Introduction
Glioblastoma multiforme (GBM) is the most frequent
malignant primary brain tumor in adults and is characterized by an
aggressive infiltrative growth (1,2). To
improve the efficacy of current multimodal therapeutic approaches
composed of neurosurgery, radiotherapy and chemotherapy (3), identification of key molecular
determinants of radio- and chemoresistance is required. Among the
plethora of potential target molecules in GBM, integrins and
integrin-associated signaling molecules, such as focal adhesion
kinase (FAK), demonstrated promising results in preclinical and
early clinical studies (4–8). Peptidomimetic cilengitide, which
blocks the RGD binding site of αv/β3 and αv/β5 integrins (9,10), is
the only target strategy that has been previously clinically
evaluated. Due to a variety of prosurvival bypass signaling
opportunities via other integrin receptors, cilengitide failed to
improve overall survival of patients with GBM in Phase IV clinical
trials (http://www.merck.com). Despite this
drawback, targeting of integrin downstream signaling mediators such
as FAK seems reasonable and likely to yield promising results.
FAK is a non-receptor protein-tyrosine kinase that
provides signaling and scaffolding functions downstream of
integrins. EGFR plays an important role in the regulation of cell
processes such as adhesion, survival, migration and invasion
(11,12). As FAK is overexpressed in a variety
of human tumors including brain, head and neck squamous cell
carcinoma (HNSCC), breast, prostate, colon, ovarian, lung, and
liver (13–19), targeting of FAK is a promising
approach to overcome cancer therapy resistance. Several FAK
inhibitors are currently under investigation in clinical trials
(http://www.clinicaltrials.gov/ct2/results?term=FAK).
Previously, radiosensitization of human pancreatic
and HNSCC was shown by siRNA-mediated FAK knockdown or
pharmacological FAK inhibition using TAE226 (20–23).
In glioblastoma cells, antisense oligonucleotides against FAK
enhanced the sensitivity of U251MG cells to chemotherapy such as
cisplatin (24). Moreover, TAE226
exhibited a variety of effects in human GBM cells such as delay in
proliferation, G2-phase cell cycle arrest, increased apoptosis and
attenuated adhesion, migration and invasion (5,7). Of
note, studies in intracranial glioblastoma xenograft models showed
prolonged survival following TAE226 treatment (5). Due to the fact that patients suffering
from GBM received radiotherapy, we assessed the radiosensitizing
potential of FAK inhibition using TAE226 in a panel of human
glioblastoma cell lines.
Materials and methods
Antibodies and reagents
Antibodies against FAK Y861 (Abcam, Cambridge, UK),
Akt, phospho-Akt S473, phospho-Akt T308, caspase-3, CTTN,
phospho-CTTN Y421, cyclin E, ERK1/2, phospho-ERK1/2, FAK,
phospho-FAK Y397, IGF-IR, phospho-IGF-IR, JNK, phospho-JNK1/2,
LC3-I/II, MEK1/2, phospho-MEK1/2, PARP, Paxillin, phospho-Paxillin
Y118, Src, phospho-Src Y118 (Cell Signaling, Danvers, MA, USA),
phospho-JNK1/2 (Invitrogen-Life Technologies, Carlsbad, CA, USA),
β-actin (Sigma, St. Louis, MO, USA), cyclin D (Zymed), horseradish
peroxidase-conjugated donkey anti-rabbit and sheep anti-mouse
(Amersham, Poole, UK) were purchased as indicated. Caspase-3
inhibitor Z-VAD (OMe)-FMK was purchased from Calbiochem (San Diego,
CA, USA). The FAK inhibitor TAE226 was kindly provided by Novartis
(Cambridge, MA, USA). Enhanced chemiluminescent reagent (ECL) was
purchased from Amersham, dimethyl sulfoxide (DMSO) from Applichem
(Darmstadt, Germany) and Vectashield/DAPI mounting medium from
Alexis (Grünberg, Germany).
Cell culture and X-ray radiation
exposure
Human A172, LN229, U87MG, U138MG and U343MG
glioblastoma cell lines were obtained from ATCC, while DD-HT7607
and DD-T4 were provided by A. Temme (University Hospital Dresden).
The cells were cultured in Dulbecco’s modified Eagle’s medium (PAA;
plus glutamax-I) supplemented with 10% fetal calf serum (PAA) and
1% non-essential amino acids (PAA). The U343MG cell line was
cultured in Basal Medium Eagle (Life Technologies), supplemented
with 10% fetal calf serum (PAA), 10 mM Hepes, 2 mM L-glutamine, 1%
penicillin/streptomycin (pen/strep) and 1% non-essential amino
acids (PAA) at 37°C in a humidified atmosphere containing 7%
CO2. Single doses of 200 kV X-rays (2, 4 or 6 Gy; Yxlon
Y.TU 320; Yxlon; 0.5 mm copper filter; ~1.3 Gy/min, 20 mA) were
applied at room temperature and measured using a Duplex dosimeter
(PTW).
Colony formation assay
Measurement of 2D cell survival was performed as
previously published (25).
Briefly, single cells were plated in 6-well cell culture dishes (BD
Biosciences, Heidelberg, Germany). After 24 h, the cells were
treated with indicated amounts of TAE226 or DMSO. In the case of
irradiation experiments, TAE226 or DMSO was applied 1, 8 or 24 h
prior to irradiation and removed 3 h after irradiation (single
X-ray doses: 2, 4, and 6 Gy). After 9–12 days, depending on the
cell line, 2D cell colonies were stained with Coomassie blue
(Merck, Darmstadt, Germany) and counted microscopically. Colonies
were defined by cell numbers >50. Plating efficiencies were
calculated as: Number of colonies formed/number of cells plated.
