Introduction
Surgical resection is the primary mode of choice in
the treatment of liver cancer, while the 5-year recurrence rate
after resection is as high as 35.4–43.5% (1). The poor prognosis associated with
liver cancer has prompted the identification and development of new
diagnostic markers and therapeutic strategies. Immunotherapy is a
potentially attractive option for patients with liver cancer.
Cancer/testis (CT) antigens are potential immunotherapeutic targets
in many types of cancers including liver cancer due to their
expression pattern, which is restrictively expressed in the testes,
yet aberrantly expressed by a variety of malignancies (2–8).
OY-TES-1 has been defined as the 23rd member of the CT antigen
family, called CT23 (9–12). OY-TES-1 was originally identified to
be the human homologue of pro-acrosin binding protein (ACRBP), a
tyrosine phosphorylated protein related to capacitation, the sp32
precursor in mouse (13).
Spontaneous humoral response against OY-TES-1 has been detected in
patients with different tumors including liver cancer (9). An HLA-A24-binding OY-TES-1 peptide
recognized by CD8 T cells was identified, and T-cell cytotoxicity
was observed against an OY-TES-1 mRNA-expressing lung tumor cell
line in vitro (14). The
above studies imply that OY-TES-1 is an attractive target for
antigen-specific immunotherapy in cancers due to its immunogenic
traits in humans (9,14). In another study in ovarian cancer
cells, a mitotic spindle protein NuMA was identified as an
ACRBP-interacting protein (12).
ACRBP depletion resulted in mitotic errors and reduced
proliferative fitness that could be rescued by NuMA co-depletion.
This indicates that ACRBP could normalize the perturbed mitotic
infrastructure responsible for disease-promoting genetic variation.
In our previous report, we demonstrated that OY-TES-1 was expressed
in human mesenchymal stem cells (MSCs) at both the mRNA and protein
levels, and downregulation of OY-TES-1 expression in these MSCs
caused cell growth inhibition, cell cycle arrest, apoptosis
induction and migration ability attenuation (15). However, whether OY-TES-1 is involved
in the biological function of liver cancer remains undetermined. In
the present study, we applied bioinformatic analysis combined with
a molecular biology assay to investigate the biological function
and protein interaction of OY-TES-1 in liver cancer. Our data
indicated that OY-TES-1 regulates biological processes of liver
cancer cells via NANOG, CD9, CCND2 and CDCA3.
Materials and methods
Motif and domain-domain interaction
analysis
The motif analysis of OY-TES-1 protein was performed
with SSDB Motif Search in Kyoto Encyclopedia of Genes and Genomes
(KEGG) online database (http://www.kegg.jp/). The protein domain interactions
were analyzed by DOMINE online database (16) (http://domine.utdallas.edu/cgi-bin/Domine) and the
Pfam protein families database (17), respectively. KEGG is a database
resource for understanding high-level functions and utilities of
the biological system from molecular-level information,
particularly large-scale molecular datasets generated by genome
sequencing and other high-throughput experimental technologies.
With KEGG motif search, a domain of unknown function with peptide
fragment usually can be found (18). DOMINE is a database of known and
predicted protein domain (domain-domain) interactions, which are
predicted by 13 different computational approaches using Pfam
domain definitions. DOMINE contains a total of 26,219 domain-domain
interactions (among 5,410 domains) out of which 6,634 are inferred
from PDB entries, of which 2,989 interactions are high-confidence
predictions (HCPs) (16,17).
Co-expressing gene analysis in liver
cancer through ONCOMINE
To identify significant OY-TES-1-co-expressing genes
in liver cancer, we searched for all relevant, publically available
microarray datasets in online cancer microarray gene expression
database, ONCOMINE (https://www.ONCOMINE.org/resource/main.html) (19). ONCOMINE database is a bioinformatics
initiative aimed at collecting, standardizing, analyzing and
delivering cancer transcriptome data to the biomedical research
community. The analysis has identified the genes, pathways and
networks deregulated across 18,000 cancer gene expression
microarrays, spanning the majority of cancer types and subtypes
(19). As there are often many
hundreds of tumor samples/microarrays within a single multi-array
result from co-expressing genes can be analyzed. ONCOMINE database
provides a potentially significant list of co-expressing genes,
which is important to define pathways in which the gene of interest
is involved (20).
Co-expressing gene annotation through
gene ontology (GO) annotator
GO annotator uses text-mining methods to extract GO
terms from scientific studies and provides this information along
with a GO term from an uncurated annotation; thus, it provides not
only facts but also their evidence (21). Based on the GO annotation, we
searched each proliferation, migration, invasion or apoptosis GO
term for the genes with high correlation and frequency to OY-TES-1
co-expression in the GO database.
Co-expressing gene literature
co-occurrence through COREMINE and PubMed
The OY-TES-1-co-expressing genes with GO terms of
cell proliferation, adhesion, migration and apoptosis in liver
cancer were fed to a literature co-occurrence tool-COREMINE online
tool (http://www.coremine.com/medical/#search) (22). COREMINE medical is a gene/protein
database and web-based tool for literature mining. It develops
automated extraction of experimental and theoretical knowledge of
biomedicine from publicly available gene and text databases to
create a gene-to-gene co-citation network for human genes in
MEDLINE records (22). The
systematic search of the literature was performed with PubMed for
studies addressing association among liver cancer, OY-TES-1 and
OY-TES-1 interacting proteins.
Oligonucleotide microarray analysis
combined with RNAi
OY-T ES-1 was down regulated in the liver cancer
cell line BEL-7404 using small interfering RNA (siRNA) with
X-tremeGENE siRNA transfection reagent (Roche Diagnostics).
OY-TES-1 siRNA and a scrambled siRNA were synthesized by Shanghai
GenePharma Co., Ltd. The sequences of the siRNAs and experimental
procedure were previously described by Cen et al (15). Total RNA extracted from
non-siRNA-treated cells and siRNA-OY-TES-1-treated cells was used
for genome-wide expression analysis with the Human Whole Genome
6×44K Microarray (Agilent Technologies, Inc., Santa Clara, CA, USA)
according to the manufacturer’s protocol (23). Data quality check and analysis were
conducted using SBC analysis system (Agilent Technologies). p-value
was calculated when duplicates were used in the experiment, and
differentially expressed genes were selected by p-value (<0.05)
(24).
Generation of biological interaction
network through GeneMANIA
Candidate genes selected from the oligonucleotide
microarray assay above were fed into a curated protein interaction
network system-GeneMANIA (http://www.genemania.org/), which is a fast web-based
tool and database for predicting gene function based on multiple
networks derived from different genomic or proteomic data/sources
with great accuracy (25). With the
GeneMANIA a gene/protein-gene/protein interaction network of
OY-TES-1 was generated.
Results
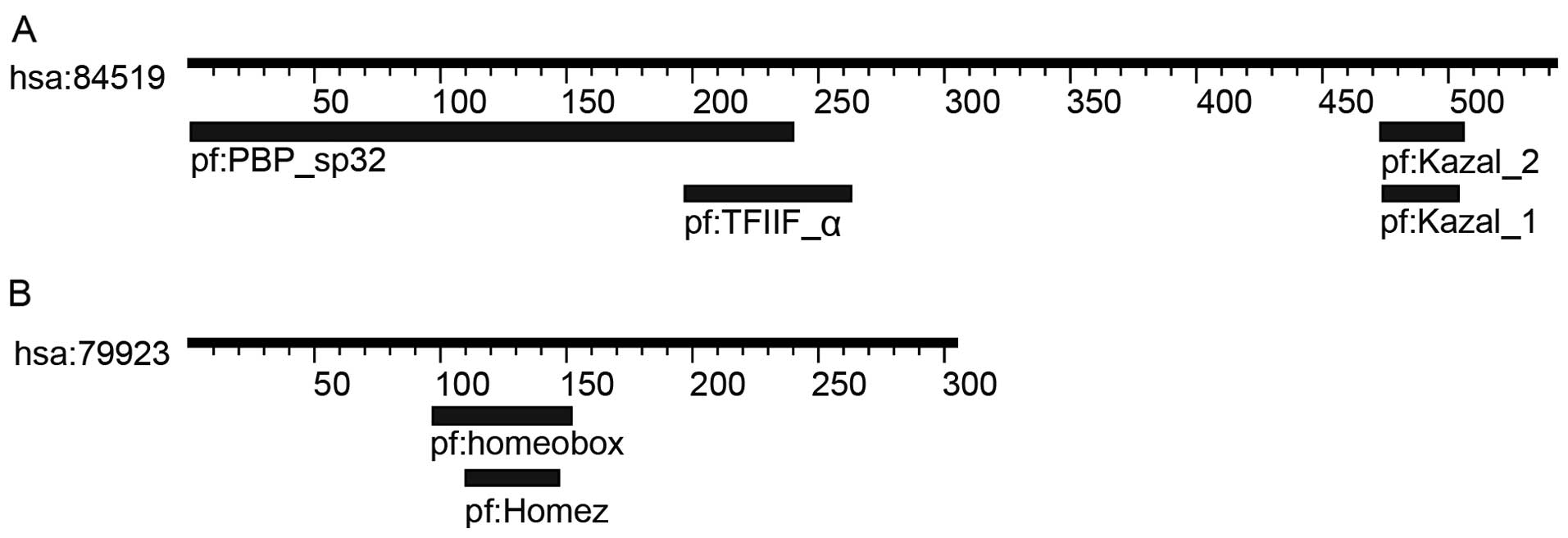
Four motifs were identified in
OY-TES-1
Following a search for ‘OY-TES-1’ in the KEGG online
database, four motifs, Kazal-1 and −2, PBP-sp32 and TFIIF-α, were
found in human OY-TES-1 on the dataset of hsa:84519 (Table I; Fig.
