Introduction
Malignant pleural mesothelioma (MPM) is an
asbestos-induced, highly aggressive tumor that was once considered
rare, but its incidence has steadily increased and new cases are
predicted to peak between 2015 and 2025 (1,2). The
disease is almost always fatal; median survival is 9 months for
patients treated with supportive care, 12.1 months for those who
receive the best available chemotherapy (3), and 11.7–13 months for those who have
maximal cytoreductive surgery, as well as chemotherapy and/or
radiation (4,5). The treatment with chemotherapy has
shown slightly improved survival, and multi-modal trials are
ongoing (1). A previous study has
revealed that pemetrexed plus cisplatin neoadjuvant chemotherapy
followed by extrapleural pneumonectomy (EPP) and hemithoracic
radiation improved the 2-year overall survival rate (6).
Known positive prognostic factors for MPM include
good performance status, young age, female, early stage and
epithelioid subtype (7–10). Several markers have been proposed
either as positive (e.g. Syndecan 1 expression) (11,12) or
negative (e.g. Cox-2 expression) (13), and new microarray technology
currently reveals batteries of genes that may have prognostic value
(14,15). Although the two principal tumor
suppressor genes, Rb (16–18)
and p53 (19), are not
commonly absent in MPM, other molecules that are important in the
Rb and p53 pathways are present in MPM (1), particularly p16 and p14
(20,21). However, whether tumor suppressor
expression is correlated with MPM prognosis is not known. Members
of the Wnt signaling family are highly conserved and secrete
glycoproteins vital to mammalian development and many aspects of
adult tissue homeostasis. The members of this family regulate
important cellular processes, such as proliferation,
differentiation and cell fate specification (22). Furthermore, the Wnt gene family
encodes transcription factors that regulate morphogenesis and cell
differentiation during embryogenesis by activating or repressing
the expression of target genes (23). Wnt signals are transduced through 7
transmembrane-type Wnt receptors encoded by Frizzled (Fz) genes to
the β-catenin-TCF pathway, the JNK pathway or the
Ca2+-releasing pathway. E-cadherin induction by
Wnt/β-catenin signaling is an evolutionarily conserved pathway
operative in lung cancer cells, and loss of Wnt7A expression
may be important in lung cancer development or progression due to
its effects on E-cadherin (24).
Therefore, Wnt7A appears to play important roles in
embryonic development and tumorigenesis (25–27).
Wnt signaling molecules are potent targets for cancer diagnosis
(susceptibility, metastasis and prognosis), cancer prevention and
treatment, and for regenerative medicine or tissue engineering
(28).
We therefore analyzed Wnt7A expression by
using quantitative RT-PCR in surgically resected MPM specimens
and/or adjacent normal tissues. We also examined the correlations
between Wnt7A expression and various clinicopathological
factors, prognosis and neoadjuvant chemotherapy.
Materials and methods
Patients
Between 1999 and 2005, 50 fresh samples of MPM
and/or the adjacent normal tissues were collected from consecutive
patients undergoing surgical resection in a study approved by the
Committee on Human Research at the University of California, San
Francisco (UCSF). Of these 50 patients, 24 had received neoadjuvant
chemotherapy before surgery. The pathologic classification of each
sample was confirmed by a review of sections stained with
hematoxylin and eosin. Clinical information was also reviewed: age,
gender, ethnicity, smoking status, Eastern Cooperative Oncology
Group performance status (ECOG PS), histological subtype,
International Mesothelioma Interest Group (IMIG) stage, surgical
procedure, chemotherapy, radiation, recurrence status, vital
status, progression status, and overall survival, which was
calculated from the date of surgery.
Tissues and RNA extraction
Tissue samples were promptly snap-frozen in liquid
nitrogen and stored at −170°C before use. Total RNA was extracted
using TRIzol LS (Invitrogen) and purified using the RNeasy Mini kit
(Qiagen).
Quantitative real-time reverse
transcription-PCR
cDNA synthesis and Taqman PCR were performed as
previously described (29).
Hybridization probes and primers (Wnt7A, Hs01114990 m1;
GAPDH, Hs00244574 m1) were purchased from Applied Biosystems (ABI).
Wnt7A expression was assayed in triplicate using an ABI 7300
real-time PCR system. Samples were normalized to the housekeeping
gene GAPDH, and expression levels were calculated using the
2−ΔΔCt method compared to total RNA of the mixed
adjacent normal pleural tissues derived from 11 patients.
Statistical analysis
Patients were divided into those with high
Wnt7A expression and those with low Wnt7A expression.
These groups were compared with respect to the clinicopathological
factors by using t-tests, Chi-square tests or Kaplan-Meier survival
curves and the Log-rank test. A correlation between Wnt7A
expression and IMIG staging system [stage, primary site (T) or
lymph node metastasis (N)] was analyzed by one-way ANOVA.
