP) was (30.96±0.57
μg/ml), the concentration of CDDP intervention in subsequent
experiments was determined as 30 μg/ml.
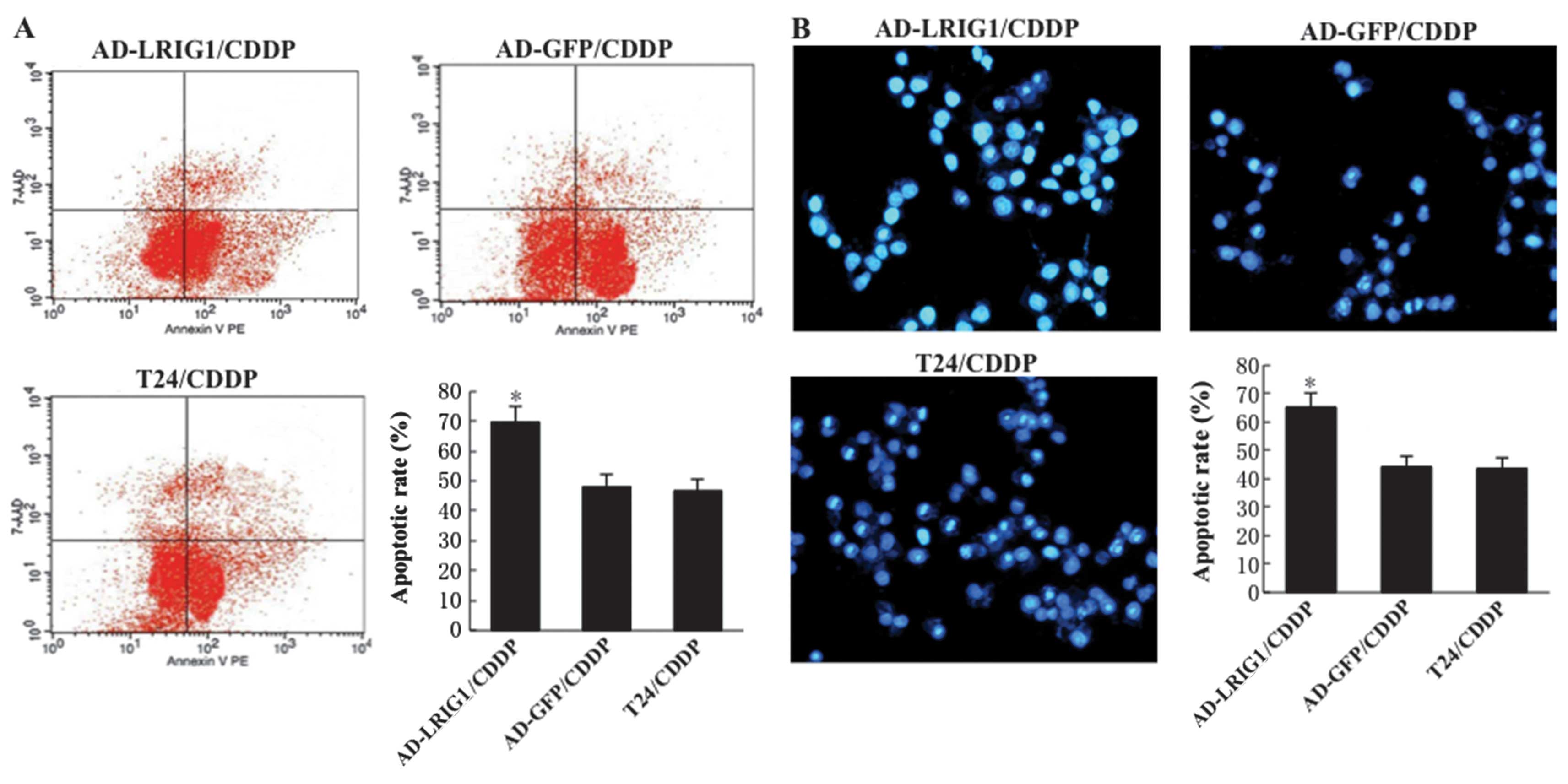
Effects of LRIG1 on CDDP-induced
apoptosis in T24 cells
To determine whether the LRIG1 gene plays an
important role in CDDP-induced apoptosis, we overexpressed LRIG1 by
Ad-LRIGl recombinant adenovirus transduction in T24 cells, followed
by treatment with 30 μg/ml CDDP for 24 h. After 24 h of CDDP
treatment, only (46.82±3.73 and 48.15±4.21%) of the cells underwent
apoptosis in T24/CDDP and Ad-GFP/CDDP cells, and the apoptotic rate
of Ad-GFP-transfected cells treated with CDDP showed no significant
difference compared with that of T24 cells treated with CDDP
(P>0.05). By contrast, (69.77±5.14%) of the Ad-LRIGl-transfected
cells underwent apoptosis (P<0.05) (Fig. 4A). Fig.