1). The Kazal motif contains two patterns, Kazal-1 and −2. The
amino terminal segment of both Kazal motifs can bind to the active
site of target proteases resulting in functional inhibition
(Table I). The family of Kazal-1
inhibitor proteins inhibits serine peptidases of the S1 family,
such as trypsin and elastase (26,27),
while the family of Kazal-2 inhibitor proteins inhibits serine
peptidases of MEROPS, such as I1, I2, I17 and I31. However,
Kazal-like domains are also seen in the extracellular part of
agrins, which are unknown to be protease inhibitor (28). TFIIF-α, a subunit of transcription
initiation factor IIF, or RNA polymerase II-associating protein 74
(RAP74) is the large subunit of transcription factor IIF. By
interacting with the proteins containing interacted motifs as
summarized in Table I, TFIIF-α
plays an essential role in accurate initiation and stimulates
elongation by RNA polymerase II (29). PBP-sp32 is a sperm-specific domain
involved in packaging acrosin zymogen into acrosomal matrix
(30). In general, OY-TES-1
interacts with the proteins containing TFIIF-α, Kazal-1 and −2
motifs or the proteins containing the interacted motifs of these 3
motifs. Thus, through these interactions, OY-TES-1 may perform its
functions in regulating the biological behavior of tumor cells.
 | Table IThe motifs of OY-TES-1 and NANOG,
interacted motifs and motif-shared proteins according to database
searcha. |
Table I
The motifs of OY-TES-1 and NANOG,
interacted motifs and motif-shared proteins according to database
searcha.
| Protein | Motif id | Location | Definition | E-value | Interacted
motif | Motif-shared
proteins |
|---|
| OY-TES-1 | pf:Kazal_1 | 474–504 | Kazal-type serine
protease inhibitor domain | 0.05 | TGF-β, Kazal-1,
Peptidase-S8, Trypsin, FOLN, SPARC-Ca_bdg, efhand, Laminin-EGF,
Thyroglobulin_1, EGF, Kunitz-BPTI, ig, Laminin-G_1, Ldl_recept_a,
Sushi, TSP-1, zf-C2H2, SRCR, PDZ, SEA, MACPF,
OATP | AGRIN, CPAMD8, FST,
FSTL3, FSTL4, FSTL5, IGFBPL1, SMOC1, SPARC, SPARCL1, SPINK1,
SPINK2, SPINK4, SPINK5, SPINK5L2, SPINK5L3, SPINK6, SPINK7, SPINK9,
TMEFF1, TMEFF2 |
| pf:Kazal_2 | 473–506 | Kazal-type serine
protease inhibitor domain | 0.0075 | TGF-β, Trypsin,
Kazal-2, BTB, Homeobox, Arrestin_N, LIM, Arrestin-C | C6, CFI, FSTL1,
FSTL3, HTRA1, HTRA3, HTRA4, IGFBP7, KAZALD1, LST3, RECK, SLC21A8,
SLCO1A2, SLCO1B1, SLCO1B3, SLCO1C1, SLCO2A1, SLCO3A1, SLCO4A1,
SLCO4C1, SLCO5A1, SLCO6A1, SMOC2, SPINK5, SPOCK1, SPOCK2, SPOCK3,
WFIKKN1, WFIKKN2 |
| pf:TFIIF_α | 197–263 | Transcription
initiation factor IIF, α subunit (TFIIF-α) | 0.13 | TFIIF_β, FCP1_C,
Tax, FlhD, Ribosomal_L7Ae, HNF-1_N, TFIIF_α | TFIIF |
| pf:PBP_sp32 | 1–240 | Proacrosin binding
protein sp32 | 9.30e-135 | Unknown | OY-TES-1
(sp32/ACRBP) |
| NANOG | pf:Homeobox | 97–152 | Homeobox
domain | 7.80E-19 | Homeobox, Pou,
SRF-TF, SBP_bac_1, CUT, HNF-1B_C, HNF-1_N, PD-C2-AF1, HLH, Pkinase,
RRM_1, zf-C2H2, PAX, WD40, MH2, EGF, Kazal_2 | Pou family |
| pf:Homez | 110–147 | Homeodomain
leucine-zipper encoding, Homez | 0.00024 | Unknown | Unknown |
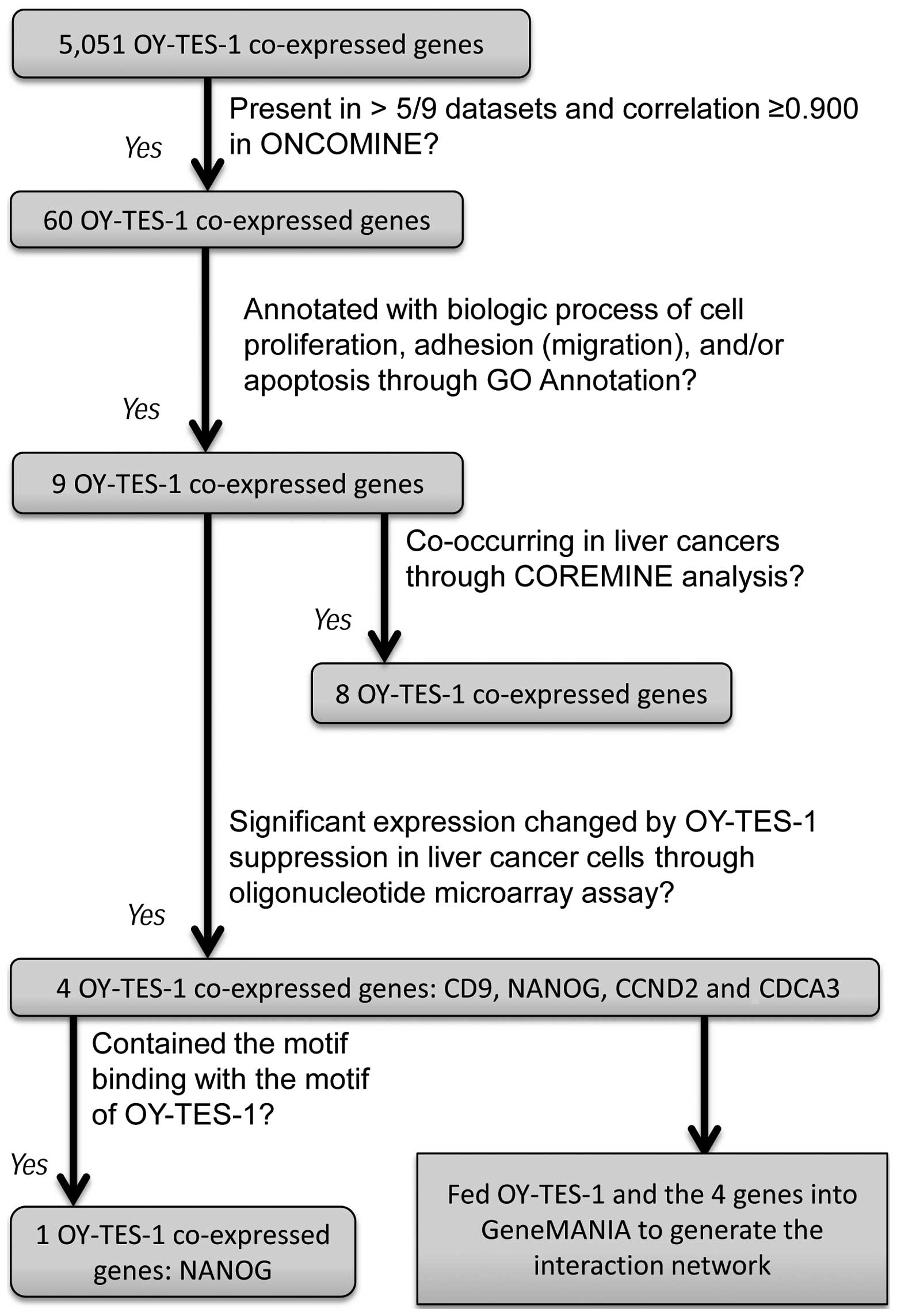
Sixty genes were found to co-express with
OY-TES-1 in liver cancer
To investigate OY-TES-1-co-expressing genes in liver
cancer, we queried the ONCOMINE database using a concept
‘co-expression genes with OY-TES-1 expression in liver cancer’.