Kaplan-Meier survival curves for overall survival were compared for
various clinicopathologic factors. Prognostic variables that were
significant on univariate analysis were entered into a Cox’s
proportional hazards model to determine the hazard ratio (HR). The
cutoff values were associated with the lowest P obtained when
comparing the two Wnt7A expression groups (the optimal P
approach). Two-sided P-values <0.05 were considered significant.
All analyses were conducted using the IBM SPSS statistics software
package, ver. 18.0.
Results
Patient characteristics
Table I summarizes
the characteristics of the 50 patients and prognosis after surgery
according to Wnt7A expression (high Wnt7A, n=30; low
Wnt7A, n=20). Performance status data were missing for 26
patients. The Wnt7A expression groups differed significantly
in regards to gender, smoking status, performance status and vital
status.
 | Table IClinicopathological characteristics
of the patients with malignant pleural mesothelioma, according to
Wnt7A expression. |
Table I
Clinicopathological characteristics
of the patients with malignant pleural mesothelioma, according to
Wnt7A expression.
| Total
(n=50) | High
Wnt7A
(n=30) | Low
Wnt7A
(n=20) | P-value |
|---|
| Age (years) | | | | |
| Mean ± SD | 66±9 | 67±10 | 67±9 | 0.942 |
| Range | (45–84) | (59–84) | (54–84) | |
| Gender | | | | |
| Male/female | 41/9 | 21/9 | 20/0 | 0.007 |
| Ethnicity | | | | |
|
Caucasian/other | 38/12 | 25/5 | 13/7 | 0.182 |
| Smoking | | | | |
| Yes/no | 16/24 | 14/11 | 2/13 | 0.024 |
| ECOG PS | | | | |
| 0/1/2/ND | 10/13/1/26 | 6/4/0/20 | 4/9/1/6 | 0.026 |
| Subtype | | | | |
|
Epithelioid/other | 42/8 | 25/5 | 17/3 | 0.875 |
| IMIG stage | | | | |
| I/II/III/IV | 1/18/24/5 | 1/13/13/2 | 0/5/11/3 | 0.363 |
| Primary tumor
(T) | | | | |
| T1/T2/T3/T4 | 1/20/22/5 | 1/16/11/3 | 0/4/11/2 | 0.550 |
| Regional lymph node
(N) | | | | |
| N0/N1/N2 | 37/5/6 | 24/1/4 | 13/4/2 | 0.293 |
| Surgical
procedure | | | | |
|
EPP/PLE/CWR/BIO | 11/35/2/2 | 8/19/2/1 | 3/16/0/1 | 0.451 |
| Chemotherapy | | | | |
| Yes/no | 30/20 | 18/12 | 12/8 | 1.000 |
| Neo/PSC/both | 24/8/2 | 15/4/1 | 11/4/1 | 0.529 |
| Neo, yes/no | 24/26 | 15/15 | 9/11 | 0.447 |
| PSC, yes/no | 8/42 | 4/26 | 4/16 | 0.351 |
| Radiation | | | | |
| Yes/no | 20/30 | 12/18 | 8/12 | 0.162 |
| Progression | | | | |
| Yes/no | 19/31 | 17/13 | 12/8 | 0.525 |
| Vital status | | | | |
|
Alive/deceased | 25/25 | 19/11 | 6/14 | 0.042 |
| Overall survival
(OS) | | | | |
| Median OS
time | 14.5±4.5 | 26.7±11.2 | 11.8±1.0 | 0.043 |
Univariate analyses
In the univariate analysis for overall survival,
gender, performance status, subtype and Wnt7A expression
were all statistically significant (Table II). Worse overall survival
correlated with low Wnt7A expression when expression was
assessed as a categorical variable [≥4.315 (log10
(Wnt7A expression) = 0.635, P=0.043; HR=2.30 (95%CI,
1.01–5.25)].
 | Table IIUnivariate analysis of overall
survival in patients with malignant pleural mesothelioma. |
Table II
Univariate analysis of overall
survival in patients with malignant pleural mesothelioma.
