Introduction
Breast cancer is a common malignant tumor that
occurs in women. Approximately 1.2 million women suffer from, and
~500,000 succumb to breast cancer annually. According to the
American Cancer Society (ACS), the incidence and mortality of
breast cancer ranked the first and second of all female cancer
types in America in 2014, accounting for 29 and 15%, respectively
(1). Approximately 50% of the
breast cancer cases and 60% of the deaths are estimated to occur in
economically developing countries (2). A significant reduction in the
mortality of breast cancer patients worldwide has been observed in
the past 20 years. This reduction has been largely due to
improvement in the early detection and development of more
effective adjuvant therapies (3).
In addition, therapies have been developed to be specifically
tailored to targeting each molecular subtype of breast cancer.
These therapies include human epidermal growth factor receptor-2
(HER2)-targeting agents for HER2-overexpressing tumors, aromatase
inhibitors, third-generation hormonal therapies for
hormone-sensitive disease, and poly(ADP-ribose) polymerase (PARP)
inhibitors for BRCA1-deficient and triple-negative breast cancers
(4). Nevertheless, a large number
of women with breast cancer experience relapse. Therefore,
identification of more effective treatment targets is needed in
breast cancer research.
Angiomotin (Amot) was first identified from its
ability to bind to angiostatin using a yeast two-hybrid screen
(5). Amot is characterized by a
conserved coiled-coil domain and a C-terminal PDZ-binding motif
(6) and is expressed as two
different isoforms, p80-Amot and p130-Amot. Compared with p80-Amot,
p130-Amot contains an extended N-terminal domain (7,8). It
was previously reported that Amot regulates endothelial cell
migration, tube formation and is important in angiogenesis
(6,9–13).
Amot is also involved in regulating permeability and the movement
of epithelial cells in tight junctions (6,9,10).
Using RT-PCR Jiang et al (14) found that Amot transcript was
significantly highly expressed in human breast cancer tissues,
particularly in highly invasive and metastatic tumor tissues, when
compared with its expression in normal mammary tissues. However,
the role and mechanism of the abnormal expression of Amot in breast
cancer remain to be elucidated.
To evaluate the function and mechanism of Amot, the
expression and location of Amot in breast cancer, adjacent
non-cancerous tissues, breast cancer and breast epithelium cell
lines, were first determined using western blotting, RT-PCR and
immunofluorescence. shRNA was applied to block and silence the
expression of Amot in the breast cancer cell line and the effect of
the silenced Amot on the biological behavior of breast cancer cell
line was studied. The results showed that breast cancer tissues had
a significantly increased Amot protein level, when compared with
adjacent non-cancerous tissues and the expression level of Amot was
closely correlated with the expression level of Ki-67 (P<0.01).
We also observed that Amot downregulation resulted in a significant
decrease in cell proliferation, cell invasiveness and migration
in vitro and was closely associated with the Hippo-YAP
pathway which regulates cell proliferation during development,
tissue regeneration and carcinogenesis. Thus, Amot acts as a
potential tumor promoter.
Materials and methods
Patients and tissue samples
A total of 242 breast cancer tissue samples and 92
adjacent non-cancerous tissue samples were obtained from the First
Affiliated Hospital of Xi’an Jiaotong University College of
Medicine and Shanghai Outdo Biotech Co. Ltd. (Shanghai, China). The
clinicopathological tumor-node-metastasis (TNM) staging was stage I
for 16 cases, stage II for 139 cases and stage III–IV for 81 cases.
Clinically relevant parameters are presented in Table I. The pathological types of all the
specimens were confirmed by independent pathologists. No patients
received radiotherapy or chemotherapy prior to surgery. TNM stages
were assigned using the 2010 Union for International Cancer Control
(UICC) criteria. The study was approved by the Human Ethics
Committee of the First Affiliated Hospital, College of Medicine of
Xi’an Jiaotong University. Informed consent was obtained from each
patient. All of the specimens were fixed in 10% buffered formalin
solution and embedded in paraffin.
 | Table IAmot expression in breast cancer and
adjacent non-cancerous tissues. |
Table I
Amot expression in breast cancer and
adjacent non-cancerous tissues.