There was a list of 5,051 genes in 9 data-sets, namely Liver
(Liao)-Cluster ID n9273 (17 genes); Multi-cancer
(Beroukhim)-Cluster ID n9385 (85 genes), Cell Line
(Rothenberg)-Cluster ID n9276 (207 genes), Cell Line (Wooster
2)-Cluster ID n9229 (209 genes), Cell Line (Barretina 2)-Cluster ID
n9313 (229 genes), Liver cancer (Bittner Multi-cancer, 978 genes),
Liver cancer (Wooster Cell Line 2, 1,875 genes), Liver cancer
(Barretina Cell Line, 1,957 genes) and Liver cancer (Bittner
Multi-cancer, 1,957 genes). As listed in Table II, 60 genes were co-expressed with
OY-TES-1 at least in 5 of 9 datasets mentioned above, and the
correlation between those genes and OY-TES-1 was >0.900.
 | Table IIGenes co-expressing with OY-TES-1 at
least in 5 out of 9 datasets. |
Table II
Genes co-expressing with OY-TES-1 at
least in 5 out of 9 datasets.
| Gene | Correlationa | Freqb | Gene | Correlation | Freq |
|---|
| EMG1 | 0.980±0.000 | 7/9 | CLSTN3 | 0.964±0.028 | 5/9 |
| CD9 | 0.980±0.005 | 7/9 | C1RL | 0.964±0.028 | 5/9 |
| ZNF384 | 0.989±0.008 | 7/9 | COPS7A | 0.990±0.009 | 5/9 |
| SCNN1A | 0.971±0.014 | 7/9 | LAG3 | 0.986±0.006 | 5/9 |
| C12orf53 | 0.991±0.010 | 7/9 | DPPA3 | 0.951±0.018 | 5/9 |
| CLEC4A | 0.951±0.018 | 6/9 | ATN1 | 0.982±0.003 | 5/9 |
| ENO2 | 0.983±0.003 | 6/9 | RIMKLB | 0.907±0.000 | 5/9 |
| CD27 | 0.977±0.006 | 6/9 | USP5 | 0.988±0.007 | 5/9 |
| MFAP5 | 0.907±0.000 | 6/9 | C12orf57 | 0.982±0.003 | 5/9 |
| FOXJ2 | 0.951±0.018 | 6/9 | TAPBPL | 0.977±0.006 | 5/9 |
| LPAR5 | 1.000±0.001 | 6/9 | C1R | 0.964±0.028 | 5/9 |
| NCAPD2 | 0.977±0.006 | 6/9 | LTBR | 0.971±0.014 | 5/9 |
| VAMP1 | 0.977±0.006 | 6/9 | LEPREL2 | 0.988±0.007 | 5/9 |
| C1S | 0.976±0.007 | 5/9 | TPI1 | 0.988±0.007 | 5/9 |
| ITFG2 | 0.861±0.015 | 5/9 | NOP2 | 0.996±0.004 | 5/9 |
| ING4 | 0.997±0.004 | 5/9 | GNB3 | 0.988±0.007 | 5/9 |
| PHB2 | 0.982±0.003 | 5/9 | MLF2 | 0.985±0.005 | 5/9 |
| NANOG | 0.951±0.018 | 5/9 | RBP5 | 0.964±0.028 | 5/9 |
| CDCA3 | 0.988±0.007 | 5/9 | LRRC23 | 0.983±0.006 | 5/9 |
| PTPN6 | 0.982±0.003 | 5/9 | LPCAT3 | 0.976±0.007 | 5/9 |
| CLEC4C | 0.951±0.018 | 5/9 | PLEKHG6 | 0.971±0.014 | 5/9 |
| SLC2A14 | 0.922±0.009 | 5/9 | GAPDH | 0.984±0.011 | 5/9 |
| TNFRSF1A | 0.971±0.014 | 5/9 | GDF3 | 0.951±0.018 | 5/9 |
| AICDA | 0.907±0.000 | 5/9 | IFFO1 | 0.988±0.012 | 5/9 |
| SLC2A3 | 0.922±0.009 | 5/9 | CD4 | 0.976±0.017 | 5/9 |
| FAM90A1 | 0.943±0.016 | 5/9 | CHD4 | 0.999±0.001 | 5/9 |
| NECAP1 | 0.951±0.018 | 5/9 | PTMS | 0.986±0.006 | 5/9 |
| CCND2 | 0.956±0.022 | 5/9 | A2ML1 | 0.907±0.000 | 5/9 |
| GPR162 | 0.988±0.007 | 5/9 | C3AR1 | 0.951±0.018 | 5/9 |
| SPSB2 | 0.988±0.007 | 5/9 | MRPL51 | 0.977±0.006 | 5/9 |
Nine OY-TES-1 co-expressing genes may
regulate biological processes
As the 60 genes identified above showed a
correlation with OY-TES-1, we further predicted their function
through GO annotator and COREMINE online tool search. As listed in
Table III, we identified 9 genes:
CD9 molecule (CD9), cyclin D2 (CCND2), CD27 molecule (CD27), cell
division cycle-associated protein (CDCA3), inhibitor of growth
family, member 4 (ING4), lymphotoxin-β receptor (LTBR), homeobox
transcription factor Nanog (NANOG), nucleolar protein 2 homolog
(NOP2) and tumor necrosis factor receptor superfamily, member 1A
(TNFRSF1A). These genes are involved in cell proliferation,
adhesion, migration and/or apoptosis.
 | Table IIIThe biological process annotation of
OY-TES-1-co-expressing genes by GO annotator. |
Table III
The biological process annotation of
OY-TES-1-co-expressing genes by GO annotator.
| Gene | GO ID | Qualified GO
term | Evidence | Ref. |
|---|
| CD9 | GO:0007155 | Cell adhesion | IDA | (38) |
| GO:0008285 | Negative regulation
of cell proliferation | IEA | No |
| CCND2 | GO:0007049 | Cell cycle | IEA | No |
| GO:0045737 | Positive regulation
of cyclin-dependent protein kinase activity | IDA | (43) |
| GO:0051301 | Cell division | IEA | No |
| CD27 | GO:0006917 | Induction of
apoptosis | ISS | No |
| GO:0008588 | Release of
cytoplasmic sequestered NF-κB | NAS | (29) |
| GO:0043066 | Negative regulation
of apoptotic process | ISS | No |
| GO:0043154 | Negative regulation
of cysteine-type endopeptidase activity involved in apoptotic
process | IDA | (29) |
| CDCA3a | GO:0007067 | Mitosis | IEA | No |
| GO:0051301 | Cell division | IEA | No |
| ING4 | GO:0006915 | Apoptotic
process | IDA | (33) |
| GO:0007050 | Cell cycle
arrest | IDA | (33) |
| GO:0008285 | Negative regulation
of cell proliferation | IDA | (33) |
| GO:0043065 | Positive regulation
of apoptotic process | IDA | (34) |
| LTBR | GO:0006915 | Apoptotic
process | IEA | No |
| GO:2001238 | Positive regulation
of extrinsic apoptotic signaling pathway | IMP | No |
| NANOG | GO:0008283 | Cell
proliferation | IMP | (50) |
| NOP2 | GO:0008284 | Positive regulation
of cell proliferation | TAS | (53) |
| TNFRSF1A | GO:0006915 | Apoptotic
process | TAS | No |
| GO:0042981 | Regulation of
apoptotic process | IEA | No |
| GO:0043123 | Positive regulation
of I-κB kinase/NF-κB cascade | IEP | (37) |
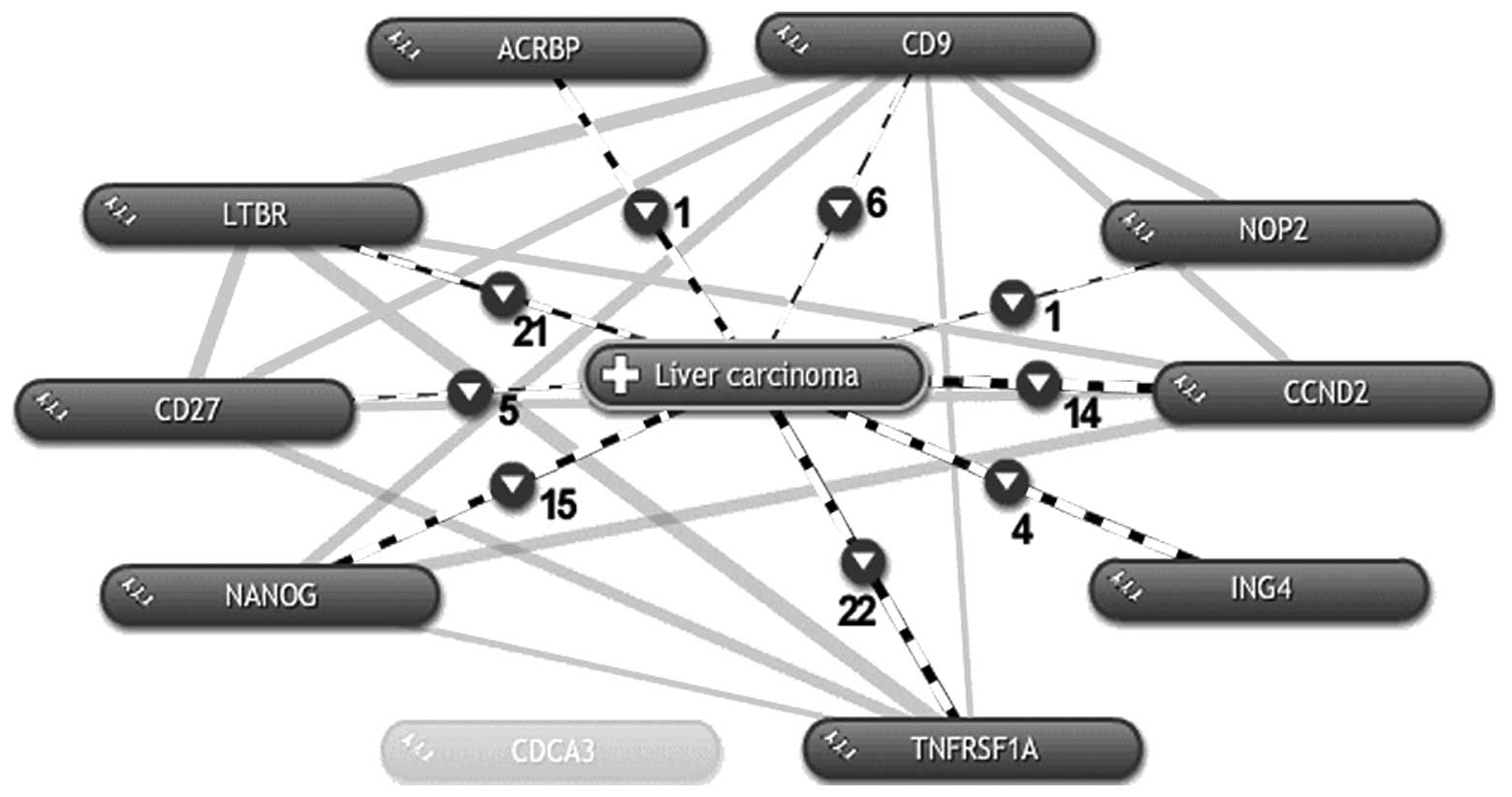
Eight OY-TES-1 co-expressing genes are
co-occurring in liver cancer
According to the above search, 9 of the
co-expressing OY-TES-1 genes are involved in the biological
behavior of cells, but whether they are related to liver cancer
remains unknown. Thus, these 9 genes and OY-TES-1 were further fed
to COREMINE online tool search using ‘liver carcinoma’ as a key
word. As shown in Fig. 2,