Surviving fractions were calculated as: number of colonies
formed/(number of cells plated (irradiated) × plating efficiency
(unirradiated). Each point on the survival curves was the mean
surviving fraction from at least three independent experiments.
Neurosphere assay
Anchorage-independent growth was enabled using
neurobasal medium (PAA), containing 32 U/ml heparine, 20 μl/ml B27
supplement, 10 μl/ml glutamax, 20 ng/ml FGF, 20 ng/ml EGF, 1%
pen/strep and 1% fungizone (26).
The cells were trypsinized and resuspended in neurobasal medium and
conferred to plates coated with Ultra-Low attachment surface
(Corning, Lowell, MA, USA). After 24 h, the cells were treated with
the indicated amounts of TAE226 or DMSO. For the irradiation
experiments, TAE226 or DMSO was applied 24 h prior to irradiation
(single X-ray doses: 2, 4, and 6 Gy). After 9–12 days, depending on
the cell line, the number of spheres with a diameter of ≥200 μm
were counted microscopically. Representative images were captured
using an Axioscope 2 microscope (Carl Zeiss, Thornwood, NY, USA) to
calculate the sphere size.
DAPI staining
For the apoptosis analysis, the cells were treated
at the indicated time points (8 or 24 h) and fixed with 80% ethanol
for at least 24 h as previously published (25). Typical apoptotic nuclear shape was
analyzed using Vectashield/DAPI mounting medium. Apoptotic nuclei
of ≥100 cells from three independent experiments were counted
microscopically using an Axioscope 2 microscope (Zeiss).
Total protein extracts and western
blotting
Total protein extracts from 2D cell cultures were
isolated as previously described (25). Therefore, the cells were lysed using
modified RIPA buffer [50 mM Tris-HCl (Carl Roth, Karlsruhe,
Germany, (pH, 7.4)], 1% Nonidet-P40 (Sigma-Aldrich, Taufkirchen,
Germany), 0.25% sodium deoxycholate (Applichem), 150 mM NaCl (VWR
International, Darmstadt, Germany), 1 mM ethylenediaminetetraacetic
acid (Merck), complete protease inhibitor cocktail (Roche,
Mannheim, Germany), 1 mM NaVO4 (Applichem), 2 mM NaF
(Applichem). The samples were stored at −80°C. The total protein