| Overall
survival |
|---|
|
|
|---|
| P-value | Hazard ratio | 95% CI |
|---|
| Age (average,
66±9) | | | |
| ≤66/>66
years | 0.347 | 1/1.47 | (0.66–3.29) |
| Gender | | | |
| Male/female | 0.096 | 1/0.46 | (0.17–1.26) |
| Ethnicity | | | |
|
Caucasian/other | 0.242 | 1/1.63 | (0.71–3.71) |
| Smoking | | | |
| No/yes | 0.163 | 1/1.99 | (0.74–5.40) |
| ECOG PS | | | |
| 0/1+2 | 0.01 | 1/3.89 | (1.29–11.8) |
| Surgical
procedure | | | |
|
EPP/PLE/CWR/BIO | 0.056 | 0/2.44/0/1 | PLE
(0.33–18.32) |
| IMIG staging
system | | | |
| 1+2/3+4 | 0.181 | 1/1.754 | (0.76–4.04) |
| Primary tumor
(T) | | | |
| T1+T2/T3+T4 | 0.241 | 1/1.63 | (0.72–3.68) |
| Regional lymph
node | | | |
|
N−/N+ | 0.816 | 1/1.13 | (0.382–3.390) |
| Subtype | | | |
|
Epithelioid/other | 0.038 | 1/2.91 | (1.01–3.34) |
| Neoadjuvant
chemotherapy | | | |
| Yes/no | 0.423 | 1/1.14 | (0.63–3.03) |
| Adjuvant
chemotherapy | | | |
| Yes/no | 0.143 | 1/0.47 | (0.17–1.32) |
| Radiation
therapy | | | |
| Yes/no | 0.395 | 1/0.71 | (0.32–1.58) |
| Wnt7A
expression | | | |
| High/low | 0.043 | 1/2.30 | (1.01–5.25) |
Multivariate analysis
Multivariate analysis using a Cox’s regression
hazard model showed no significant prognostic factors for overall
survival, but did show that low Wnt7A expression (P=0.051,
HR=2.283) and histological subtype (non-epithelioid, P=0.05,
HR=2.898) were likely to be negative prognostic factors for overall