| Group | Negative expression
(%) | Weak expression
(%) | Moderate expression
(%) | Strong expression
(%) |
|---|
| Cancer tissues | 37 (15.3) | 54 (22.3) | 106 (43.8) | 45 (18.6) |
| Non-cancerous
tissues | 80 (87) | 8 (8.6) | 4 (4.3) | 0 (0) |
| Total | 117 | 62 | 110 | 45 |
| H | 129.4 | | | |
| P | 0.000a | | | |
Immunohistochemistry
Fixed tumor tissue samples were sectioned,
deparaffinized, rehydrated and subjected to heat-induced antigen
retrieval in EDTA buffer (1.0 mM, pH 8.0) for 10 min in a microwave
oven. The samples were blocked with 3% hydrogen peroxide. After
being blocked with 1% bovine serum albumin (BSA), the sections were
incubated overnight at 4°C with a primary antibody specific for
Amot (Genemed Synthesis Inc., San Antonio, TX, USA). Control
sections were incubated with an isotype-matched polypeptide control
antibody and phosphate-buffered saline (PBS). Subsequently, the
sections were incubated with HRP-conjugated secondary antibody for
30 min. The sections were stained with 3,3′-diaminobenzidine, and
then counter-stained with hema toxylin and examined under a
microscope (Olympus CX21; Tokyo, Japan). To evaluate Amot protein
expression, the staining intensity was graded as 0 for no staining;
1 for weak staining; 2 for moderate staining; or 3 for strong
staining. The extent of staining was scored according to the
percentage of positively stained cells as follows: 0 (≤5%), 1
(6–25%), 2 (26–50%), 3 (51–75%) and 4 (76–100%). The number of
positively stained cells was determined by counting cells from 10
random fields at ×400 magnification. The final immunohistochemical
staining score was obtained by multiplying the staining intensity
and the extent of staining (negative expression, scores 0–2; weak
expression, scores 3–5, moderate expression, scores 6–9; and strong
expression, scores 10–12).
Antibodies
The Amot antibody was produced by Genemed Synthesis
Inc. The synthetic peptide sequence (C+LVKSSSKRE ALEKAMR and
C-KTPIQILGQEPDAEMVEYLI) was conjugated to keyhole limpet hemocyanin
(KLH) for immunizations. GAPDH and rabbit flag were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). YAP, YAP/TAZ,
LATS1, MOB, MST1 and SAV1 were purchased from Cell Signaling
Technology (Danvers, MA, USA).
Cell culture and lentiviral
transfection
The MCF-7, T-47D, BT-474, MDA-MB-453, MDA-MB-231
breast cancer cell lines and MCF-10A breast epithelial cell line
were purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). MCF-10A cells were cultured in Dulbecco’s
modified Eagle’s medium (DMEM)/F12 (Invitrogen, Carlsbad, CA, USA)
supplemented with 5% horse serum (HyClone, Logan, UT, USA), 1%
penicillin/streptomycin, 0.5 μg/ml hydro-cortisone, 10 μg/ml
insulin (both from Sigma, Santa Clara, CA, USA) and 20 ng/ml
recombinant human EGF (Invitrogen). MCF-7, T47D and MDA-MB-435
breast cancer cell lines were cultured in DMEM supplemented with
10% fetal bovine serum (FBS) (both from HyClone). BT474 was
cultured in RPMI-1640 medium supplemented with 10% FBS (both from
HyClone). All the cell cultures were maintained at 37°C in a
humidified atmosphere containing 5% CO2.
Lentivirus vectors for human Amot small hairpin RNA
(shRNA) encoding a green fluorescent protein (GFP) and a
puromycin-resistant gene were constructed, packed and purified by
GeneChem Co., Ltd. (Shanghai, China). The RNA interference
sequences used were: shAMOT (8489-1R), 5′-TGCAGAGATGGTGGAATAT-3′;
shAMOT (8491-2R) 5′-ACACATCGAAATCCGAGAT-3′; negative control: shNC,
5′-TTCTCCGAATGTGTCACGT-3′.
MCF-7 cells were transfected with lentivirus
according to the manufacturer’s instructions (GeneChem Co., Ltd.).
For transfection, the lentiviruses mixed with medium containing
Polybrene were added to the cells at the confluence of 30–40%.
After 8 h of transfection, the medium was replaced by fresh DMEM
medium containing 10% FBS. Seventy-two hours after transfection,
the cells were selected with 3.5 μg/ml puromycin for 2 weeks. Amot
knockdown was verified by western blot analysis. The cells were
divided into three groups: CON (control; the uninfected breast
cancer cells); KD (knockdown; cells transfected with the Amot shRNA
lentivirus); NC (negative control; cells transfected with the mock
control lentivirus).