4B demonstrates the results of the Hoechst staining assay,
which confirmed the results of the flow cytometric assay. The
results indicated that LRIG1 was able to promote the CDDP-induced
apoptosis in T24 cells.
The mechanism of upregulation of LRIG1
enhances CDDP- induced apoptosis in T24 cells
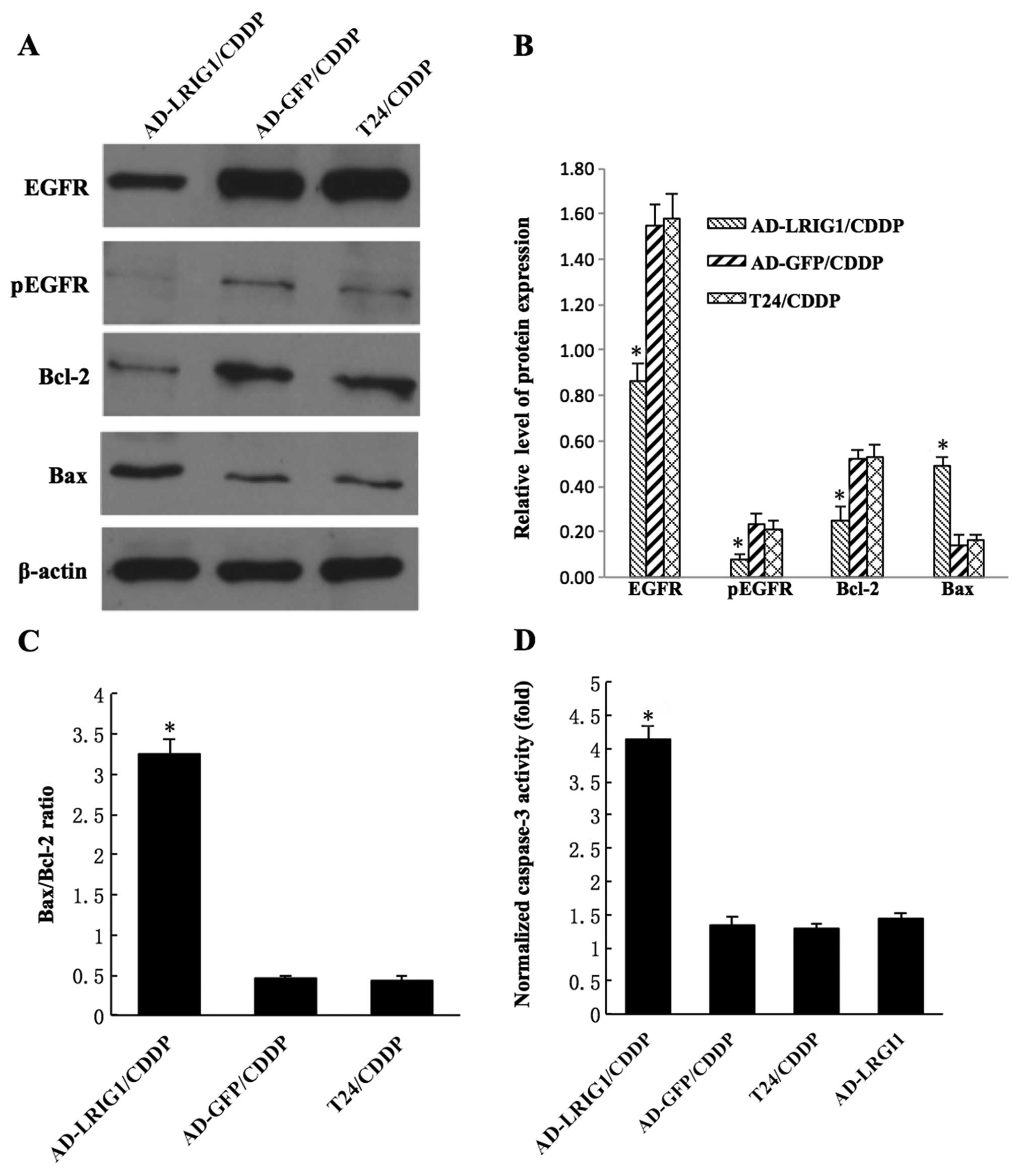
As shown in Fig. 5A and
B, following treatment with 30 μg/ml CDDP for 24 h, the level
of pEGFR in the AD-LRIG1/CDDP cells (0.08±0.02) was markedly lower
than those in AD-GFP/CDDP (0.23±0.05) and T24/CDDP (0.21±0.04)
cells (P<0.05). Similarly, the EGFR protein expression in
AD-LRIG1/CDDP cells (0.86±0.08) was markedly lower than that in
AD-GFP/CDDP (1.55±0.09) and T24/CDDP (1.58±0.11) cells (P<0.05).
AD-LRIG1/CDDP cells had a higher level of the Bax protein and a
lower level of the Bcl-2 protein compared with the AD-GFP/CDDP and
T24/CDDP cells (P<0.05). The Bax/Bcl-2 ratio in AD-LRIG1/CDDP
cells (3.25±0.19) was higher than that in the AD-GFP/CDDP
(0.46±0.04) and T24/CDDP (0.43±0.06) cells (P<0.05)(Fig. 5C). Caspase-3 activity in
AD-LRIG1/CDDP cells (4.14±0.21) was markedly increased compared
with Ad-GFP/CDDP (1.34±0.12), T24/CDDP (1.29±0.07) and Ad-LRIGl
(not treated with CDDP) (1.44±0.08) cells (P<0.05) (Fig. 5D). The results suggest that the
mitochondria-mediated apoptotic pathway is involved in the
upregulation of LRIG1 expression and enhances CDDP-induced
apoptosis of T24 cells.
Discussion
As one of the most effective chemotherapeutic
agents, CDDP is widely used in the treatment of many malignancies,
including bladder cancer (19).
However, the serious side effects of CDDP and inherent or acquired
resistance of tumor cells to CDDP are obstacles that have to be