amount was measured using the bicinchoninic acid assay (Pierce,
Bonn, Germany). The 2D monolayer cells were treated with FAK
inhibitor (TAE226, 0.3–10 μM) for 1, 8 or 24 h and subsequently
with Caspase 3 inhibitor [Z-VAD (OMe)-FMK, 100 μM] for 1 h
pretreatment, where appropriate.
siRNA-mediated knockown of FAK
FAK siRNA (sense 5′-CCAAAUUCGAGUACUAAGAtt-3′) was
obtained from Ambion (Austin, TX, USA). A non-specific siRNA (sense
5′-GCAGCUAUAUGAAUGUUGUtt-3′; MWG) was used as the control. The
cells were prepared for siRNA transfection (20 nmol/l) with
oligofectamine and were subjected 24 h after transfection to a
colony formation assay and western blotting.
Statistical analysis
Data are presented as the mean ± SD values. The
level of significance was determined by the Student’s t-test
(Microsoft Excel 2010). P<0.05 was considered statistically
significant.
Results
TAE226 effectively reduces FAK
phosphorylation in human glioblastoma cells
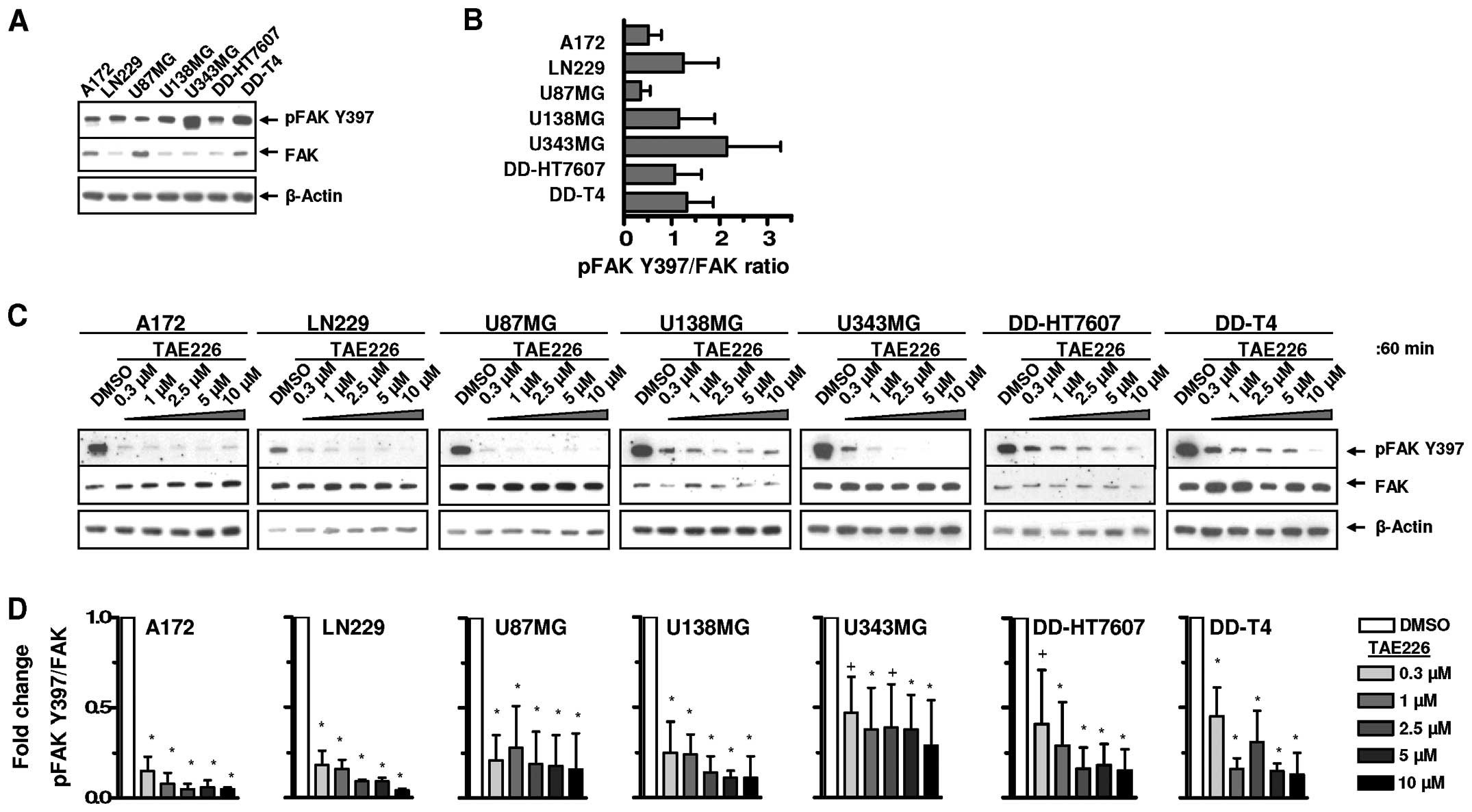
Basal FAK expression and Y397 phosphorylation were
examined using a panel of human glioblastoma cell lines (Fig. 1A and B). The inhibitory potential of
TAE226 on FAK Y397 autophosphorylation was subsequently assessed
(Fig. 1C). TAE226-mediated FAK Y397
dephosphorylation was, although cell line-dependent, not clearly
concentration-dependent over the range of 0.3–10 μM (Fig. 1C and D).
TAE226 efficiently reduces FAK
autophosphorylation and modulates downstream signaling
molecules
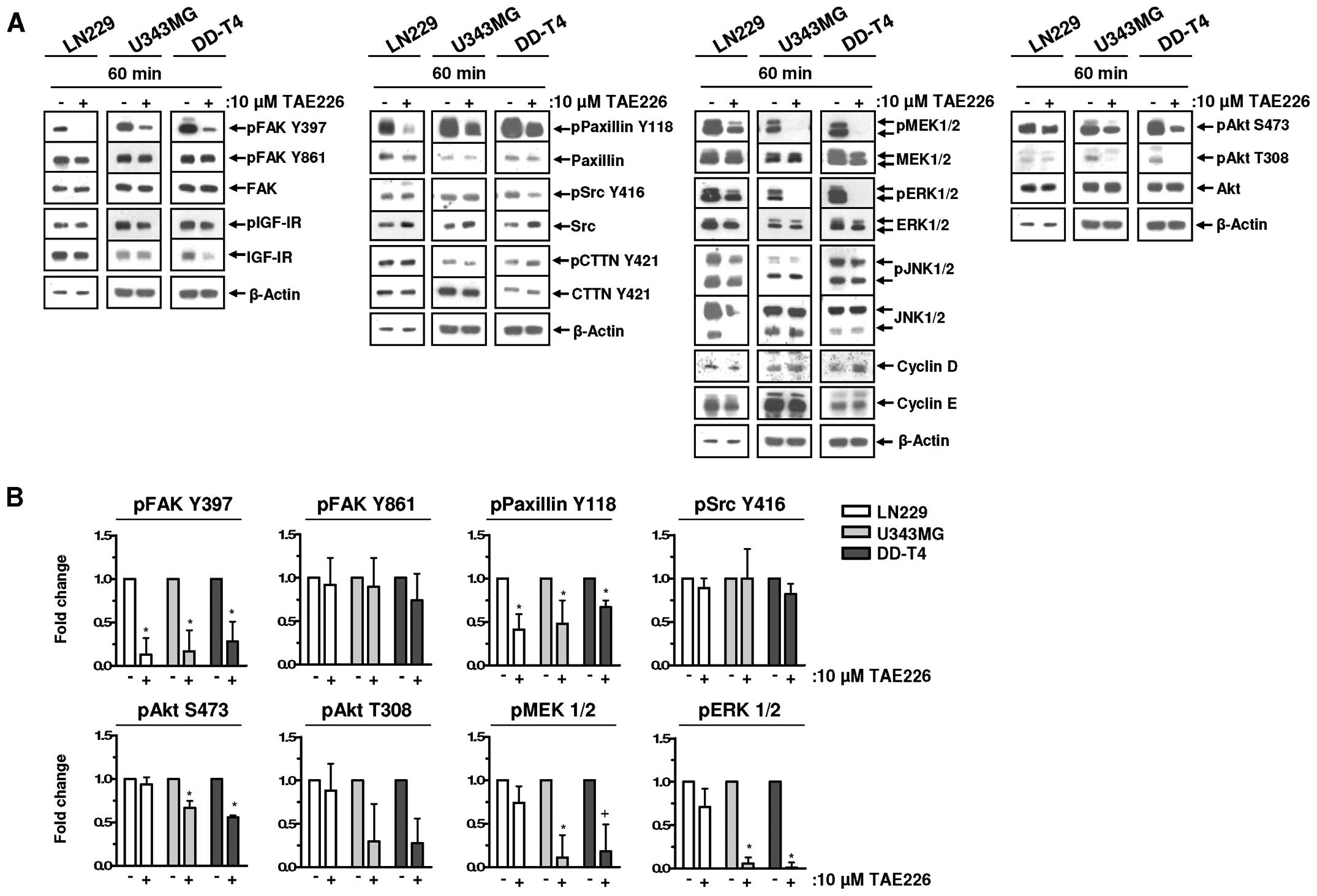
We systematically analyzed the potential of TAE226
on various signaling pathways in the LN229, U343MG and DD-T4
glioblastoma cells. FAK and insulin-like growth factor-I receptor
(IGF-IR), both reported as targets of TAE226 (27), showed a different response to TAE226
treatment. FAK was dephosphorylated, whereas no modification was
detected for IGF-IR (Fig. 2A and
B). In the three glioma cells, Paxillin Y118 phosphorylation
was reduced by TAE226, while only U343MG and DD-T4 cells revealed
reduced phosphorylation of Akt S473/T308, MEK1/2 and ERK1/2
following TAE226 relative to DMSO (Fig.