co-occurrence with liver cancer was demonstrated for OY-TES-1 and 8
of the 9 genes except for CDCA3, and these 8 genes also co-occur
with each other. Among those 8 genes, CD27, ING4, LTBR and TNFRSF1A
are known to be involved in apoptosis; CD27 negatively regulates
the apoptotic process (31–34), while ING4 (35–37),
LTBR (38) and TNFRSF1A (39) positively regulate the apoptotic
process. CD9 is also considered to regulate migration and adhesion
of cells (40,41). In addition, CD9 and ING4 are thought
to negatively regulate cell proliferation (35,44).
The others, CCND2 (45–51), NANOG (52–54)
and NOP2 (55,56), positively regulate cell
proliferation. With regard to CDCA3, a G1 phase
controlling gene which prevents G1 arrest, there is no
current literature that shows that it is involved in liver cancer.
However, considering that CDCA3 has a high expression frequency and
a high co-expression correlation with OY-TES-1 in liver cancer
datasets, further investigation of CDCA3 is needed. To date, there
is no report of the involvement of OY-TES-1 in apoptosis,
migration, adhesion and cell proliferation of liver cancer. We here
demonstrated that OY-TES-1 is co-expressed with 9 genes (CD9,
CCND2, ING4, CDCA3, NANOG, NOP2, CD27, LTBR and TNFRSF1A) with a
high correlation and frequency, inferring that OY-TES-1 may be
involved in the cell adhesion/migration regulated by CD9, cell
proliferation mediated by CD9, CCND2, ING4, CDCA3, NANOG and NOP2,
and apoptosis modulated by CD27, ING4, LTBR and TNFRSF1A in liver
cancer, respectively.
Four candidate genes are significantly
altered by OY-TES-1 downregulation
As the above results identified 9
OY-TES-1-co-expressing genes with functions of cell proliferation,
adhesion, migration and/or apoptosis, we further screened an
oligonucleotide microarray following OY-TES-1 down-regulation in a
liver cancer cell line. It was found that a total of 8,870 genes
were significantly altered (p<0.05) in the
siRNA-OY-TES-1-treated cell as compared with the control. Notably,
these 9 OY-TES-1 co-expressing genes (CD9, CCND2, CDCA3, NANOG,
ING4, NOP2, CD27, LTBR and TNFRSF1A) revealed a differential
expression profile. CD9, CCND2 and CDCA3 were upregulated, whereas
NANOG was downregulated. Another 5 genes had no expression change
(p>0.05, Table IV).
Furthermore, after searching the motif of CD9, CCND2, CDCA3 and
NANOG in SSDB, DOMINE and Pfam database, an interacted motif of
Kazal-2 contained in OY-TES-1, homeobox, was found in human NANOG
on the dataset of hsa:79,923 (Table
I; Fig. 1). Therefore, NANOG
may be considered as the most likely candidate protein interacting
with OY-TES-1 in liver cancers.
 | Table IVExpression profile of OY-TES-1
co-expressing candidate genes and their interacing genes by
OY-TES-1 suppression in the cell line BEL-7404a. |
Table IV
Expression profile of OY-TES-1
co-expressing candidate genes and their interacing genes by
OY-TES-1 suppression in the cell line BEL-7404a.
| Gene symbol | Gene name | Biological function
of encoded protein | Fold-change | P-value |
|---|
| OY-TES-1
co-expressing candidate genes |
| CD9 | CD9 molecule | Negative regulation
of cell proliferation; suppressor of cancer cell motility and
metastasis | 2.2263 | 2.00E-04 |
| CCND2 | Cyclin D2 | Positive regulation
of cell proliferation. Regulatory subunit of CDK4 or CDK6, required
for cell cycle G1/S transition | 2.0956 | 0.0017 |
| CDCA3 | Cell division cycle
associated 3 | F-box-like protein
required for entry into mitosis. Acts by participating in E3 ligase
complexes that mediate the ubiquitination and degradation of WEE1
kinase at G2/M phase | 2.0355 | 0.0208 |
| NANOG | Nanog homeobox | Transcription
regulator involvesin embryo stem (ES) cells proliferation and
self-renewal. When overexpressed, promotes cells to enter into S
phase and proliferation | 0.4258 | 0.0064 |
| TNFRSF1A | Tumor necrosis
factor receptor superfamily, member 1A | Major receptor for
the tumor necrosis factor-α, mediate apoptosis by activating
NF-κB | 1.2090 | 0.065 |
| NOP2 | NOP2 nucleolar
protein homolog | Positive regulation
of cell proliferation; increase nucleolar activity associated with
cell proliferation | 1.2410 | 0.1695 |
| ING4 | Inhibitor of growth
family, member 4 | Tumor suppressor
protein, involves in the TP53-dependent regulatory pathway;
negative regulation of cell proliferation, positive regulation of
apoptotic process | 1.2089 | 0.1978 |
| LTBR | Lymphotoxin β
receptor | Receptor for
heterotrimeric lymphotoxin and TNFS14/LIGHT. Promotes apoptosis via
TRAF3 and TRAF5 | 1.3154 | 0.2497 |
| CD27 | CD27 molecule | Receptor for
CD70/CD27L. Negative regulation of cysteine-type endopeptidase
activity involved in apoptotic process | 0.8403 | 0.3803 |
| Interacting genes
of OY-TES-1-co-expressing candidate genes |
| CCND3 | Cyclin D3 | Regulatory
component of the cyclin D3-CDK4 (DC) complex that inhibits members
of the retinoblastoma (RB) protein family, and regulates the
cell-cycle during G1/S transition | 1.4241 | 0.0032 |
| CDK6 | Cyclin-dependent
kinase 6 |
Serine/threonine-protein kinase involved
in the control of the cell cycle and differentiation; promotes
G1/S transition; negatively regulates cell
differentiation | 1.2173 | 0.0205 |
| WEE1 | WEE1 homolog | Negative regulator
of entry into mitosis (G2 to M transition) by protecting
the nucleus from cytoplasmically activated cyclin B1-complexed
CDK1 | 1.5128 | 0.0031 |
| CD44 | CD44 molecule | Receptor for
hyaluronic acid (HA) and possibly matrix metalloproteinases (MMPs).