survival (Table III).
 | Table IIIResults of the multivariate analysis
Cox’s regression hazard model for overall and progression-free
survival. |
Table III
Results of the multivariate analysis
Cox’s regression hazard model for overall and progression-free
survival.
| | | | | | 95% CI for HR |
|---|
| | | | | |
|
|---|
| β | SE of β | Wald | P-value | HR | Lower | Upper |
|---|
|
Non-epithelioid | 1.064 | 0.544 | 3.827 | 0.05 | 2.898 | 0.998 | 8.416 |
| Low Wnt7A | 0.825 | 0.422 | 3.824 | 0.051 | 2.283 | 0.998 | 5.22 |
Wnt7A expression and its relationship
with clinicopathological characteristics and overall survival
Wnt7A expression differed significantly
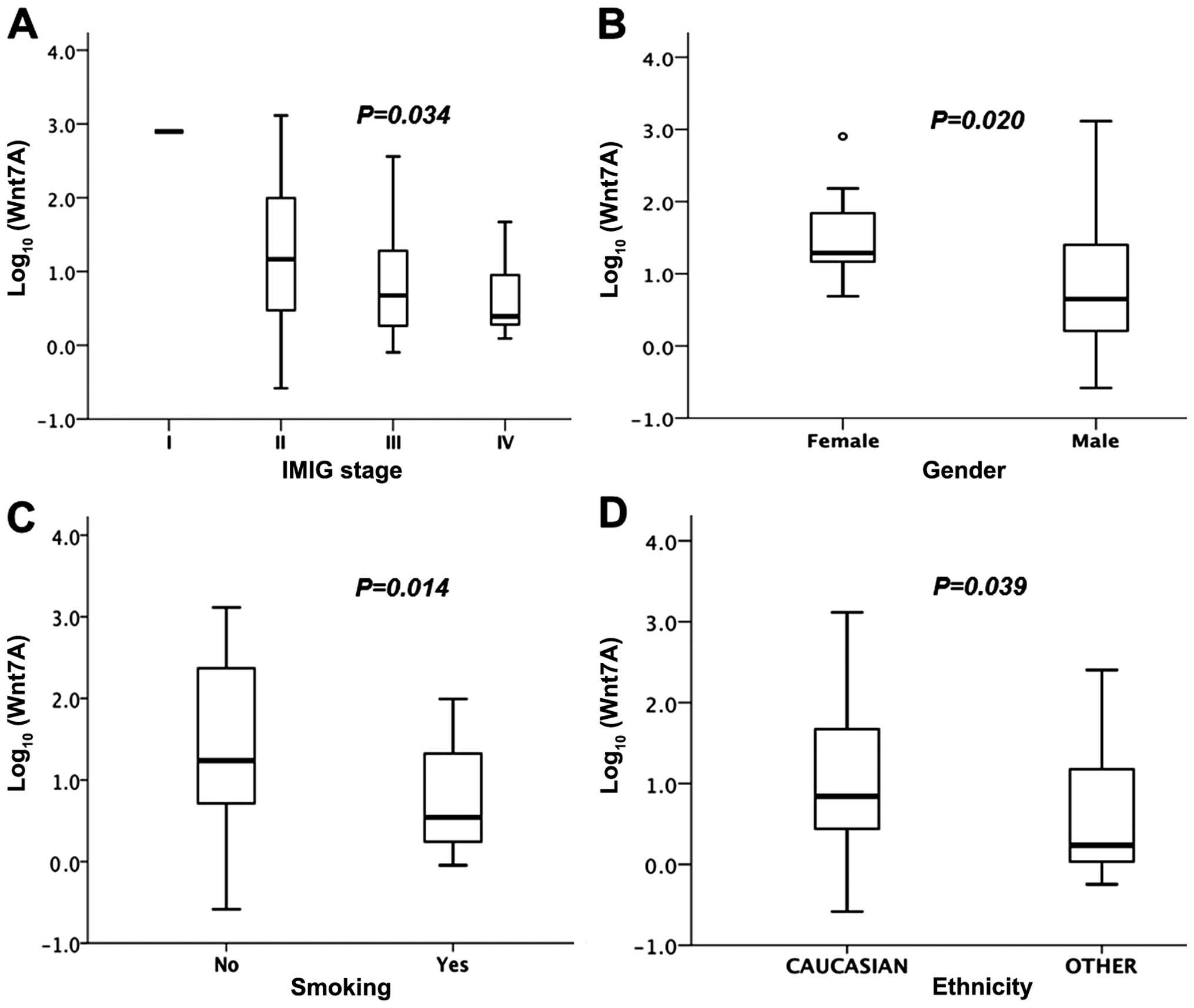
between the 11 paired normal and tumor tissues (P=0.005, Fig. 5), and by tumor stage (Fig. 1A), gender (Fig. 1B), smoking status (Fig. 1C) and ethnicity (Fig. 1D). The logarithm of Wnt7A
expression values in the tumors ranged from −0.58 to +0.89 (mean ±
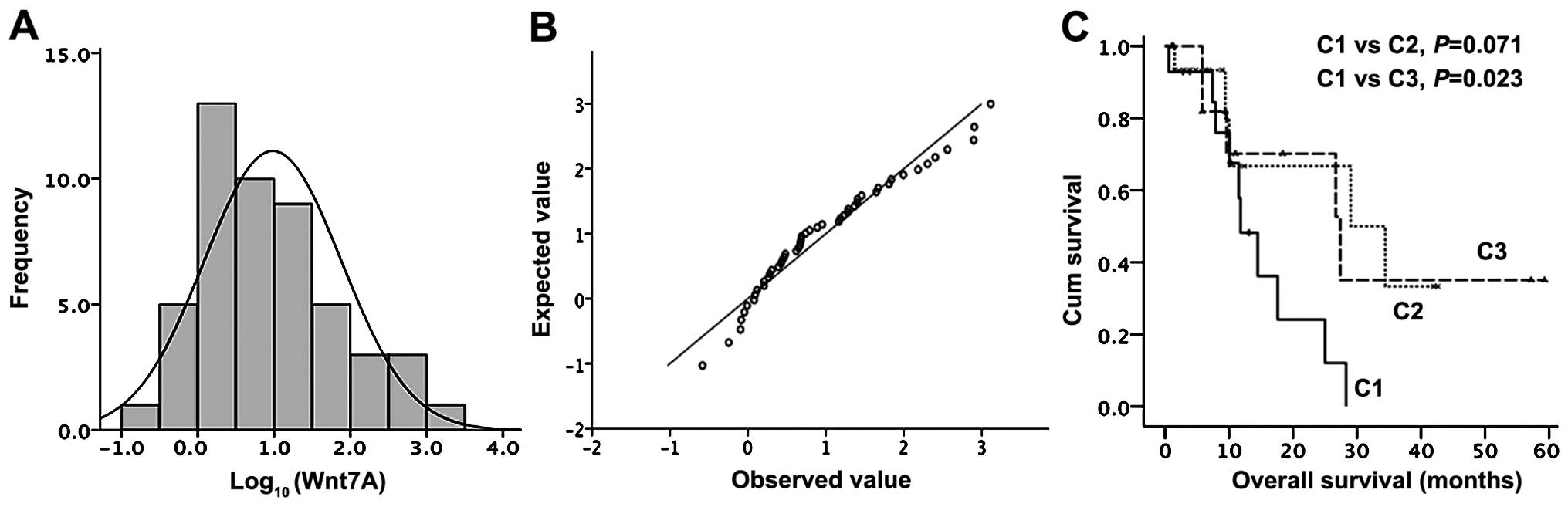
SD, 0.98±0.14). The frequency distribution graph showed two peaks
(Fig. 6), which allowed two
subgroups, high-risk (low Wnt7A) and low risk (high
Wnt7A) to be defined. A cutoff point for distribution of
Wnt7A expression into 2 groups was optimized at 4.67 in the
logarithm of Wnt7A expression to acquire a minimum P-value
in the difference of overall survival between the 2 groups.