overcome by clinicians. To overcome cellular resistance of tumor to
CDDP, even a small increase in dose can cause severe cytotoxicity
to normal cells. Thus, it is imperative to examine novel
chemosensitizers to reduce drug dosage, minimize adverse reactions,
improve the efficacy of therapy and promote the application of CDDP
in cancer chemotherapy. Over the past decade, chemogene
therapy-chemotherapy agents combined with gene therapy, has been
developed as a novel adjuvant therapeutic strategy for cancer
treatment (7,20).
Previous findings have confirmed that EGFR and its
signaling pathways played an important role in the chemoresistance
of cancer cells against CDDP-induced cell apoptosis (21,22).
CDDP-resistant cancer cells have altered response to the EGF ligand
and enhanced the activation of EGFR (23). pEGFR is the activated form of EGFR
(24), CDDP induces EGFR activation
through the phosphorylation of tyrosine 845, which stabilizes the
activation loop of EGFR, maintains the enzyme in the active state,
provides a binding surface for protein substrates and leads to cell
survival (25). Thus, the
upregulation of pEGFR expression may be a survival response to
exposure of tumor cells to chemotherapeutic drugs. In the present
study, we found that after exposure to CDDP, pEGFR expression was
significantly increased, suggesting a potential mechanism through
which T24 cells demonstrate chemoresistance to CDDP. According to
the findings of Zhang et al (21), EGFR-tyrosine kinase inhibitor
(AG1478) increased the apoptosis of the human U87 glioma cell line
induced by CDDP. Thus, inhibition of the EGFR signaling pathway and
reduction of the pEGFR expression may enhance the chemosensitivity
of bladder cancer cells to CDDP.