Adhesion with HA plays an important role in cell migration, tumor
growth and progression | 1.2606 | 0.0055 |
| ITGA2 | Integrin, α2 | Receptor for
laminin, collagen, collagen C-propeptides, fibronectin and
E-cadherin. It is responsible for adhesion of platelets and other
cells, modulation of collagen and collagenase gene expression | 2.1047 | 0.0084 |
| ITGB1 | Integrin, β1 | Membrane receptors
involved in cell adhesion and recognition in a variety of processes
including embryogenesis, hemostasis, tissue repair, immune response
and metastatic diffusion of tumor cells | 1.2910 | 0.0297 |
| ITGA5 | Integrin, α5 | Receptor for
fibronectin and fibrinogen. Enhance angiogenesis in Kaposi’s
sarcoma lesions when HIV-I infected | 2.0167 | 0.0029 |
| EGR1 | Early growth
response 1 | Transcriptional
regulator recognizes and binds to EGR-site. Activates the
transcription of target genes whose products are required for
mitogenesis and differentiation | 2.3115 | 1.00E-04 |
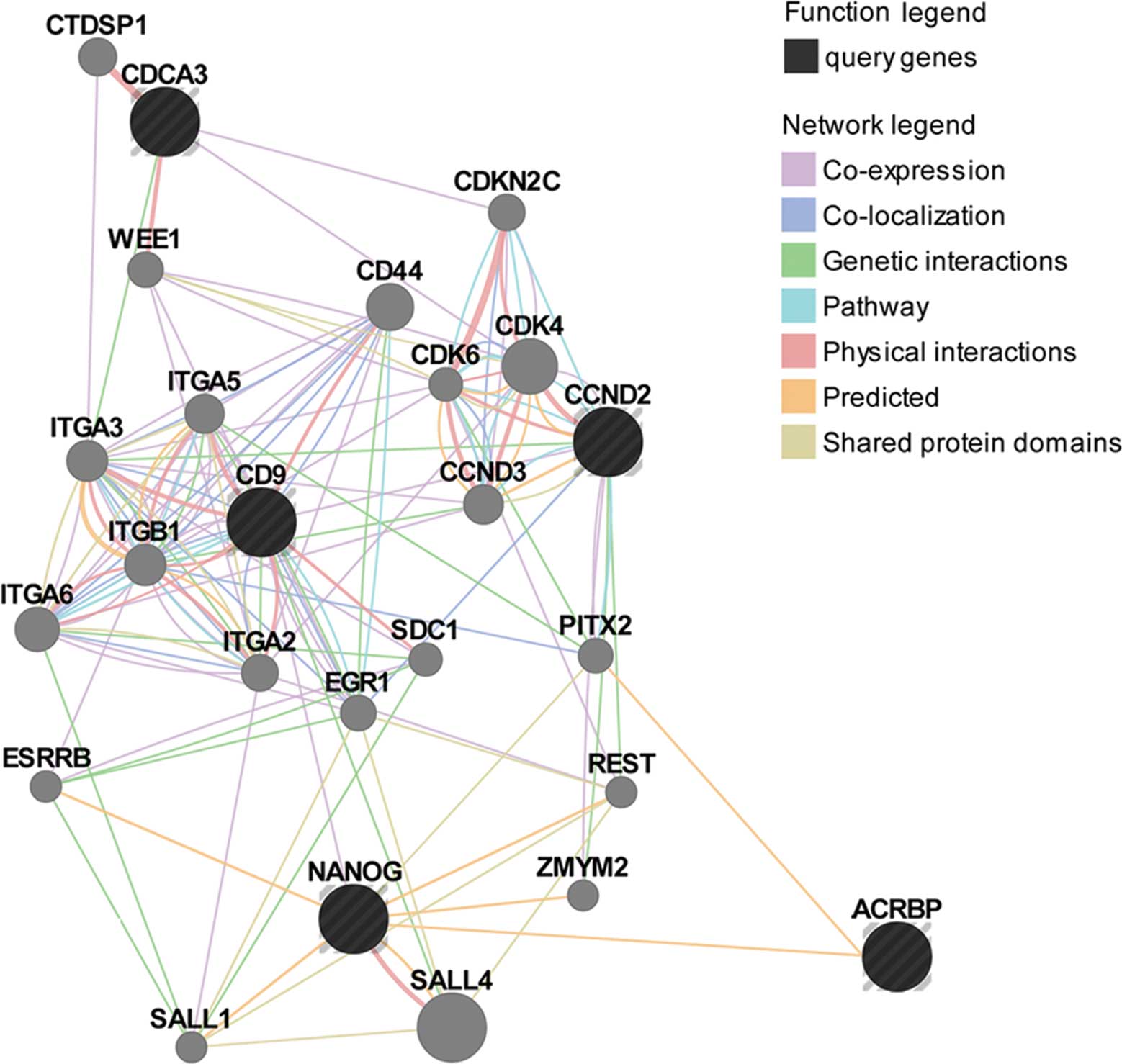
OY-TES-1 may be functionally related to
NANOG, CD9, CCND2 and CDCA3 by various interactions
Due to the unclear functions of OY-TES-1 and its
co-expressing proteins, OY-TES-1, NANOG, CDCA3, CD9 and CCND2 were
fed into GeneMANIA to predict their functions and interactions. As
shown in Fig. 3, OY-TES-1, NANOG,
CD9, CCND2 and CDCA3 were co-expressed, co-localized, physically
and genetically interacted, and/or shared protein domains and
pathways with each other and a number of other proteins, such as
CCND3, CDK4, CDK6, CD44, ITGA2, ITGA3, ITGB1, ESRRB, EGR1, PITX2,
REST, CDKN2C and WEE1 (Table V).
Therefore, it can be suggested that OY-TES-1, NANOG, CDCA3, CD9 and
CCND2 may be functionally related. Although OY-TES-1 was
considerably less interactive with other proteins involving in cell
proliferation, adhesion, migration and apoptosis in comparing the
results, it contains a Kazal-2 domain that could bind with the
homeobox domain shared by NANOG and PITX2. Thus, we added
interactions between OY-TES-1, NANOG and PITX2, and predicted these
interactions with cell proliferation, adhesion, motility and
apoptosis in liver cancer. Based on the annotated functions in
accordance with the GeneMANIA network, OY-TES-1, NANOG, CD9, CCND2
and CDCA3, along with other proteins listed in Table V, may play important roles in the
regulation of cell adhesion, the cell cycle, kinase activity,
apoptosis (or anoikis) and DNA binding.
 | Table VThe biologic process annotated
functions of OY-TES 1-co-expressing proteins and their interacting
proteins in the GeneMANIA network. |
Table V
The biologic process annotated
functions of OY-TES 1-co-expressing proteins and their interacting
proteins in the GeneMANIA network.