2A and B). TAE226 treatment did not affect Src, CTTN and JNK1/2
or the expression of cell cycle proteins Cyclin D or E (Fig. 2A and B).
TAE226 differentially radiosensitizes
glioblastoma cells
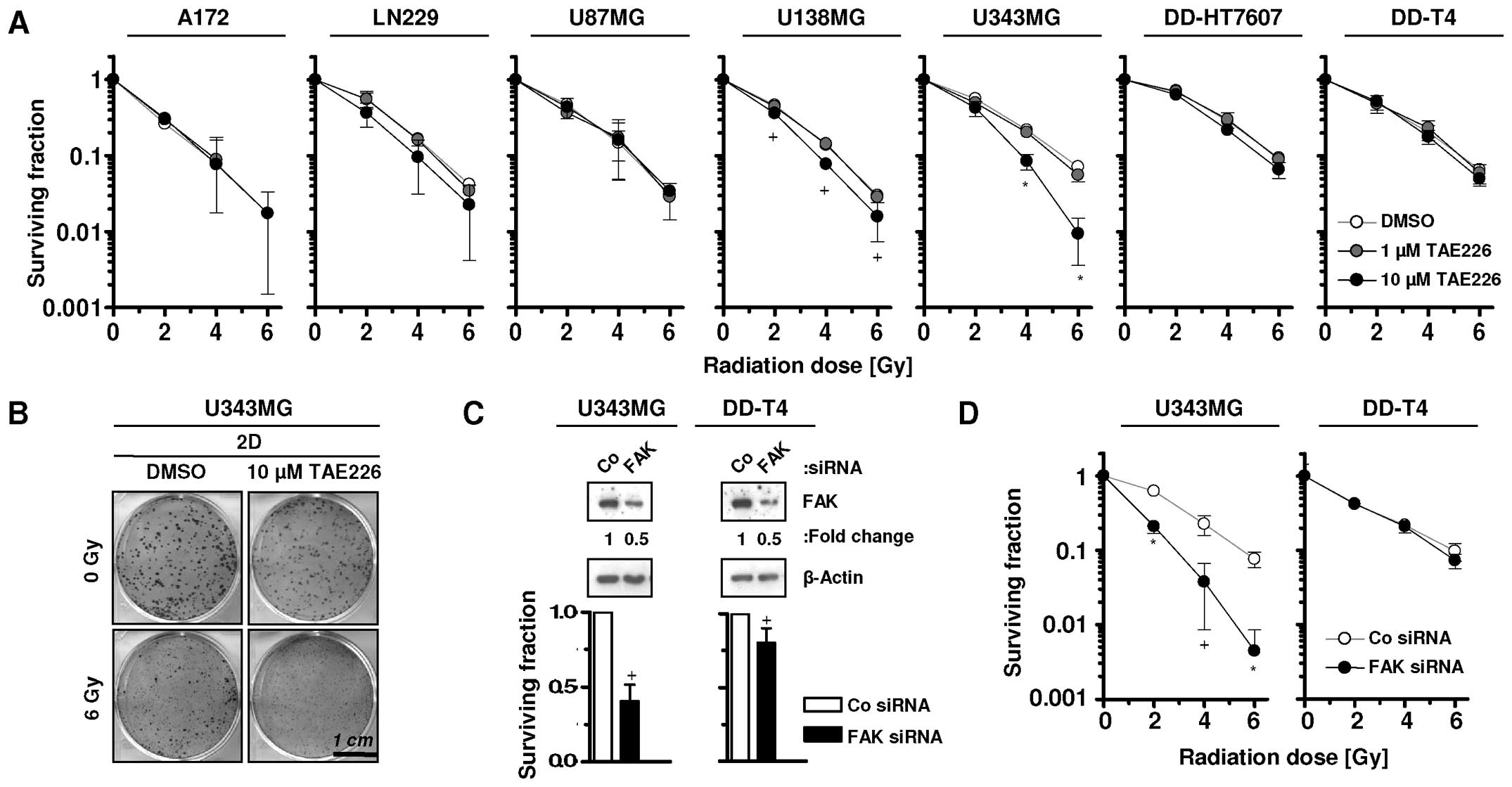
Cytotoxic effects of TAE226 have been previously
reported (4–8). In the present study, we examined the
radiosensitizing potential of TAE226 in glioblastoma cells. TAE226
failed to radiosensitize the tested glioma cells when pretreated
with 1 μM TAE226, whereas 10 μM TAE226 pretreatment significantly
radiosensitized U138MG and U343MG cells (Fig. 3A and B). Additionally, whether
siRNA-mediated FAK targeting provides similar effects, was assessed
in U343MG and DD-T4 glioma cells (Fig.