Overall survival was significantly more favorable
for patients with high Wnt7A expression than for those with
low expression (26.7±11.1 months vs. 11.8±4.9; P=0.043, Fig. 2A). To confirm a correlation between
Wnt7A expression and survival, we divided the distribution
of Wnt7A expression into thirds (low Wnt7A,
intermediate Wnt7A, high Wnt7A) using the 33rd and
66th percentiles. We found that Kaplan-Meier curves plotted for
epithelioid tumors showed better prognosis (P=0.023, Fig. 6). Previously Fennel et al
estimated in a similar way (30).
Overall survival was also significantly more
favorable for patients with epithelioid tumors than for those with
non-epithelioid tumors (P=0.038). In the 42 patients who had
epithelioid tumors, overall survival was significantly worse when
the tumors had low Wnt7A expression (Fig. 1C). MST of high Wnt7A was
25±5.2 (95% CI, 8.8 to 41.2) months, whereas MST of low
Wnt7A was 10.4±2.6 (95% CI, 5.2 to 15.6) months.
All 9 female patients with MPM had high Wnt7A
expression. They also had a more favorable overall survival than
the male patients (P=0.096, Fig.
3A), including the subgroup with epithelioid tumors (P=0.034,
Fig. 3B). Among the men with
epithelioid tumors, those with high Wnt7A expression had
better survival than those with low Wnt7A expression
(P=0.092, Fig. 3D).
Survival analysis of the 42 patients with
epithelioid tumors showed that overall survival for the 20 patients
who received neoadjuvant chemotherapy was not significantly better
than that for the 22 patients who did not (Fig. 4A). Overall survival was
significantly better for patients with high Wnt7A-expressing
epithelioid tumors than for those with low Wnt7A-expressing
tumors (27.4±0 vs. 10.1±1.7 vs. 10.1±1.7; 95% CI, 6.6 to 13.6;
P=0.019, Fig. 4B). In the patients
with high Wnt7A-expressing epithelioid tumors, overall
survival did not differ between those who underwent neoadjuvant
chemotherapy and those who did not (P=0.902, Fig. 4C). However, in patients with low
Wnt7A-expressing epithelioid tumors, overall survival was
significantly better in those who underwent neoadjuvant
chemotherapy than in those who did not (P=0.024; HR=4.31, 95% CI of
HR, 1.1 to 16.9; Fig. 4E). In the
subset of the 20 patients who received neoadjuvant chemotherapy,
overall survival did not differ significantly between those with
low Wnt7A vs. high Wnt7A tumors (17.6±5.25; 95% CI,
7.3 to 27.9 months vs. 34.4±13.3; 95% CI, 8.2 to 60.5 months,
P=0.425; Fig. 4D). In the subset of
22 patients who did not have neoadjuvant chemotherapy, overall
survival was significantly better for patients with high
Wnt7A-expressing tumors than for those with low
Wnt7A-expressing tumors (P=0.031; 95% HR=1.03 to 15.06;
Fig. 4F).
Discussion
The Wnt genes compose a large gene family encoding a
group of secreted signaling molecules that have been implicated in
oncogenesis and a number of developmental processes. Expression of
Wnt7A is restricted to certain tissues: placenta, kidney,
testis, uterus, fetal lung and fetal and adult brain. Why study it
in MPM? Our PCR analysis of Wnt7A expression in MPM showed
that low Wnt7A expression (P=0.051, HR=2.283) and
histological subtype (non-epithelioid, P=0.05, HR=2.898) were
likely to be negative prognostic factors for overall survival. Our
survival analyses indicated that Wnt7A expression was
correlated with overall survival in the univariate analysis, but
not in the multivariate Cox’s regression.
In our study, Wnt7A expression was
significantly higher in women with MPM than in men (Fig. 1B), and gender was a positive
prognostic factor in patients with epithelioid tumors (Fig. 3B). Wnt7A is required for
proper differentiation and gland formation during uterine
development. Following post-natal growth, Wnt7A expression
becomes restricted primarily to the luminal epithelium and is
responsible for maintaining expression of other Wnts in the stroma
(31–33). The Wnt7A gene is known to
guide the development of the anterior-posterior axis in the female
reproductive tract, and to play a critical role in uterine smooth
muscle patterning and maintenance of adult uterine function. This
gene is also responsive to changes in the levels of sex steroid
hormone in the female reproductive tract. An inverse association
for mRNA expression was found between Wnt7A and estrogen
receptor α (ER-α) (34).