| GO annotation | FDR (n/a) | Genes/proteins in
the network |
|---|
| Cyclin-dependent
protein kinase holoenzyme complex | 2.93E-05 | CCND2, CCND3, CDK4,
CDK6 |
| Cell-matrix
adhesion | 1.47E-03 | CD9, CD44, CDK6,
ITGA2, ITGA3, ITGB1 |
| Cell-substrate
adhesion | 5.14E-03 | CD9, CD44, CDK6,
ITGA2, ITGA3, ITGB1 |
| Transcription
regulatory region sequence-specific DNA binding | 1.77E-02 | NANOG, ESRRB, EGR1,
PITX2, REST |
| G1
phase/G1 phase of mitotic cell cycle | 6.28E-02 | CDKN2C, CDK4,
CDK6 |
| Regulation of
cyclin-dependent protein serine/threonine kinase activity | 1.46E-01 | CCND2, CCND3,
CDKN2C |
| G1/S
transition of mitotic cell cycle | 1.68E-01 | CDCA3, CDK6, CDK4,
WEE1, CDKN2C |
| Negative regulation
of anoikis | 2.06E-01 | ITGB1, ITGA5 |
Discussion
Functional prediction of genes/proteins based on
bioinformatic analysis is a feasible and valuable technique for the
mining of gene/protein functions, and many large-scale networks of
protein interactions within the cell have made it possible to
multi-dimensionally study the functions in the context of a network
(57). Thus, mining and exploring
potentially OY-TES-1-interacting genes via bioinformatic methods
would be a first, necessary, feasible and reasonable way to reveal
its function in liver cancer. Based on the motif, co-expression
profile, GO and literature co-occurrence analysis, we found 60
genes to be co-expressing with OY-TES-1 in liver cancer, and 9 out
of 60 of these genes are involved in cell proliferation, adhesion
(migration) and/or apoptosis. OY-TES-1 and 8 out of 9 genes were
found to co-occur in liver cancer, and these 8 genes co-occur with
each other. Furthermore, with RNAi and oligonucleotide microarray
analysis, we confirmed that, of these 9 genes, expression of CD9,
CCND2 and CDCA3 was significantly increased, and NANOG was markedly
decreased. The expression levels of the other 5 genes did not
change when OY-TES-1 was suppressed in liver cancer cells
(p>0.05, Table IV). GeneMANIA
network analysis demonstrated that OY-TES-1, NANOG, CD9, CCND2 and
CDCA3 were co-expressed, co-localized, physically and genetically
interacted and/or shared protein domains and pathways with each
other (Fig. 3). Annotated functions
(Table V) suggested that OY-TES-1
may participate in tumor cell proliferation, migration, invasion
and apoptosis through regulation of CCND2, CDCA3, CD9 and
NANOG.
Both CCND2 and CDCA3 are G1 phase
controlling genes. CCND2 overexpression is associated with the
tumorigenesis and progression of various types of cancers including
liver cancers by affecting the cell cycle, particularly in the
G1 phase (G1 cell cycle transition) with
G1 CCND2/cyclin-dependent kinase (CDK)4 (or 6) complexes
(58–61). Exhibiting a difference with CCND2,
CDCA3 can increase the capacity of proliferation by preventing
G1 arrest via decreased expression of the CDK inhibitor
(CDKI) (62,63). In the present study, downregulation
of OY-TES-1 in BEL-7404 cells was accompanied by an increase in
CCND2 and CDCA3 as well as their interacting genes CCND3 and CDK6
(p<0.05, Table IV, Fig. 3), which are able to accelerate cell
proliferation by promoting G1/S transition, CDK activity
regulation or cyclin/CDK complex formation (60,61,64).
However, as a negative regulator of entry into mitosis
(G2 to M transition) (65), WEE1 was significantly increased
(p<0.05) (Table IV, Fig. 3); the other cell cycle involved
genes CD4 and CDKN2C were not altered (data not shown). Therefore,
it is reasonable to infer that downregulation of OY-TES-1 may
accelerate the cell cycle and promote proliferation in liver cancer
cells through increased expression of CCND2, CDCA3 and their
interacing genes CCND3 and CDK6.
CD9 and NANOG are also thought to be associated with
the malignant behavior of cells. The absence and low expression of
CD9 in small cell lung cancer may contribute to the highly invasive
and metastatic phenotype, while ectopic expression of CD9 reduced
cell proliferation and motility, attenuated metastasis (66,67)
and promoted apoptosis (68,69).
Therefore, CD9 has been regarded as an important tumor progression
suppressor (70). To date, there is
paucity in the research of the correlation between CD9 and liver
cancer. As regard to NANOG, it is one of the most important core
markers of cancer stem cells (CSCs) due to its capacity to maintain
pluripotency, regulate proliferation and prevent differentiation
(71,72). NANOG-positive CSCs in liver cancer
exhibit drug resistance and a high capacity for tumor invasion and
metastasis (73,74). The same situation is present in
other cancers. For example, upregulation of NANOG enhances
malignant behaviors in esophageal cancer (52,75);
adversely, its downregulation causes inhibitive effects on ovarian
and gastric cancer (76). Here, we
demonstrated that suppression of OY-TES-1 in a cancer cell line
significantly increased expression of CD9 and its interacting genes
(CD44, ITGA2, ITGB1 and ITGA5), which negatively regulate
proliferation and migration in cancer cells (40–43).
Meanwhile, we also found a decrease in NANOG and elevation in EGR1
which interacts with NANOG (Fig. 4;
Table IV). EGR1 is thought to be a
cancer suppressor (77). There was
no change in the other genes listed in Table V, which are involved in cell
differentiation and proliferation and are related with CD9 or
NANOG. Notably, in the present study downregulation of OY-TES-1 in
liver cancer cells caused two opposite effects, namely, promotion
of cell proliferation with increase in CCND2 and CDCA3, and
inhibition of cell proliferation with CD9 upregulation and NANOG
downregulation. Therefore, it was speculated that OY-TES-1 may play
multiple roles in liver cancer. Experiments should be conducted to
elucidate the function of OY-TES-1 with CD9, NANOG, CCND2, CDCA3
and their interacted proteins in the future.
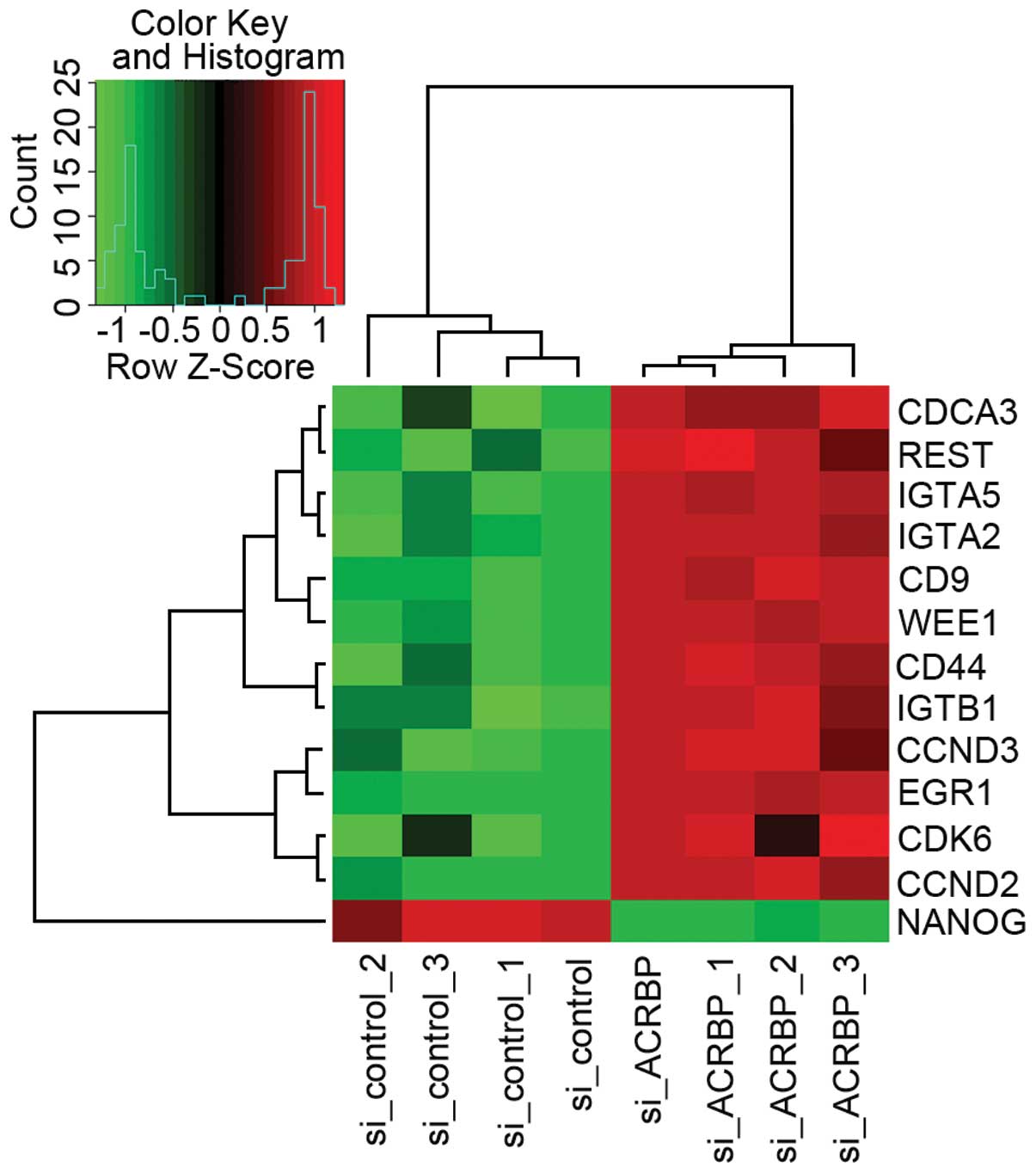
Collectively, as shown in Fig. 5, we first report that OY-TES-1
suppression results in significant expression changes of its
co-expressing genes, CCND2, CDCA3, CD9 and NANOG. As it contains a
Kazal-2-interacting motif, homeobox, NANOG may be considered to be
the most likely candidate protein interacting with OY-TES-1 in
liver cancer. Thus, the present study may set the stage for further
investigation of the role of OY-TES-1 in liver cancer.