Real-time polymerase chain reaction
analysis
Total mRNA was extracted using the Fast 200 reagent
(Pioneer Biotechnology Inc., Shaanxi, China) and reverse
transcription was performed using an RT-PCR kit (Takara, Dalian,
China). Complementary DNA synthesis was conducted using a SYBR
ExScript RT-PCR kit (Takara) according to the manufacturer’s
instructions. RT-PCR was conducted using the iQ5 Multicolor
Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) and
SYBR Premix Ex Taq™ II (Takara). The primer sequences used
for amplification were: Amot 5′-CCAGAATATCCCTTCAAG-3′ and
5′-GAGTTCCTGGCTGACAAT-3′, GAPDH: 5′-CTCCTCCACCTTTGACGCTG-3′
and 5′-TCCTCTTGTGCTCTTGCTGG-3′. GAPDH was applied as the
internal housekeeping gene control. Each reaction was performed in
a final volume of 10 μl containing 1.0 μl of appropriately diluted
cDNA, 0.4 μl (10 μM) of forward and reverse primers specific for
human Amot or GAPDH, 5 μl of SYBR Premix Ex Taq and 3.2 μl
of water. The PCR consisted of 1 min at 95°C followed by 40 cycles
of denaturation for 15 sec at 95°C, annealing for 15 sec at 55°C
and a primer extension for 45 sec at 72°C. The ΔΔCt
method was used for the relative quantification of Amot
expression.
Western blotting
Cells were lysed with RIPA buffer [50 mmol/l Tris
(pH 7.5), 100 mmol/l NaCl, 1 mmol/l EDTA, 0.5% NP40, 0.5% Triton
X-100, 2.5 mmol/l sodium orthovanadate, 10 μl/ml protease inhibitor
cocktail and 1 mmol/l PMSF] by incubating for 20 min at 4°C.
Protein concentrations were determined by a BCA assay (Pierce,
Rockford, IL, USA). Equal amounts of the cell lysate protein were
subjected to 10% SDS-PAGE, transferred to nitrocellulose membranes
(Millipore, Boston, MA, USA), blocked with 5% non-fat dry milk in
Tris-buffered saline with Tween-20 for 1 h, and incubated with the
indicated antibody overnight at 4°C. The reactive bands were
developed by chemiluminescence with the luminol reagent
(Millipore). The blots were re-probed with GAPDH antibody as
a loading control.
Immunofluorescent staining
MCF-7, BT-474 and MDA-MB-231 cells were cultured on
coverslips to the appropriate densities. The cells were fixed with
4% paraformaldehyde solution for 10 min at room temperature, washed
three time with PBST and then permeabilized with 0.1% Triton X-100
for 10 min. The slides were blocked with 5% BSA and 10% horse serum
in PBST for 1 h at room temperature and incubated with antibodies
against Amot (1:100) overnight at 4°C. After being washed with PBS,
cells were incubated with IgG-HRP secondary antibody (1:100;
ZhongShan Jinqiao Biological Company, Peking, China) for 1 h at RT.
The cells were then washed three times and visualized using a laser
scanning confocal microscope (Leica, Germany).
Cell proliferation assay
Cells were seeded in 1% gelatin-coated 96-well
plates with 5×103 cells/well. Relative cell numbers were
quantified each day using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma) assay. The absorbance was measured at 492 nm using a
multifunction microplate reader (POLARstar OPTIMA, Germany).
Plate colony formation assay
The cells were trypsinized and resuspended in DMEM
containing 10% FBS. One handred cells were cultured in 6-well
plates until visible cell colonies were formed. After the cells
were fixed and stained, the number of cell colonies was counted
under a microscope (Leica, Germany).
5-Bromodeoxyuridine (Brdu) incorporation
assay
Brdu assay was used to detect the variation of the S
phase of breast cancer cells and evaluate the effect of Amot
downregulation on cell proliferation. Brdu (10 μmol/l) was added to
the medium, cultured in a constant-temperature incubator for 40
min, and fixed by 35% ethanol at 4°C for 1–2 h. Then cells were
resuspended in 2N Hcl and 0.1 M sodium borate successively. After
centrifugation at 800 × g for 5 min, the supernatant was decanted
and the cells were cultured in 5 μl anti-Brdu antibody (BD
Biosciences, Bedford, MA, USA) and 45 μl PBS (containing 0.5%
Tween-20 + 0.5% BSA) at room temperature for 30 min away from
light. The S phase of the cells were observed by flow cytometry (BD
Biosciences).