3C). The results showed that U343MG, but not DD-T4, cells were
significantly radiosensitized by this approach (Fig. 3D).
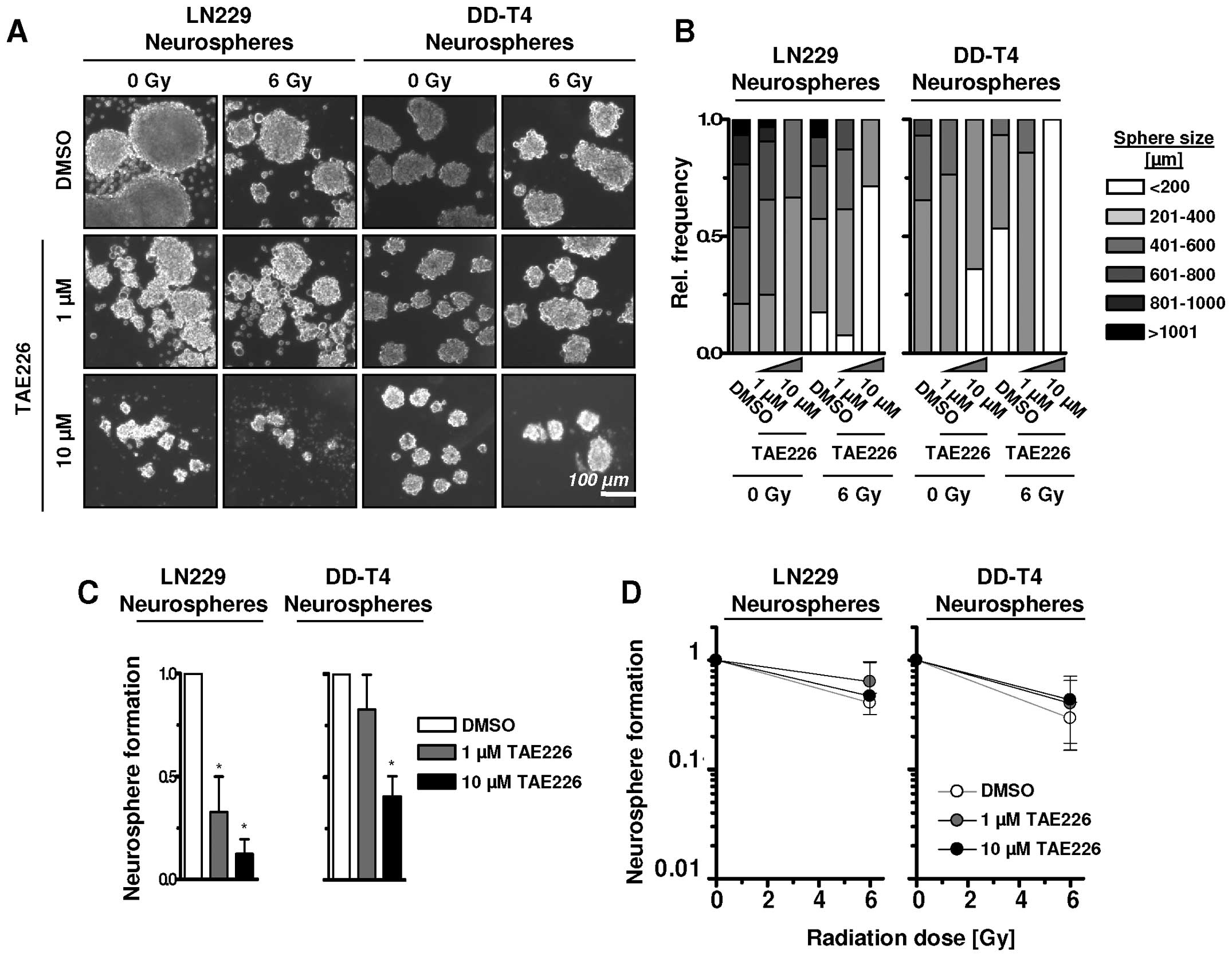
TAE226 reduces sphere growth without
changing glioblastoma radiosensitivity
TAE226 efficacy in glioblastoma suspension cell
cultures in neural basal media was examined. Under these
conditions, treatment of single GBM cells with TAE226 led to a
shift from larger to smaller spheres with diameters ranging from
>1001 to <200 μm (Fig. 4A and
B). Combined with a 6-Gy single X-ray dose, the frequency of
smaller spheres further increased in LN229 and DD-T4 cells
(Fig. 4A and B). In line with
sphere size, sphere number of LN229 and DD-T4 cells was
significantly reduced by 1 or 10 μM TAE226 alone (Fig. 4C) while radiosensitization in the
GBM cells grown as spheres was lacking (Fig. 4D). Thus, under 3D suspension growth
conditions, TAE226 has a strong potential for growth inhibition but
not for radiosensitization of GBM cells.