Acknowledgements
We thank Ms. Fang Chen, Ms. Chengxiao Chen from
Guangxi Medical University for their excellent technical
assistance. The present study was supported by the National Natural
Science Foundation of China (nos. 81360371, 30760055 and 81360374),
the Natural Science Foundation of Guangxi (nos. 2011GXNSFA018275
and 2014GXNSFAA118172), and the Innovative Project for Postgraduate
of Guangxi Educational Bureau (nos. YCBZ2013017 and
YCSZ2014103).
References
|
1
|
Mo QG, Liang AM, Yang NW, et al:
Surgery-predominant comprehensive therapy for 134 patients with
small hepatocellular carcinoma. Ai Zheng. 22:189–191. 2003.(In
Chinese). PubMed/NCBI
|
|
2
|
Yoon H, Lee H, Kim HJ, et al: Tudor
domain-containing protein 4 as a potential cancer/testis antigen in
liver cancer. Tohoku J Exp Med. 224:41–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song MH, Choi KU, Shin DH, et al:
Identification of the cancer/testis antigens AKAP3 and CTp11 by
SEREX in hepatocellular carcinoma. Oncol Rep. 28:1792–1798.
2012.PubMed/NCBI
|
|
4
|
Xing Q, Pang XW, Peng JR, et al:
Identification of new cytotoxic T-lymphocyte epitopes from cancer
testis antigen HCA587. Biochem Biophys Res Commun. 372:331–335.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao L, Mou DC, Leng XS, et al: Expression
of cancer-testis antigens in hepatocellular carcinoma. World J
Gastroenterol. 10:2034–2038. 2004.PubMed/NCBI
|
|
6
|
Pang PH, Chan KT, Tse LY, et al: Induction
of cytotoxic T cell response against HCA661 positive cancer cells
through activation with novel HLA-A*0201 restricted epitopes.
Cancer Lett. 256:178–185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang XA, Dong XY, Qiao H, et al:
Immunohistochemical analysis of the expression of FATE/BJ-HCC-2
antigen in normal and malignant tissues. Lab Invest. 85:205–213.
2005. View Article : Google Scholar
|
|
8
|
Yin YH, Li YY, Qiao H, et al: TSPY is a
cancer testis antigen expressed in human hepatocellular carcinoma.
Br J Cancer. 93:458–463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ono T, Kurashige T, Harada N, et al:
Identification of proacrosin binding protein sp32 precursor as a
human cancer/testis antigen. Proc Natl Acad Sci USA. 98:3282–3287.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan R, Huang W, Xiao SW, et al: OY-TES-1
expression and serum immunoreactivity in hepatocellular carcinoma.
World Chi J Digest. 17:3307–3312. 2009.(In Chinese).
|
|
11
|
Tammela J, Uenaka A, Ono T, et al:
OY-TES-1 expression and serum immunoreactivity in epithelial
ovarian cancer. Int J Oncol. 29:903–910. 2006.PubMed/NCBI
|
|
12
|
Whitehurst AW, Xie Y, Purinton SC, et al:
Tumor antigen acrosin binding protein normalizes mitotic spindle
function to promote cancer cell proliferation. Cancer Res.
70:7652–7661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanemori Y, Ryu JH, Sudo M, et al: Two
functional forms of ACRBP/sp32 are produced by pre-mRNA alternative
splicing in the mouse. Biol Reprod. 88:1052013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okumura H, Noguchi Y, Uenaka A, et al:
Identification of an HLA-A24-restricted OY-TES-1 epitope recognized
by cytotoxic T-cells. Microbiol Immunol. 49:1009–1016. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cen YH, Guo WW, Luo B, et al: Knockdown of
OY-TES-1 by RNAi causes cell cycle arrest and migration decrease in
bone marrow-derived mesenchymal stem cells. Cell Biol Int.
36:917–922. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yellaboina S, Tasneem A, Zaykin DV, et al:
DOMINE: a comprehensive collection of known and predicted
domain-domain interactions. Nucleic Acids Res. 39:D730–D735. 2011.
View Article : Google Scholar :
|
|
17
|
Finn RD, Bateman A, Clements J, et al:
Pfam: the protein families database. Nucleic Acids Res.
42:D222–D230. 2014. View Article : Google Scholar :
|
|
18
|
Kumar B, Sharma D, Sharma P, et al:
Proteomic analysis of Mycobacterium tuberculosis isolates resistant
to kanamycin and amikacin. J Proteomics. 94:68–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno
V, et al: Oncomine 3.0: genes, pathways, and networks in a
collection of 18,000 cancer gene expression profiles. Neoplasia.
9:166–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wilson BJ and Giguère V: Identification of
novel pathway partners of p68 and p72 RNA helicases through
Oncomine meta-analysis. BMC Genomics. 8:4192007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Ma B, Lu M, et al: Construction of
network for protein kinases that play a role in acute pancreatitis.
Pancreas. 42:607–613. 2013. View Article : Google Scholar
|
|
22
|
Melaiu O, Cristaudo A, Melissari E, et al:
A review of transcriptome studies combined with data mining reveals
novel potential markers of malignant pleural mesothelioma. Mutat
Res. 750:132–140. 2012. View Article : Google Scholar
|
|
23
|
Smith IM, Glazer CA, Mithani SK, et al:
Coordinated activation of candidate proto-oncogenes and cancer
testes antigens via promoter demethylation in head and neck cancer
and lung cancer. PLoS One. 4:e49612009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suyama T, Shiraishi T, Zeng Y, et al:
Expression of cancer/testis antigens in prostate cancer is
associated with disease progression. Prostate. 70:1778–1787.
2010.PubMed/NCBI
|
|
25
|
Warde-Farley D, Donaldson SL, Comes O, et
al: The GeneMANIA prediction server: biological network integration
for gene prioritization and predicting gene function. Nucleic Acids
Res. 38:W214–W220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williamson MP, Marion D and Wüthrich K:
Secondary structure in the solution conformation of the proteinase
inhibitor IIA from bull seminal plasma by nuclear magnetic
resonance. J Mol Biol. 173:341–359. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Laskowski M Jr, Kato I, Ardelt W, et al:
Ovomucoid third domains from 100 avian species: isolation,
sequences, and hypervariability of enzyme-inhibitor contact
residues. Biochemistry. 26:202–221. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schlott B, Wöhnert J, Icke C, et al:
Interaction of Kazal-type inhibitor domains with serine
proteinases: biochemical and structural studies. J Mol Biol.
318:533–546. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Funk JD, Nedialkov YA, Xu D and Burton ZF:
A key role for the α1 helix of human RAP74 in the initiation and
elongation of RNA chains. J Biol Chem. 277:46998–47003. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baba T, Niida Y, Michikawa Y, et al: An
acrosomal protein, sp32, in mammalian sperm is a binding protein
specific for two proacrosins and an acrosin intermediate. J Biol
Chem. 269:10133–10140. 1994.PubMed/NCBI
|
|
31
|
Hase H, Kanno Y, Kojima H, et al: CD27 and
CD40 inhibit p53-independent mitochondrial pathways in apoptosis of
B cells induced by B cell receptor ligation. J Biol Chem.
277:46950–46958. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi JY, Gao Q, Wang ZC, et al:
Margin-infiltrating CD20+ B cells display an atypical
memory phenotype and correlate with favorable prognosis in
hepatocellular carcinoma. Clin Cancer Res. 19:5994–6005. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang XD, Wang L, Ji FJ, et al: Decreased
CD27 on B lymphocytes in patients with primary hepatocellular
carcinoma. J Int Med Res. 40:307–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang ZQ, Yang ZY, Zhang LD, et al:
Increased liver-infiltrating CD8+FoxP3+
regulatory T cells are associated with tumor stage in
hepatocellular carcinoma patients. Hum Immunol. 71:1180–1186. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang X, Xu LS, Wang ZQ, et al: ING4
induces G2/M cell cycle arrest and enhances the chemosensitivity to
DNA-damage agents in HepG2 cells. FEBS Lett. 570:7–12. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Doyon Y, Cayrou C, Ullah M, et al: ING
tumor suppressor proteins are critical regulators of chromatin
acetylation required for genome expression and perpetuation. Mol
Cell. 21:51–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Cai L, Chen H, et al: Inhibitor of
growth 4 induces growth suppression and apoptosis in glioma U87MG.