Wound-healing assay
The cells were seeded in 6-well plates and cultured
with DMEM containing 10% FBS until the cells reached subconfluence.
Following removal of the culture medium, a monolayer of the
sub-confluent cells was scratched with a 200 μl pipette tip to
create a wound area. The wounded monolayer was washed with PBS
twice and cultured in FBS-free medium or 2% FBS medium for 48 h.
Cell migration into the wound area was monitored by inverted
microscopy, and photographed at the indicated time points until the
wound was completely closed.
Cell migration and invasion assays
Migration and invasion assays were performed using
the BioCoat cell migration chamber (BD Biosciences), which consists
of a 24-well companion plate with cell culture inserts containing a
filter with 8-μm-diameter pores. The Transwell for the invasion
assay was coated with Matrigel (1:3 dilution with DMEM free of
serum; BD Biosciences). The cells were trypsinized and suspended
with DMEM without FBS at 2×105/ml for the migration
assay and with DMEM without FBS at 2×106/ml for the
invasion assay. Cell suspension (100 μl) was added to the upper
well and 600 μl DMEM medium containing 10% FBS was added to the
lower well. Cells in the wells were incubated in 5% CO2
at 37°C for 24 h for the migration assay and 48 h for the invasion
assay. After incubation, cells in the upper wells were gently
removed by scrubbing, fixed in 95% ethanol for 15 min and stained
with 0.4% crystal violet for 30 min. Invasive or migrated cells
were subsequently photographed with a microscope.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
(Chicago, IL, USA). Data were presented as the mean ± SD for at
least three replicates for each group. Statistical differences
between groups were determined using the ANOVA, Student’s t-test,
Wilcoxon and Chi-square tests. P<0.05 was considered to indicate
a statistically significant result.
Results
Amot expression and localization in
breast cancer and adjacent non-cancerous tissues
To verify the specificity of the Amot antibody,
immunohistochemistry of breast cancer tissues was performed using
Amot antibody, polypeptide-enclosed Amot antibody and PBS (negative
control), respectively. The results showed that Amot was highly
expressed in breast cancer tissues with Amot antibody, and
negatively expressed in breast cancer tissues with
polypeptide-enclosed Amot antibody or PBS (Fig. 1A–C). The results suggested that Amot
antibody was specific and sensitive.
Immunohistochemistry was performed on 242 breast
cancer and 92 adjacent non-cancerous tissues, to assess the
expression and localization of Amot. The results showed that Amot
was expressed in 205 breast cancer and 12 adjacent non-cancerous
tissues. Amot was highly expressed in breast cancer tissues, but
weakly expressed in adjacent non-cancerous tissues. The difference
was statistically significant (P<0.001; Table I). Notably, Amot expression was
observed in the nucleus and cytoplasm of breast cancer tissues. In
particular, Amot was strongly positively expressed in the nucleus.
However, Amot showed a weak positive expression in the cytoplasm of
adjacent non-cancerous tissues (Fig.
1D–G). We examined whether the strong positive expression of
Amot in breast cancer tissues was connected with the clinically
relevant parameters including age, tumor size, clinical stage,
pathological grade, local lymph node status, the expression of ER,
PR, Her-2 and Ki-67 (Table II). We
found that the expression level of Amot was increased in specimens
from patients with a high level of Ki-67, which is an
immunohistochemical proliferation marker in many types of cancer
(Fig. 1H; Table II; P<0.01). This finding
indicated that Amot was significantly correlated with cell
proliferation and invasion.
 | Table IIThe relationship between the
expression of Amot protein and clinicopathological factors. |
Table II
The relationship between the
expression of Amot protein and clinicopathological factors.