LRIG1 protein is a negative regulator of the EGFR
signaling pathway (26). The
inactivation of LRIG1 in rodents promotes skin epidermal cell
hyperplasia, suggesting involvement in EGFR signaling regulation
(27). The upregulation of the
LRIG1 transcript and protein levels was found to promoted
ubiquitylation and degradation of EGFR by the receptor combination
of leucine-rich repeat (LRR) as well as immunoglobulin-like (Ig)
domains of the LRIG1 protein (12).
In a preliminary study we showed that upregulation of LRIG1
expression by plasmid transfection in the human BIU87 bladder
cancer cell line resulted in cell cycle arrest, inhibition of cell
proliferation, promotion of cell apoptosis and attenuation of cell
invasive and metastatic abilities in vitro by downregulating
the expression of EGFR (10).
In this study, the expression of LRIG1 was
upregulated by the adenovirus vector AD-LRIG1. The present
experiments have demonstrated that the increase in LRIG1 expression
strengthened the apoptosis-inducing effects of CDDP by Annexin
V-FITC/PI-positive flow cytometry and Hoechst staining assays. The
results were consistent with the study of Guo et al
(28), which reported that the
upregulation of LRIG enhanced apoptosis in glioma cells induced by
CDDP in vitro. In addition, the western blot assay was used
to detect the level of protein expression of LRIG1, EGFR, pEGFR,
Bcl-2 and Bax and caspase-3 activity was evaluated using a
caspase-3 colorimetric assay kit. The expression of EGFR and pEGFR
protein in T24 cells infected with AD-LRIG1 was markedly lower than
that of T24 cells infected with AD-GFP and uninfected cells.
Upregulation of the LRIG1 expression in combination with CDDP
decreased the cellular activity level of Bcl-2 while increasing the
cellular activity levels of Bax and caspase-3. This is consistent
with the functional mechanism of upregulation of LRIG1 alone, which
inactivated the EGFR signaling pathway and activated the
mitochondrial pathway of apoptosis. These findings have
demonstrated that the upregulation of LRIG1 expression may lead to
inactivation of the EGFR signaling pathway, thereby sensitizing T24
cells to CDDP-induced apoptosis and minimizing resistance of T24
cells to CDDP.
The mitochondrial pathway of apoptosis is mainly
regulated by mitochondria-related Bcl-2 family members, such as
Bcl-2 and Bax (29). Bcl-2 is a
28-kDa integral, intracellular membrane protein that prevents cells
from undergoing apoptosis in response to a variety of cell death
signals. It negatively regulates the activation of caspase-3, the
key executioner of apoptosis, which functions as an effector of
mammalian cell death pathways. Overexpression of Bcl-2 inhibits
caspase activities and apoptosis (30,31).
Bax is a 23-kDa proapoptotic protein, and decreased levels of Bax
in tumor cells lead to resistance to apoptosis (32). Previous studies have shown that
Bcl-2 acts on mitochondria to stabilize membrane integrity.
However, Bax acts to damage the mitochondrial membrane structure
directly. The ratio of Bax/Bcl-2 can function as a margin to
determine cell fate. When the ratio of Bax/Bcl-2 increases,
caspase-3 is activated and cells undergo apoptosis (33). The present study results show
upregulation of the LRIG1-inactivated EGFR singaling pathway,
resulting in a decrease in the Bcl-2 level, with a concurrent
increase in the Bax level and caspase-3 activation. CDDP-induced
apoptosis is generally considered to result from an increase in the
Bax/Bcl-2 ratio and caspase-3 activation (34). However, the ratio of Bax/Bcl-2 and
activity of caspase-3 in the presence of combined upregulation of
LRIG1 expression with CDDP administration was much higher than that
of the upregulation of LRIG1 expression or CDDP administration
alone. The results suggest that CDDP-induced apoptosis may be
enhanced by upregulation of LRIG1 via the mitochondrial pathway of
apoptosis.