Pathobiology. 76:181–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Karabulut B, Karaca B, Atmaca H, et al:
Regulation of apoptosis-related molecules by synergistic
combination of all-trans retinoic acid and zoledronic acid in
hormone-refractory prostate cancer cell lines. Mol Biol Rep.
38:249–259. 2011. View Article : Google Scholar
|
|
39
|
Matsuda A, Suzuki Y, Honda G, et al:
Large-scale identification and characterization of human genes that
activate NF-κB and MAPK signaling pathways. Oncogene. 22:3307–3318.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Masellis-Smith A and Shaw AR:
CD9-regulated adhesion. Anti-CD9 monoclonal antibody induces pre-B
cell adhesion to bone marrow fibroblasts through de novo
recognition of fibronectin. J Immunol. 152:2768–2777.
1994.PubMed/NCBI
|
|
41
|
Leung KT, Chan KY, Ng PC, et al: The
tetraspanin CD9 regulates migration, adhesion, and homing of human
cord blood CD34+ hematopoietic stem and progenitor
cells. Blood. 117:1840–1850. 2011. View Article : Google Scholar
|
|
42
|
Powner D, Kopp PM, Monkley SJ, et al:
Tetraspanin CD9 in cell migration. Biochem Soc Trans. 39:563–567.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kanetaka K, Sakamoto M, Yamamoto Y, et al:
Overexpression of tetraspanin CO-029 in hepatocellular carcinoma. J
Hepatol. 35:637–642. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li J and Li G: Cell cycle regulator ING4
is a suppressor of melanoma angiogenesis that is regulated by the
metastasis suppressor BRMS1. Cancer Res. 70:10445–10453. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Meyerson M and Harlow E: Identification of
G1 kinase activity for cdk6, a novel cyclin D partner.
Mol Cell Biol. 14:2077–2086. 1994.PubMed/NCBI
|
|
46
|
Yadav S, Pandey A, Shukla A, et al:
miR-497 and miR-302b regulate ethanol-induced neuronal cell death
through BCL2 protein and cyclin D2. J Biol Chem. 286:37347–37357.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou J, Tian Y, Li J, et al: miR-206 is
down-regulated in breast cancer and inhibits cell proliferation
through the up-regulation of cyclinD2. Biochem Biophys Res Commun.
433:207–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang L, Liu X, Jin H, et al: miR-206
inhibits gastric cancer proliferation in part by repressing
cyclinD2. Cancer Lett. 332:94–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen BB, Glasser JR, Coon TA, et al: F-box
protein FBXL2 targets cyclin D2 for ubiquitination and degradation
to inhibit leukemic cell proliferation. Blood. 119:3132–3141. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Igawa T, Sato Y, Takata K, et al: Cyclin
D2 is overexpressed in proliferation centers of chronic lymphocytic
leukemia/small lymphocytic lymphoma. Cancer Sci. 102:2103–2107.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dong Q, Meng P, Wang T, et al: MicroRNA
let-7a inhibits proliferation of human prostate cancer cells in
vitro and in vivo by targeting E2F2 and CCND2. PLoS One.
5:e101472010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Darr H, Mayshar Y and Benvenisty N:
Overexpression of NANOG in human ES cells enables feeder-free
growth while inducing primitive ectoderm features. Development.
133:1193–1201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang L, Zhang X, Zhang M, et al: Increased
Nanog expression promotes tumor development and cisplatin
resistance in human esophageal cancer cells. Cell Physiol Biochem.
30:943–952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Siu MK, Wong ES, Kong DS, et al: Stem cell
transcription factor NANOG controls cell migration and invasion via
dysregulation of E-cadherin and FoxJ1 and contributes to adverse
clinical outcome in ovarian cancers. Oncogene. 32:3500–3509. 2013.
View Article : Google Scholar
|
|
55
|
Valdez BC, Perlaky L, Saijo Y, et al: A
region of antisense RNA from human p120 cDNA with high homology to
mouse p120 cDNA inhibits NIH 3T3 proliferation. Cancer Res.
152:5681–5686. 1992.
|
|
56
|
Siggers RH and Hackam DJ: The role of
innate immune-stimulated epithelial apoptosis during
gastrointestinal inflammatory diseases. Cell Mol Life Sci.
68:3623–3634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sharan R, Ulitsky I and Shamir R:
Network-based prediction of protein function. Mol Syst Biol.
3:882007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dodurga Y, Oymak Y, Gündüz C, et al:
Leukemogenesis as a new approach to investigate the correlation
between up regulated gene 4/upregulator of cell proliferation
(URG4/URGCP) and signal transduction genes in leukemia. Mol Biol
Rep. 40:3043–3048. 2013. View Article : Google Scholar
|
|
59
|
Faussillon M, Monnier L, Junien C and
Jeanpierre C: Frequent overexpression of cyclin D2/cyclin-dependent
kinase 4 in Wilms’ tumor. Cancer Lett. 221:67–75. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Park TJ, Chun JY, Bae JS, et al: CCND2
polymorphisms associated with clearance of HBV infection. J Hum
Genet. 55:416–420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Takano Y, Kato Y, van Diest PJ, et al:
Cyclin D2 overexpression and lack of p27 correlate positively and
cyclin E inversely with a poor prognosis in gastric cancer cases.
Am J Pathol. 156:585–594. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Uchida F, Uzawa K, Kasamatsu A, et al:
Overexpression of cell cycle regulator CDCA3 promotes oral cancer
progression by enhancing cell proliferation with prevention of G1
phase arrest. BMC Cancer. 12:3212012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen J, Zhu S, Jiang N, et al: HoxB3
promotes prostate cancer cell progression by transactivating CDCA3.
Cancer Lett. 330:217–224. 2013. View Article : Google Scholar
|
|
64
|
Bunt J, de Haas TG, Hasselt NE, et al:
Regulation of cell cycle genes and induction of senescence by
overexpression of OTX2 in medulloblastoma cell lines. Mol Cancer
Res. 8:1344–1357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Visconti R, Palazzo L, Della Monica R and
Grieco D: Fcp1-dependent dephosphorylation is required for
M-phase-promoting factor inactivation at mitosis exit. Nat Commun.
3:8942012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Funakoshi T, Tachibana I, Hoshida Y, et
al: Expression of tetraspanins in human lung cancer cells: frequent
downregulation of CD9 and its contribution to cell motility in
small cell lung cancer. Oncogene. 22:674–687. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ovalle S, Gutiérrez-López MD, Olmo N, et
al: The tetraspanin CD9 inhibits the proliferation and
tumorigenicity of human colon carcinoma cells. Int J Cancer.
121:2140–2152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Saito Y, Tachibana I, Takeda Y, et al:
Absence of CD9 enhances adhesion-dependent morphologic
differentiation, survival, and matrix metalloproteinase-2
production in small cell lung cancer cells. Cancer Res.
66:9557–9565. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Murayama Y, Miyagawa J, Oritani K, et al:
CD9-mediated activation of the p46 Shc isoform leads to apoptosis
in cancer cells. J Cell Sci. 117:3379–3388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zheng R, Yano S, Zhang H, et al: CD9
overexpression suppressed the liver metastasis and malignant
ascites via inhibition of proliferation and motility of small-cell
lung cancer cells in NK cell-depleted SCID mice. Oncol Res.
15:365–372. 2005.
|
|
71
|
Kim JS, Kim J, Kim BS, et al:
Identification and functional characterization of an alternative
splice variant within the fourth exon of human nanog. Exp Mol Med.
37:601–607. 2005. View Article : Google Scholar
|
|
72
|
Oh JH, Do HJ, Yang HM, et al:
Identification of a putative trans-activation domain in human
Nanog. Exp Mol Med. 37:250–254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Shan J, Shen J, Liu L, et al: Nanog
regulates self-renewal of cancer stem cells through the
insulin-like growth factor pathway in human hepatocellular
carcinoma. Hepatology. 56:1004–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sun C, Sun L, Jiang K, et al: NANOG
promotes liver cancer cell invasion by inducing
epithelial-mesenchymal transition through NODAL/SMAD3 signaling
pathway. Int J Biochem Cell Biol. 45:1099–1108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Du Y, Shi L, Wang T, Liu Z and Wang Z:
Nanog siRNA plus Cisplatin may enhance the sensitivity of
chemotherapy in esophageal cancer. J Cancer Res Clin Oncol.
138:1759–1767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ji W and Jiang Z: Effect of shRNA-mediated
inhibition of Nanog gene expression on the behavior of human
gastric cancer cells. Oncol Lett. 6:367–374. 2013.PubMed/NCBI
|
|
77
|
Yu J, Zhang SS, Saito K, et al: PTEN
regulation by Akt-EGR1-ARF-PTEN axis. EMBO J. 28:21–33. 2009.
View Article : Google Scholar :
|