| | Expression level | | |
|---|
| |
| | |
|---|
| Variables | N | Neg. | Pos. | χ2 | P-value |
|---|
| Age (years) | | | | 0.000 | 1.000 |
| <35 | 11 | 2 | 9 | | |
| ≥35 | 229 | 36 | 19 | | |
| Missinga | 2 | | | | |
| Histological
grade | | | | 2.424 | 0.298 |
| 1 | 18 | 5 | 13 | | |
| 2 | 184 | 26 | 158 | | |
| 3 | 40 | 7 | 33 | | |
| Missinga | 0 | | | | |
| Clinical stage | | | | 0.497 | 0.781 |
| I | 16 | 3 | 13 | | |
| II | 139 | 24 | 115 | | |
| III–IV | 81 | 11 | 70 | | |
| Missinga | 6 | | | | |
| Size (cm) | | | | 1.158 | 0.560 |
| <2 | 41 | 7 | 34 | | |
| ≥2 | 199 | 31 | 168 | | |
| Missinga | 2 | | | | |
| Lymph node | | | | 0.057 | 0.811 |
| Negative | 93 | 14 | 79 | | |
| Positive | 141 | 22 | 117 | | |
| Missinga | 8 | | | | |
| ER stage | | | | 1.518 | 0.218 |
| ER (−) | 91 | 17 | 74 | | |
| ER (+) | 134 | 17 | 117 | | |
| Missinga | 17 | | | | |
| PR stage | | | | 0.033 | 0.857 |
| PR (−) | 122 | 19 | 103 | | |
| PR (+) | 102 | 15 | 87 | | |
| Missinga | 18 | | | | |
| Her-2 stage | | | | 2.741 | 0.098 |
| Her-2 (−) | 165 | 21 | 144 | | |
| Her-2 (+) | 60 | 13 | 47 | | |
| Missinga | 17 | | | | |
| Ki-67 stage | | | | 6.790 | 0.009b |
| <14% | 114 | 20 | 94 | | |
| ≥14% | 46 | 1 | 45 | | |
| Missinga | 82 | | | | |
Amot expression and localization in
breast cancer cell lines
The expression of Amot in MCF-7, T-47D, BT-474,
MDA-MB-453 and MDA-MB-231 breast cancer cell lines and the MCF-10A
breast epithelial cell line was detected by western blotting and
RT-PCR. Our findings showed that Amot mRNA and protein were
expressed in all the breast cancer cell lines, while the Amot
expression level was significantly higher in MCF-7 cells than in
the remaining cell lines (Fig. 2A and
C). The specificity of the Amot antibody was verified by
western blotting (Fig. 2B).
Notably, Amot was expressed in the nucleus and cytoplasm of breast
cancer cells. In particular, a strong positive Amot expression was
observed in the nucleus. The localization of Amot in breast cancer
cell lines was similar to those in breast cancer tissues (Fig. 2D).
Amot downregulation decreased
proliferative, invasive and metastatic capacity of MCF-7 cells in
vitro
The Amot protein expression was effectively
suppressed in MCF-7 cells by the shAmot lentivirus (Fig. 3A). We then conducted MTT assay,
plate colony formation and BrdU incorporation assay to estimate the
effect of Amot silencing on cell proliferation. MTT assay showed
that compared to CON and NC cells, the cells in the Amot knockdown
group grew gradually, with decreased cell viability (Fig. 3B). The plate colony formation assay
demonstrated that in MCF-7 Amot KD cells, the number of colonies
were reduced significantly (Fig. 3C and
D; P<0.001). BrdU incorporation assay further verified the
above findings. The Amot knockdown cells exhibited a significant
decrease in the percentage of S-phase cells, when compared with the
CON and NC cells (Fig. 3E and F;
P<0.001). The above results consistently suggested that Amot
downregulation inhibited MCF-7 cell proliferation.