Hypersensitivity of leiomyoma cells to estrogen may deregulate
Wnt7A expression (34).
Decreased Wnt7A expression may lead to loss of control in
the patterning of the myometrium and result in the development of
leiomyoma (34). In particular, the
recent finding that ER-β acts as a tumor suppressor has great
potential relevance to predicting disease progression and
therapeutic response in patients with MPM (35).
E-cadherin induction by Wnt/β-catenin signaling is
an evolutionarily conserved pathway operative in lung cancer cells,
and loss of Wnt7A expression may be important in lung cancer
development or progression through its effects on E-cadherin.
Apparent physiologic levels of Wnt7A positively regulate
E-cadherin expression in lung cancer (24).
During development, the Wnt pathway affects cell
fate, polarity and proliferation, and Wnt7A has been
implicated in the maintenance of HOX expression. In contrast to
what occurs in normal lung and mortal short-term bronchial
epithelial cultures, Wnt7A was frequently less expressed or
absent in lung cancers. It is possible that those genes that are
normally expressed in undifferentiated cells are upregulated in
cancer, similar with other Homeobox genes (23,36).
In our study, Wnt7A expression was highly
correlated with IMIG stage (Fig.
1A). The primary tumor (T) seemed to contribute to stage than
lymph node metastasis (N) (data not shown). These results indicate
that the role of Wnt7A is to have cells differentiated and
kept normal or restored to original specific morphology of the
organ, and if it is lost or decreased, it may have consequence for
worse prognosis in MPM.
In our study, Wnt7A expression might have
been affected by smoking in normal or precancerous events such
K-ras mutation, loss of EGFR mutation, or P53 mutation, even in
pleural tissues. We found that MPM patients with a smoking history
had lower Wnt7A expression than those who did not (Fig. 1C). Smoking considerably increases
the risk of developing mesothelioma. A smoker who is exposed to
asbestos has a 50- to 90-fold greater chance of developing
mesothelioma whereas a non-smoker exposed to asbestos has a 5-fold
greater chance (37,38). It is possible that smoking might
decrease Wnt7A expression associated with tumor-associated
macrophages (39).
The results of our univariate analysis showed that
gender, Eastern Cooperative Oncology Group performance status (ECOG
PS), surgical procedure, and Wnt7A expression were not
significant predictors of overall survival. With the exception of
performance status, these findings are identical to those reported
previously (7–10). ECOG PS, usually one of the most
reliable prognostic factors, was not a significant predictor of
survival in our study. However, ECOG PS data were not available for
more than half of our patients, so we could not compare the
relative contribution of ECOG PS to prognosis with that of
Wnt7A expression. We did find that Wnt7A expression
and histological subtype were similarly prognostic in the Cox’s
model, indicating that they could be prognostic markers for
MPM.
The use of neoadjuvant chemotherapy before surgery
for MPM has recently become common. However, neoadjuvant
chemotherapy remains investigational because it has not
definitively showed a survival benefit (40–42). A
recent multicenter phase II trial using pemetrexed plus cisplatin
neoadjuvant chemotherapy followed by extrapleural pneumonectomy
(EPP) and hemithoracic radiation demonstrated feasibility and a
reasonable long-term survival rate (6). On the other hand, neoadjuvant
chemotherapy appeared to affect compliance with surgery and
radiation therapy. These findings point to the need to determine
which patients will benefit from neoadjuvant chemotherapy before
surgery. The European Organization for Research and Treatment of
Cancer (EORTC) prognostic score (EPS) for MPM, based on three
consecutive phase II trials using different chemotherapy agents,
found no association between objective tumor response and EPS
classification (30). In our study,
the survival analysis of 42 patients with epithelioid tumors showed
that overall survival for the 20 patients who received neoadjuvant
chemotherapy was not significantly better than that for 22 patients
who did not. In the patients with high Wnt7A epithelioid
tumors, overall survival did not differ between those who underwent
neoadjuvant chemotherapy and those who did not (P=0.902, Fig. 4C) while in patients with low
Wnt7A epithelioid tumors, overall survival was significantly
better in those who underwent neoadjuvant chemotherapy than in
those who did not. These results indicate the patients with low
Wnt7A expression do benefit, while patients with high
Wnt7A expression do not benefit. Thus, we recommend
neoadjuvant chemotherapy for patients with low Wnt7A
tumors.
Among patients who did not have neoadjuvant
chemotherapy, those with high Wnt7A expression had a
significantly better prognosis than those with low Wnt7A
expression. However, among patients who did have neoadjuvant
chemotherapy, those with low Wnt7A expression had a
significantly better prognosis than those with high Wnt7A
expression. This indicates that Wnt7A is a putative novel
prognostic factor for MPM and a novel predictor for determining
whether neoadjuvant chemotherapy will be beneficial for patients
with MPM. This means that patients with high Wnt7A
expression have better prognosis, and patients with high
Wnt7A expression might be more sensitive to neoadjuvant
chemotherapy and they could have a better prognosis than those
without neoadjuvant chemotherapy.