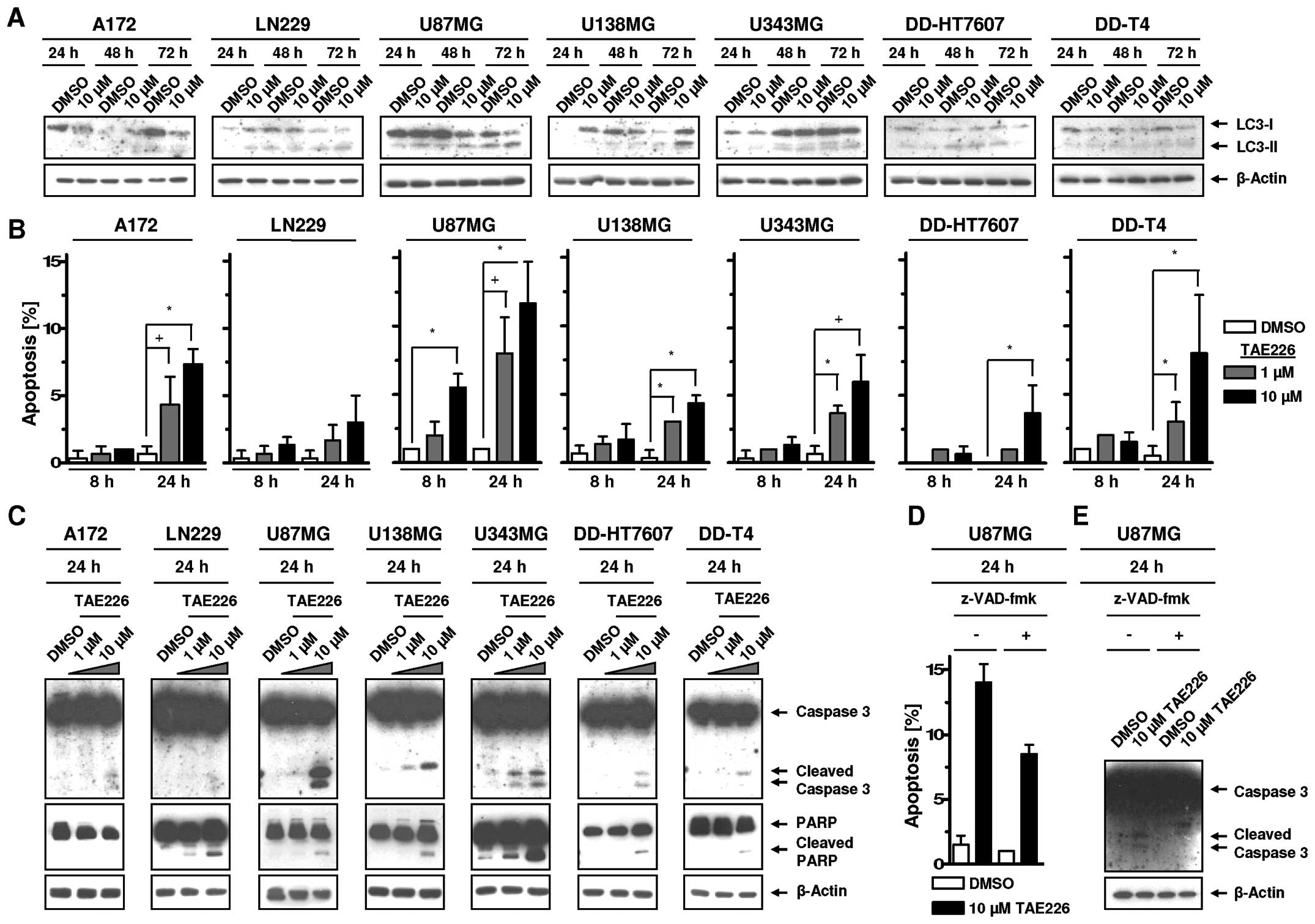
TAE226-mediated FAK inhibition induces
apoptosis but not autophagic cell death in glioblastoma cells
To assess whether TAE226 induces autophagy and
apoptosis in glioblastoma cell lines, we measured LC3-I and LC3-II
expression, apoptotic cell nuclei, Caspase 3 and PARP cleavage. In
our analysis, LC3-I to LC3-II conversion was not clearly observable
following TAE226 indicating autophagy was not an essential cell
death process in the examined glioma cells (Fig. 5A). However, TAE226 increased
apoptosis relative to DMSO controls in a cell line-, dose- and
time-dependent manner (Fig. 5B).
The magnitude of apoptosis was not reflected by the degree of
cleaved Caspase 3 and PARP (Fig.
5C). By means of the Caspase inhibitor Z-VAD (OMe)-fmk, we
showed in U87MG cells with the highest rate of TAE226-induced
apoptosis that Caspase 3 cleavage can be completely prevented while
the number of apoptotic nuclei is only reduced by ~40% (Fig. 5D and E). These data suggested
apoptosis induction, but not autophagy, contributed to the
cytotoxicity of TAE226.
TAE226 strongly attenuates clonogenic
cell survival of glioblastoma cells
The impact of TAE226 to reduce clonogenicity of
glioblastoma cells was investigated. Increasing TAE226
concentrations significantly reduced the clonogenic survival of all
tested glioblastoma cells in a time-dependent manner in comparison
to DMSO control.
Discussion
Optimization of multimodal therapy concepts for GBM
is necessary. In this study, we investigated the radiosensitizing
and cytotoxic potential of the pharmacological FAK inhibitor TAE226
in glioblastoma cells. We found significant dose- and
time-dependent cytotoxicity of TAE226 in a panel of seven human
glioblastoma cell lines.