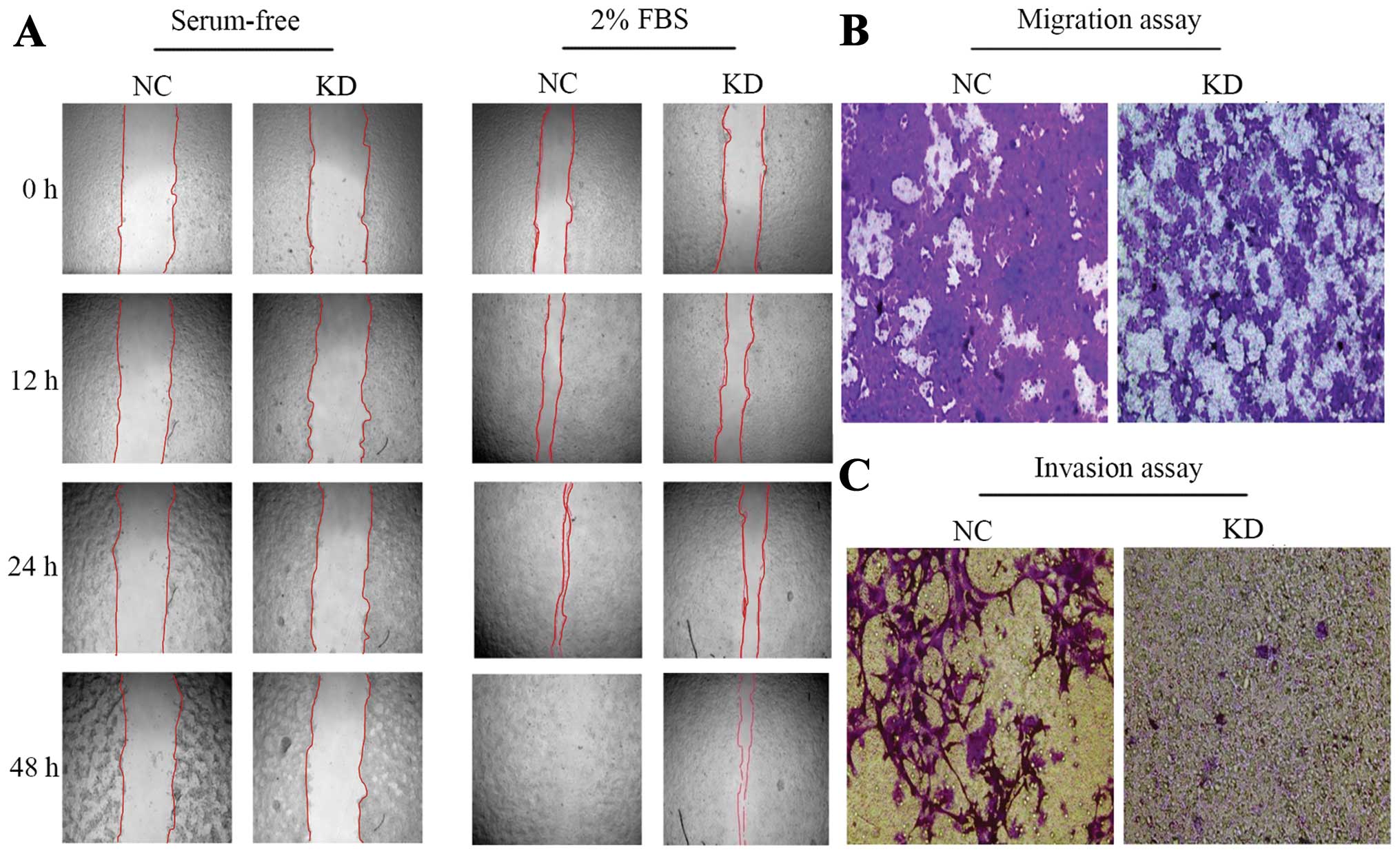
The effect of Amot downregulation on invasion and
metastasis was explored using wound healing and Transwell assays.
In the absence of the serum, cell migration showed no significant
change between the Amot KD and NC groups. Of note, the NC cells
exhibited characteristic morphological changes of apoptosis after
the serum was decanted 24 h, while the Amot knockdown cells were
allowed to grow in the serum-free medium. In the presence of 2%
FBS, the migration of the Amot knockdown cells was slower than that
of the NC cells (Fig. 4A).
Transwell assay was conducted to further confirm the abovementioned
results. It was found that the number of invading and migrating
Amot knockdown cells were significantly reduced, when compared to
those of NC cells (Fig. 4B and C),
suggesting that Amot downregulation significantly decelerated the
invasion and migration of MCF-7 cells in vitro.
Our results revealed that Amot knockdown
significantly decreased the proliferative, invasive and metastatic
capacity of MCF-7 cells in vitro.
Association of Amot and Hippo-YAP
pathway
Amot has been shown to participate in the activities
of the Hippo-YAP pathway (15). To
elucidate the relationship of Amot and Hippo-YAP pathway, we
examined the expression of YAP, YAP/TAZ, LATS1, MOB, MST1 and SAV1
in MCF-7 cells following the downregulation of Amot using western
blot analysis. The results showed that the expression of YAP,
YAP/TAZ and LATS1 was significantly decreased in MCF-7 cells
following the knockdown of Amot (Fig.
5A). However, the expression of MOB, MST1 and SAV1 did not
exhibit any notable change. Additionally, the expression of YAP was
obviously decreased in the nucleoprotein (Fig. 5B and C).