In conclusion, our study revealed that Wnt7A
is a putative novel prognostic factor for MPM and a novel predictor
for determining whether neoadjuvant chemotherapy will be beneficial
for patients with MPM. Finally, our results suggest that
Wnt7A is possibly a novel tumor-suppressor gene in MPM.
Acknowledgements
The present study was supported by NIH/NCI
R01CA125030, and the Eileen D. Ludwig Endowed Fund for Thoracic
Oncology Research (to B.H.); the Kazan, McClain, Abrams, Fernandez,
Lyons, Greenwood, Harley and Oberman Foundation; the Jeffrey and
Karen Peterson Family Foundation; and Paul and Michelle Zygielbaum
Research Fund (to D.J.).
References
|
1
|
Robinson BW and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Musk AW and de Klerk NH: Epidemiology of
malignant mesothelioma in Australia. Lung Cancer. 45:S21–S23. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
et al: Phase III study of pemetrexed in combination with cisplatin
versus cisplatin alone in patients with malignant pleural
mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borasio P, Berruti A, Bille A, et al:
Malignant pleural mesothelioma: clinicopathologic and survival
characteristics in a consecutive series of 394 patients. Eur J
Cardiothorac Surg. 33:307–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Flores RM, Zakowski M, Venkatraman E, et
al: Prognostic factors in the treatment of malignant pleural
mesothelioma at a large tertiary referral center. J Thorac Oncol.
2:957–965. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krug LM, Pass HI, Rusch VW, et al:
Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin
followed by extrapleural pneumonectomy and radiation for malignant
pleural mesothelioma. J Clin Oncol. 27:3007–3013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herndon JE, Green MR, Chahinian AP, Corson
JM, Suzuki Y and Vogelzang NJ: Factors predictive of survival among
337 patients with mesothelioma treated between 1984 and 1994 by the
Cancer and Leukemia Group B. Chest. 113:723–731. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Neumann V, Rutten A, Scharmach M, Muller
KM and Fischer M: Factors influencing long-term survival in
mesothelioma patients-results of the German mesothelioma register.
Int Arch Occup Environ Health. 77:191–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Metintas M, Metintas S, Ucgun I, et al:
Prognostic factors in diffuse malignant pleural mesothelioma:
effects of pretreatment clinical and laboratory characteristics.
Respir Med. 95:829–835. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baas P: Predictive and prognostic factors
in malignant pleural mesothelioma. Curr Opin Oncol. 15:127–130.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar-Singh S, Jacobs W, Dhaene K, et al:
Syndecan-1 expression in malignant mesothelioma: correlation with
cell differentiation, WT1 expression, and clinical outcome. J
Pathol. 186:300–305. 1998. View Article : Google Scholar
|
|
12
|
Edwards JG, Swinson DE, Jones JL, Waller
DA and O’Byrne KJ: EGFR expression: associations with outcome and
clinicopathological variables in malignant pleural mesothelioma.
Lung Cancer. 54:399–407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Edwards JG, Faux SP, Plummer SM, et al:
Cyclooxygenase-2 expression is a novel prognostic factor in
malignant mesothelioma. Clin Cancer Res. 8:1857–1862.
2002.PubMed/NCBI
|
|
14
|
Lopez-Rios F, Chuai S, Flores R, et al:
Global gene expression profiling of pleural mesotheliomas:
overexpression of aurora kinases and P16/CDKN2A deletion as
prognostic factors and critical evaluation of microarray-based
prognostic prediction. Cancer Res. 66:2970–2979. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gordon GJ, Rockwell GN, Godfrey PA, et al:
Validation of genomics-based prognostic tests in malignant pleural
mesothelioma. Clin Cancer Res. 11:4406–4414. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimizu E, Coxon A, Otterson GA, et al: RB
protein status and clinical correlation from 171 cell lines
representing lung cancer, extrapulmonary small cell carcinoma, and
mesothelioma. Oncogene. 9:2441–2448. 1994.PubMed/NCBI
|
|
17
|
Modi S, Kubo A, Oie H, Coxon AB,
Rehmatulla A and Kaye FJ: Protein expression of the RB-related gene
family and SV40 large T antigen in mesothelioma and lung cancer.