Taken together, our data have demonstrated that
upregulation of the expression of LRIG1 inhibited EGFR signaling
pathway, activated the mitochondrial pathway of apoptosis and
eventually increased the sensitivity of bladder cancer cells to
CDDP. LRIG1 is a promising target for chemogene therapy with CDDP,
suggesting that the upregulation of LRIG1 expression and
appropriate combination of CDDP administration is a potential
strategy for the treatment of human bladder cancer in future.
Acknowledgements
This study was supported by grants from the China
Postdoctoral Science Foundation funded project (no. 2013M541524),
the Zhejiang Provincial Natural Science Foundation of China (no.
LY12H05002), the Zhejiang Provincial Medicines Health Science and
Technology Program Foundation of China (no. 2013KYA180), and the
Ningbo Municipal Natural Science Foundation of China (no.
2013A610208).
Abbreviations:
|
CDDP
|
cisplatin
|
|
EGFR
|
epidermal growth factor receptor
|
|
FCM
|
flow cytometry
|
|
GFP
|
green fluorescence protein
|
|
LRIG1
|
leucine-rich repeats and
immunoglobulin-like domains 1
|
|
MOI
|
multiplicity of infection
|
|
pEGFR
|
phosphorylation EGFR
|
|
RT-PCR
|
reverse-transcription polymerase chain
reaction
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herr HW, Dotan Z, Donat SM and Bajorin DF:
Defining optimal therapy for muscle invasive bladder cancer. J
Urol. 177:437–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Als AB, Sengelov L and von der Maase H:
Long-term survival after gemcitabine and cisplatin in patients with
locally advanced transitional cell carcinoma of the bladder: focus
on supplementary treatment strategies. Eur Urol. 52:478–486. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Basu A and Krishnamurthy S: Cellular
responses to cisplatin-induced DNA damage. J Nucleic Acids.
2010:2013672010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ciarimboli G: Membrane transporters as
mediators of cisplatin side-effects. Anticancer Res. 34:547–550.
2014.PubMed/NCBI
|
|
6
|
Li QQ, Wang G, Liang H, Li JM, Huang F,
Agarwal PK, Zhong Y and Reed E: β-Elemene promotes
cisplatin-induced cell death in human bladder cancer and other
carcinomas. Anticancer Res. 33:1421–1428. 2013.PubMed/NCBI
|
|
7
|
Sekine I, Minna JD, Nishio K, Saijo N and
Tamura T: Genes regulating the sensitivity of solid tumor cell
lines to cytotoxic agents: a literature review. Jpn J Clin Oncol.
37:329–336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nilsson J, Vallbo C, Guo D, Golovleva I,
Hallberg B, Henriksson R and Hedman H: Cloning, characterization,
and expression of human LIG1. Biochem Biophys Res Commun.
284:1155–1161. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hedman H, Nilsson J, Guo D and Henriksson
R: Is LRIG1 a tumour suppressor gene at chromosome 3p14.3? Acta
Oncol. 41:352–354. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang WM, Yan ZJ, Ye ZQ and Guo DS: LRIG1,
a candidate tumour-suppressor gene in human bladder cancer cell
line BIU87. BJU Int. 98:898–902. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hedman H and Henriksson R: LRIG inhibitors
of growth factor signalling - double-edged swords in human cancer?
Eur J Cancer. 43:676–682. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gur G, Rubin C, Katz M, et al: LRIG1
restricts growth factor signaling by enhancing receptor
ubiquitylation and degradation. EMBO J. 23:3270–3281. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yarden Y: The EGFR family and its ligands
in human cancer: signalling mechanisms and therapeutic
opportunities. Eur J Cancer. 37:3–8. 2001. View Article : Google Scholar
|
|
14
|
Pedersen MW, Melthorn M, Damstrup L and
Poulsen HS: The type III epidermal growth factor receptor mutation.