Discussion
Amot is an angiostatin-binding protein that promotes
endothelial cell migration and angiogenesis (5) and is expressed as the protein
isoforms, p80-Amot and p130-Amot. The angiostatin-responsive
migration-promoting function has been observed in the Amot p80
splicing variant, but not in the YAP-binding p130 variant (11). YAP-binding p130 Amot has been found
to be involved in tumorigenesis. It was observed for the first time
in 2011 that AmotL2 knockdown can activate YAP and induce cell
transformation of MDCK epithelial cells, suggesting that Amot
family proteins may play tumor suppressive roles (15). However, using RT-PCR Jiang et
al (14) found that breast
cancer tissues expressed significantly higher levels of Amot
transcript, compared with normal mammary tissues. The Amot
expression was significantly increased with the increasing degree
of invasion and metastasis. Amot expression showed close
relationships with VE-cadherin and PECAM-1. Findings of Jiang et
al suggested that Amot was closely related to angiogenesis,
invasiveness and poor survival of breast cancer (14). In 2008, Levchenko found that a DNA
vaccine targeting Amot induced an antibody response and
significantly inhibited angiogenesis and tumor growth (16). In addition, it has been reported
that Amot expression likely promotes cell growth by prolonging the
activation of MAPK signaling. Amot expression enhanced the
proliferation rate of MCF-7 cells and induced MCF-10A cells to form
large, disorganized spheroids in Matrigel. On the other hand, a
reduced expression of Amot resulted in decreased ERK1/2-associated
growth of MDA-MB-468 and SKBR3 cells (17). The above studies have indicated that
Amot acts as a potential tumor promoter, which was well supported
by our results.
In the present study, Amot was highly expressed in
breast cancer tissues and cells, but weakly expressed in normal
controls, while the expression level of Amot was increased in
specimens from patients with a high level of Ki-67. The results
indicated that Amot may be involved in breast cancer proliferation
and invasion. Amot knockdown, not only retarded growth and
viability of MCF-7 cells, but also significantly decreased the
percentage of S-phase cells. In addition, lentiviral-mediated Amot
silencing significantly reduced the number of colonies formed by
MCF-7 cells. Our results show that Amot downregulation inhibited
MCF-7 cell proliferation. We also found that Amot knockdown in
cells retarded the migration and invision of MCF-7 cells in
vitro. Taken together, Amot downregulation decreased the
proliferative, invasive and metastatic capacity of MCF-7 cells,
indicating that Amot is a potential tumor promoter in breast
cancer.
The Hippo-YAP signaling pathway regulates cell
proliferation during development, tissue regeneration and
carcinogenesis. Amot family proteins have been recently identified
as negative regulators of YAP by promoting YAP phosphorylation to
preventing their nuclear translocations (16–21).
However, it has been shown that Amot-p130 may promote nuclear
translocation of YAP and act as a transcriptional cofactor of the
YAP-TEAD complex to facilitate biliary epithelial cell
proliferation and liver cancer development either in response to
tissue injury or in the absence of the tumor suppressor Merlin
(22,23). The different results were obtained
probably since different organs and cells were used in those
studies. In the present study, Amot knockdown significantly
decreased the YAP and LATS1 expression in MCF-7 cells. Notably, the
expression of YAP was obviously decreased in the nucleoprotein. The
results suggest that Amot is involved in regulation of the
proliferation of MCF-7 cells by modulating the nucleoprotein
expression of YAP in the Hippo-YAP pathway. Future studies are
needed to reveal the mechanism for a clear role of Amot in the
Hippo-YAP pathway.
The respective roles of p80-Amot and p130-Amot were
not studied in the present study since we did not make a clear
distinction between p80 and p130 splicing variants of the Amot
antibody and shRNA. Future studies are to focus mainly on the
specific mechanisms of Amot and Hippo-YAP pathway, including the
expression changes of total YAP, phosphorylated YAP, p80-Amot, and
p130-Amot in the nucleus and cytoplasm, the activity of the
downstream transcription factor TEAD, as well as the relationship
between Amot and other signaling pathways associated with cell
proliferation, invasion and metastasis.
In conclusion, our results have shown that Amot was
highly expressed in breast cancer tissues and played an important
role in promoting breast cancer cell proliferation and invasion. In
addition, there was a more intimate connection between Amot and the
Hippo-YAP pathway. Further studies are needed to reveal the
mechanisms underlying the effect of Amot-induced Hippo-YAP pathway
on breast cancer growth and invasion.