Oncogene. 19:4632–4639. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giacinti C and Giordano A: RB and cell
cycle progression. Oncogene. 25:5220–5227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cote RJ, Jhanwar SC, Novick S and Pellicer
A: Genetic alterations of the p53 gene are a feature of malignant
mesotheliomas. Cancer Res. 51:5410–5416. 1991.PubMed/NCBI
|
|
20
|
Wong L, Zhou J, Anderson D and Kratzke RA:
Inactivation of p16INK4a expression in malignant mesothelioma by
methylation. Lung Cancer. 38:131–136. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang CT, You L, Yeh CC, et al:
Adenovirus-mediated p14(ARF) gene transfer in human mesothelioma
cells. J Natl Cancer Inst. 92:636–641. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boersma CJ, Bloemen M, Hendriks JM, van
Berkel EA, Olijve W and van Zoelen EJ: Homeobox proteins as signal
transduction intermediates in regulation of NCAM expression by
recombinant human bone morphogenetic protein-2 in osteoblast-like
cells. Mol Cell Biol Res Commun. 1:117–124. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohira T, Gemmill RM, Ferguson K, et al:
WNT7a induces E-cadherin in lung cancer cells. Proc Natl Acad Sci
USA. 100:10429–10434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Riddle RD, Ensini M, Nelson C, Tsuchida T,
Jessell TM and Tabin C: Induction of the LIM homeobox gene Lmx1 by
WNT7a establishes dorsoventral pattern in the vertebrate limb.
Cell. 83:631–640. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Timmreck LS, Pan HA, Reindollar RH and
Gray MR: WNT7A mutations in patients with Mullerian duct
abnormalities. J Pediatr Adolesc Gynecol. 16:217–221. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bui TD, Lako M, Lejeune S, et al:
Isolation of a full-length human WNT7A gene implicated in limb
development and cell transformation, and mapping to chromosome
3p25. Gene. 189:25–29. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kirikoshi H and Katoh M: Expression of
WNT7A in human normal tissues and cancer, and regulation of WNT7A
and WNT7B in human cancer. Int J Oncol. 21:895–900. 2002.PubMed/NCBI
|
|
29
|
Raz DJ, Ray MR, Kim JY, et al: A multigene
assay is prognostic of survival in patients with early-stage lung
adenocarcinoma. Clin Cancer Res. 14:5565–5570. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fennell DA, Parmar A, Shamash J, et al:
Statistical validation of the EORTC prognostic model for malignant
pleural mesothelioma based on three consecutive phase II trials. J
Clin Oncol. 23:184–189. 2005. View Article : Google Scholar
|
|
31
|
Miller C, Degenhardt K and Sassoon DA:
Fetal exposure to DES results in de-regulation of Wnt7a during
uterine morphogenesis. Nat Genet. 20:228–230. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mericskay M, Kitajewski J and Sassoon D:
Wnt5a is required for proper epithelial-mesenchymal interactions in
the uterus. Development. 131:2061–2072. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Parr BA and McMahon AP: Sexually dimorphic
development of the mammalian reproductive tract requires Wnt-7a.
Nature. 395:707–710. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li S, Chiang TC, Davis GR, Williams RM,
Wilson VP and McLachlan JA: Decreased expression of Wnt7a mRNA is
inversely associated with the expression of estrogen receptor-alpha
in human uterine leiomyoma. J Clin Endocrinol Metab. 86:454–457.
2001.PubMed/NCBI
|
|
35
|
Pinton G, Brunelli E, Murer B, et al:
Estrogen receptor-beta affects the prognosis of human malignant
mesothelioma. Cancer Res. 69:4598–4604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abate-Shen C: Deregulated homeobox gene
expression in cancer: cause or consequence? Nat Rev Cancer.
2:777–785. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Andujar P, Wang J, Descatha A, et al:
p16INK4A inactivation mechanisms in non-small-cell lung cancer
patients occupationally exposed to asbestos. Lung Cancer. 67:23–30.
2010. View Article : Google Scholar
|
|
38
|
Becklake MR, Thomas D, Liddell F and
McDonald JC: Follow-up respiratory measurements in Quebec
chrysotile asbestos miners and millers. Scand J Work Environ
Health. 8:105–110. 1982.PubMed/NCBI
|
|
39
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Flores EM: Induction chemotherapy,
extrapleural pneumonectomy, and radiotherapy in the treatment of
malignant pleural mesothelioma: the Memorial Sloan-Kettering
experience. Lung Cancer. 49:S71–S74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tsao AS, Mehran R and Roth JA: Neoadjuvant
and intrapleural therapies for malignant pleural mesothelioma. Clin
Lung Cancer. 10:36–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Weder W, Kestenholz P, Taverna C, et al:
Neoadjuvant chemotherapy followed by extrapleural pneumonectomy in
malignant pleural mesothelioma. J Clin Oncol. 22:3451–3457. 2004.
View Article : Google Scholar : PubMed/NCBI
|