Biological significance and potential target for anti-cancer
therapy. Ann Oncol. 12:745–760. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sebastian S, Settleman J, Reshkin SJ,
Azzariti A, Bellizzi A and Paradiso A: The complexity of targeting
EGFR signalling in cancer: from expression to turnover. Biochim
Biophys Acta. 1766:120–139. 2006.PubMed/NCBI
|
|
16
|
Kim WT, Kim J, Yan C, et al: S100A9 and
EGFR gene signatures predict disease progression in muscle invasive
bladder cancer patients after chemotherapy. Ann Oncol. 25:974–979.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hiraishi Y, Wada T, Nakatani K, Tojyo I,
Matsumoto T, Kiga N, Negoro K and Fujita S: EGFR inhibitor enhances
cisplatin sensitivity of oral squamous cell carcinoma cell lines.
Pathol Oncol Res. 14:39–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li F, Yang W, Guo D, Hu Z, Xu H and Ye Z:
LRIG1 combined with cisplatin enhances bladder cancer lesions via a
novel pathway. Oncol Rep. 25:1629–1637. 2011.PubMed/NCBI
|
|
19
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ta HT, Dass CR, Larson I, Choong PF and
Dunstan DE: A chitosan hydrogel delivery system for osteosarcoma
gene therapy with pigment epithelium-derived factor combined with
chemotherapy. Biomaterials. 30:4815–4823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Xing X, Zhan H, Li Q, Fan Y, Zhan
L, Yu Q and Chen J: EGFR inhibitor enhances cisplatin sensitivity
of human glioma cells. J Huazhong Univ Sci Technolog Med Sci.
31:773–778. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshida T, Okamoto I, Iwasa T, Fukuoka M
and Nakagawa K: The anti-EGFR monoclonal antibody blocks
cisplatin-induced activation of EGFR signaling mediated by HB-EGF.
FEBS Lett. 582:4125–4130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Geng X, Ye H, Feng Z, Lao X, Zhang L,
Huang J and Wu ZR: Synthesis and characterization of
cisplatin-loaded, EGFR-targeted biopolymer and in vitro evaluation
for targeted delivery. J Biomed Mater Res A. 100:2839–2848. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Magkou C, Nakopoulou L, Zoubouli C, Karali
K, Theohari I, Bakarakos P and Giannopoulou I: Expression of the
epidermal growth factor receptor (EGFR) and the phosphorylated EGFR
in invasive breast carcinomas. Breast Cancer Res. 10:R492008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tice DA, Biscardi JS, Nickles AL and
Parsonss SJ: Mechanism of biological synergy between cellular Src
and epidermal growth factor receptor. Proc Natl Acad Sci USA.
96:1415–1420. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sheu JJ, Lee CC, Hua CH, et al: LRIG1
modulates aggressiveness of head and neck cancers by regulating
EGFR-MAPK-SPHK1 signaling and extracellular matrix remodeling.
Oncogene. 33:1375–1384. 2014. View Article : Google Scholar
|
|
27
|
Suzuki Y, Miura H, Tanemura A, et al:
Targeted disruption of LIG-1 gene results in psoriasiform epidermal
hyperplasia. FEBS Lett. 521:67–71. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo Z, Chen Q, Liu B, Tian D, Zhang S and
Li M: LRIG1 enhances chemosensitivity by modulating BCL-2
expression and receptor tyrosine kinase signaling in glioma cells.
Yonsei Med J. 55:1196–1205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Williams GT and Smith CA: Molecular
regulation of apoptosis: genetic controls on cell death. Cell.
74:777–779. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakashima T, Miura M and Hara M:
Tetrocarcin A inhibits mitochondrial functions of Bcl-2 and
suppresses its anti-apoptotic activity. Cancer Res. 60:1229–1235.
2000.PubMed/NCBI
|
|
31
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou W, Fu XQ, Liu J and Yu HG: RNAi
knockdown of the Akt1 gene increases the chemosensitivity of
gastric cancer cells to cisplatin both in vitro and in vivo. Regul
Pept. 176:13–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen C, Zhou H, Xu L, Xu D, Wang Y, Zhang
Y, Liu X, Liu Z, Ma D, Ma Q and Chen Y: Recombinant human PDCD5
sensitizes chondrosarcomas to cisplatin chemotherapy in vitro and
in vivo. Apoptosis. 15:805–813. 2010. View Article : Google Scholar : PubMed/NCBI
|