Acknowledgements
This study was supported by the National Natural
Science Fund of China (no. 81172171).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berry DA, Cronin KA, Plevritis SK, et al:
Effect of screening and adjuvant therapy on mortality from breast
cancer. N Engl J Med. 353:1784–1792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fong PC, Boss DS, Yap TA, et al:
Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA
mutation carriers. N Engl J Med. 361:123–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Troyanovsky B, Levchenko T, Månsson G,
Matvijenko O and Holmgren L: Angiomotin: an angiostatin binding
protein that regulates endothelial cell migration and tube
formation. J Cell Biol. 152:1247–1254. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patrie KM: Identification and
characterization of a novel tight junction-associated family of
proteins that interacts with a WW domain of MAGI-1. Biochim Biophys
Acta. 1745:131–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levchenko T, Aase K, Troyanovsky B, Bratt
A and Holmgren L: Loss of responsiveness to chemotactic factors by
deletion of the C-terminal protein interaction site of angiomotin.
J Cell Sci. 116:3803–3810. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ernkvist M, Aase K, Ukomadu C, et al:
p130-Angiomotin associates to actin and controls endothelial cell
shape. FEBS J. 273:2000–2011. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bratt A, Birot O, Sinha I, et al:
Angiomotin regulates endothelial cell-cell junctions and cell
motility. J Biol Chem. 280:34859–34869. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wells CD, Fawcett JP, Traweger A, et al: A
Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity
proteins in epithelial cells. Cell. 125:535–548. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ernkvist M, Birot O, Sinha I, et al:
Differential roles of p80- and p130-angiomotin in the switch
between migration and stabilization of endothelial cells. Biochim
Biophys Acta. 1783:429–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ernkvist M, Luna Persson N, Audebert S, et
al: The Amot/Patj/Syx signaling complex spatially controls RhoA
GTPase activity in migrating endothelial cells. Blood. 113:244–253.
2009. View Article : Google Scholar :
|
|
13
|
Zheng Y, Vertuani S, Nyström S, et al:
Angiomotin-like protein 1 controls endothelial polarity and
junction stability during sprouting angiogenesis. Circ Res.
105:260–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang WG, Watkins G, Douglas-Jones A,
Holmgren L and Mansel RE: Angiomotin and angiomotin like proteins,
their expression and correlation with angiogenesis and clinical
outcome in human breast cancer. BMC Cancer. 6:162006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao B, Li L, Lu Q, et al: Angiomotin is a
novel Hippo pathway component that inhibits YAP oncoprotein. Genes
Dev. 25:51–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Levchenko T, Veitonmäki N, Lundkvist A, et
al: Therapeutic antibodies targeting angiomotin inhibit
angiogenesis in vivo. FASEB J. 22:880–889. 2008. View Article : Google Scholar
|
|
17
|
Ranahan WP, Han Z, Smith-Kinnaman W, et
al: The adaptor protein AMOT promotes the proliferation of mammary
epithelial cells via the prolonged activation of the extracellular
signal-regulated kinases. Cancer Res. 71:2203–2211. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chan SW, Lim CJ, Chong YF, Pobbati AV,
Huang C and Hong W: Hippo pathway-independent restriction of TAZ
and YAP by angiomotin. J Biol Chem. 286:7018–7026. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Huang J and Chen J:
Angiomotin-like proteins associate with and negatively regulate
YAP1. J Biol Chem. 286:4364–4370. 2011. View Article : Google Scholar :
|
|
20
|
Dai X, She P, Chi F, et al:
Phosphorylation of angiomotin by Lats1/2 kinases inhibits F-actin
binding, cell migration, and angiogenesis. J Biol Chem.
288:34041–34051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan SW, Lim CJ, Guo F, Tan I, Leung T and
Hong W: Actin-binding and cell proliferation activities of
angiomotin family members are regulated by Hippo pathway-mediated
phosphorylation. J Biol Chem. 288:37296–37307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong W: Angiomotin’g YAP into the nucleus
for cell proliferation and cancer development. Sci Signal.
6:pe272013. View Article : Google Scholar
|
|
23
|
Yi C, Shen Z, Stemmer-Rachamimov A, et al:
The p130 isoform of angiomotin is required for Yap-mediated hepatic
epithelial cell proliferation and tumorigenesis. Sci Signal.
6:ra772013. View Article : Google Scholar : PubMed/NCBI
|