These results are in concordance with previous
studies in which TAE226 caused growth inhibition and apoptosis
induction in different tumor entities such as glioblastoma
(7), neuroblastoma (28), head and neck squamous cell carcinoma
(HNSCC) (21,23), esophageal tumors (29) and breast cancer (30). However, monotherapy using a FAK
inhibitor is unlikely to occur in the clinic and, thus, knowledge
concerning the radiosensitizing potential of TAE226 is of great
relevance. TAE226 is described as a potent inhibitor of the tyrosin
protein kinases FAK and IGF-IR. Contrary to previously published
data (5,23,27),
we found significantly reduced FAK Y397 autophosphorylation without
modification of IGF-IR phosphorylation following TAE226 application
in glioblastoma cells. Similar to other studies (5,23), FAK
inhibition led to alterations of adapter and signaling molecules
directly or partly associated with FAK such as Paxillin, Akt,
MEK1/2 and ERK1/2.
Owing to this multitarget deactivation, TAE226
mediated strong cytotoxicity that may be attributed to apoptosis
but not autophagy. Similar data have been published by other
authors (7,24). Moreover, this apoptosis induction
presented partly Caspase-dependent and partly Caspase-independent,
thus further mechanistic examination is required. Of note,
apoptosis or anoikis is stimulated following TAE226-dependent FAK
Y397 autophosphorylation in GBM cells indicating the importance of
integrin-mediated anchorage-dependent growth of GBM cells. The
broad inhibitory spectrum of TAE226 is beneficial to induced cell
death in suspension cell cultures, in which cell-ECM interactions
are less relevant for cell survival. Hypothesizing TAE226 to
activate different cell death modes under adhesion vs. suspension
seems of high interest for the clinical scenario where different
GBM cell populations compose the tumor heterogeneity and are likely
to be differentially dependent on adhesion.
Based on the fact that small molecules are
administered in combination with conventional radiotherapy or
radiochemotherapy, we investigated TAE226 plus irradiation and
found that only a small number of GBM cell lines are
radiosensitized. Previous studies testing TAE226/irradiation showed
that head and neck cancer cells, but not carcinoma cells, from
lung, colorectum and pancreas can be sensitized to radiotherapy
(21,23). Targeting of β1 integrin, located
upstream of FAK, mediated less cytotoxicity than TAE226 but
radiosensitization in human GBM cell lines (4). These observations suggest that: i) GBM
cell survival is highly FAK-dependent; ii) the molecular circuitry
controlled by targets of TAE226 does not essentially contribute to
the DNA damage response of GBM cells.
In conclusion, our findings demonstrate that the
pharmacological FAK inhibitor TAE226 efficiently reduces clonogenic
survival without radiosensitization in a panel of glioblastoma cell
lines. In the future, the identification of the integrin-related
adhesome seems to be key for the optimization of therapeutic
strategies for GBM as the multiprotein-multifunctional adhesome
complexes control prosurvival signaling and invasion processes.
Acknowledgements
This study was supported by The German Federal
Ministry of Education and Research; contract grant number: BMBF
Contract 03ZIK041 (to N.C.) and by The Saxon Ministry of Science
and Arts and the EFRE Europäische Fonds für regionale Entwicklung,
Europa fördert Sachsen; Contract grant no. 100066308. We are
grateful to A. Temme (University Hospital Dresden, Germany) for
providing U343MG, DD-HT7607 and DD-T4 cells. The pharmacological
FAK inhibitor TAE226 was kindly provided by Novartis Institutes of
Biomedical Research, Inc. (Cambridge, MA, USA).
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bleeker FE, Molenaar RJ and Leenstra S:
Recent advances in the molecular understanding of glioblastoma. J
Neurooncol. 108:11–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Becker KP and Yu J: Status quo -
standard-of-care medical and radiation therapy for glioblastoma.
Cancer J. 18:12–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eke I, Storch K, Kastner I, Vehlow A,
Faethe C, Mueller-Klieser W, Taucher-Scholz G, Temme A, Schackert G
and Cordes N: Three-dimensional invasion of human glioblastoma
cells remains unchanged by X-ray and carbon ion irradiation in
vitro. Int J Radiat Oncol Biol Phys. 84:e515–e523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu TJ, LaFortune T, Honda T, Ohmori O,
Hatakeyama S, Meyer T, Jackson D, de Groot J and Yung WK:
Inhibition of both focal adhesion kinase and insulin-like growth
factor-I receptor kinase suppresses glioma proliferation in vitro
and in vivo. Mol Cancer Ther. 6:1357–1367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rieken S, Habermehl D, Mohr A, Wuerth L,
Lindel K, Weber K, Debus J and Combs SE: Targeting ανβ3 and ανβ5
inhibits photon-induced hypermigration of malignant glioma cells.
Radiat Oncol. 6:1322011. View Article : Google Scholar
|
|
7
|
Shi Q, Hjelmeland AB, Keir ST, Song L,
Wickman S, Jackson D, Ohmori O, Bigner DD, Friedman HS and Rich JN:
A novel low-molecular weight inhibitor of focal adhesion kinase,
TAE226, inhibits glioma growth. Mol Carcinog. 46:488–496. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang M, Li Y, Chilukuri K, Brady OA,
Boulos MI, Kappes JC and Galileo DS: L1 stimulation of human glioma
cell motility correlates with FAK activation. J Neurooncol.
105:27–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reardon DA, Nabors LB, Stupp R and
Mikkelsen T: Cilengitide: an integrin-targeting
arginine-glycine-aspartic acid peptide with promising activity for
glioblastoma multiforme. Expert Opin Investig Drugs. 17:1225–1235.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nabors LB, Mikkelsen T, Hegi ME, Ye X,
Batchelor T, Lesser G, Peereboom D, Rosenfeld MR, Olsen J, Brem S,
Fisher JD and Grossman SA: A safety run-in and randomized phase 2
study of cilengitide combined with chemoradiation for newly
diagnosed glioblastoma (NABTT 0306). Cancer. 118:5601–5607. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McLean GW, Carragher NO, Avizienyte E,
Evans J, Brunton VG and Frame MC: The role of focal-adhesion kinase
in cancer - a new therapeutic opportunity. Nat Rev Cancer.
5:505–515. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao J and Guan JL: Signal transduction by
focal adhesion kinase in cancer. Cancer Metastasis Rev. 28:35–49.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cance WG, Harris JE, Iacocca MV, Roche E,
Yang X, Chang J, Simkins S and Xu L: Immunohistochemical analyses
of focal adhesion kinase expression in benign and malignant human
breast and colon tissues: correlation with preinvasive and invasive
phenotypes. Clin Cancer Res. 6:2417–2423. 2000.PubMed/NCBI
|
|
14
|
Glukhova M, Koteliansky V, Sastre X and
Thiery JP: Adhesion systems in normal breast and in invasive breast
carcinoma. Am J Pathol. 146:706–716. 1995.PubMed/NCBI
|
|
15
|
Gutenberg A, Brück W, Buchfelder M and
Ludwig HC: Expression of tyrosine kinases FAK and Pyk2 in 331 human
astrocytomas. Acta Neuropathol. 108:224–230. 2004.PubMed/NCBI
|
|
16
|
Han NM, Fleming RY, Curley SA and Gallick
GE: Overexpression of focal adhesion kinase (p125FAK) in human
colorectal carcinoma liver metastases: independence from c-src or
c-yes activation. Ann Surg Oncol. 4:264–268. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsu NY, Chen CY, Hsu CP, Lin TY, Chou MC,
Chiou SH and Chow KC: Prognostic significance of expression of
nm23-H1 and focal adhesion kinase in non-small cell lung cancer.
Oncol Rep. 18:81–85. 2007.PubMed/NCBI
|
|
18
|
Kornberg LJ: Focal adhesion kinase
expression in oral cancers. Head Neck. 20:634–639. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Owens LV, Xu L, Craven RJ, Dent GA, Weiner
TM, Kornberg L, Liu ET and Cance WG: Overexpression of the focal
adhesion kinase (p125FAK) in invasive human tumors. Cancer Res.
55:2752–2755. 1995.PubMed/NCBI
|
|
20
|
Cordes N, Frick S, Brunner TB, Pilarsky C,
Grützmann R, Sipos B, Kloppel G, McKenna WG and Bernhard EJ: Human
pancreatic tumor cells are sensitized to ionizing radiation by
knockdown of caveolin-1. Oncogene. 26:6851–6862. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eke I and Cordes N: Dual targeting of EGFR
and focal adhesion kinase in 3D grown HNSCC cell cultures.
Radiother Oncol. 99:279–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hehlgans S, Eke I and Cordes N: Targeting
FAK radiosensitizes 3-dimensional grown human HNSCC cells through
reduced Akt1 and MEK1/2 signaling. Int J Radiat Oncol Biol Phys.
83:e669–e676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hehlgans S, Lange I, Eke I and Cordes N:
3D cell cultures of human head and neck squamous cell carcinoma
cells are radiosensitized by the focal adhesion kinase inhibitor
TAE226. Radiother Oncol. 92:371–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu ZM, Yuan XH, Jiang PC, Li ZQ and Wu T:
Antisense oligonucleodes targeting the focal adhesion kinase
inhibit proliferation, induce apoptosis and cooperate with
cytotoxic drugs in human glioma cells. J Neurooncol. 77:117–123.
2006. View Article : Google Scholar
|
|
25
|
Storch K, Eke I, Borgmann K, Krause M,
Richter C, Becker K, Schröck E and Cordes N: Three-dimensional cell
growth confers radioresistance by chromatin density modification.
Cancer Res. 70:3925–3934. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Günther HS, Schmidt NO, Phillips HS,
Kemming D, Kharbanda S, Soriano R, Modrusan Z, Meissner H, Westphal
M and Lamszus K: Glioblastoma-derived stem cell-enriched cultures
form distinct subgroups according to molecular and phenotypic
criteria. Oncogene. 27:2897–2909. 2008. View Article : Google Scholar
|
|
27
|
Wang ZG, Fukazawa T, Nishikawa T, Watanabe
N, Sakurama K, Motoki T, Takaoka M, Hatakeyama S, Omori O, Ohara T,
Tanabe S, Fujiwara Y, Shirakawa Y, Yamatsuji T, Tanaka N and
Naomoto Y: TAE226, a dual inhibitor for FAK and IGF-IR, has
inhibitory effects on mTOR signaling in esophageal cancer cells.
Oncol Rep. 20:1473–1477. 2008.PubMed/NCBI
|
|
28
|
Beierle EA, Trujillo A, Nagaram A,
Golubovskaya VM, Cance WG and Kurenova EV: TAE226 inhibits human
neuroblastoma cell survival. Cancer Invest. 26:145–151. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watanabe N, Takaoka M, Sakurama K, Tomono
Y, Hatakeyama S, Ohmori O, Motoki T, Shirakawa Y, Yamatsuji T,
Haisa M, Matsuoka J, Beer DG, Nagatsuka H, Tanaka N and Naomoto Y:
Dual tyrosine kinase inhibitor for focal adhesion kinase and
insulin-like growth factor-I receptor exhibits anticancer effect in
esophageal adenocarcinoma in vitro and in vivo. Clin Cancer Res.
14:4631–4639. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Golubovskaya VM, Virnig C and Cance WG:
TAE226-induced apoptosis in breast cancer cells with overexpressed
Src or EGFR. Mol Carcinog. 47:222–234. 2008. View Article : Google Scholar
|