Introduction
Cervical cancer is the second most common malignant
tumor in females worldwide. It has been estimated to affect 500,000
women/year and lead to 270,000 deaths (1). In recent years, there has been
considerable progress in the diagnosis and comprehensive treatment
of cervical cancer. Nevertheless, there have been no fundamental
changes in cervical cancer incidence and mortality. Cervical cancer
occurrence and development is a gradual process. Cervical
intraepithelial neoplasia (CIN) is one of the common precancerous
lesions of cervical cancer. High-risk human papilloma virus (HPV)
infection is closely related to cervical precancerous lesions and
cervical cancer. However, a single HPV infection will not
necessarily develop into cervical cancer (2). Cervical cancer is also related to the
genetic and immune system of the host and other factors. It is
believed that the occurrence and development of cervical cancer is
a complex multi-factor and multi-step process, often involving
proto-oncogenes and activation or inhibition of tumor-suppressor
genes (3). Therefore, there is a
great demand to identify reliable prognostic biomarkers, to predict
the tendency of cervical cancer metastasis, to detect recurrent
lesions early, and to standardize treatment, all of which may
improve the overall survival rate and reduce mortality.
ATPase family AAA domain-containing protein 2
(ATAD2), a member of the AAA+ ATPase family, also known as ANCCA
(AAA+ nuclear coregulator cancer-associated), was identified by
microarray analysis (4). ATAD2 is a
remarkably conserved protein that is expressed predominantly in
germ cells, but is systematically overexpressed in many different
types of unrelated cancers (5).
ATAD2 contains both an ATPase domain and bromodomain. The presence
of a bromodomain adjacent to an AAA type ATPase domain indicates
that ATAD2 is a factor acting primarily on chromatin structure and
function (6). The structure of
ATAD2 suggests that it has functions associated with genome
regulation, including cell proliferation, differentiation and
apoptosis (7). ATAD2 can regulate
downstream genes such as cyclin D1, c-Μyc and E2F1, which
contribute directly or indirectly to cell proliferation (8). Studies have revealed that ATAD2 is
highly expressed in several types of tumors, such as breast,
ovarian, endometrial and lung cancer, hepatocellular carcinoma and
large B-cell lymphoma (9–14). However, there have been no studies
concerning the gene function and prognosis related to ATAD2 in
cervical cancer.
In the present study, we compared ATAD2 levels in
cervical cancer specimens and matched normal/tumor-adjacent
cervical tissues, in order to investigate the impact of ATAD2 on
cell proliferation, invasion and migration. Different methods were
used to determine the relationships between the ATAD2 level and
clinicopathological characteristics and to elucidate its prognostic
value in cervical cancer patients based on survival data.
Materials and methods
Patient tissue samples
One hundred and thirty-five cervical cancer (CC) and
sixty CIN tissues were collected from patients who underwent
routine cervical resection at the First Affiliated Hospital of
China Medical University. None of the patients had received
preoperative radiotherapy or chemotherapy prior to surgical
resection. Clinical stage and grade were evaluated independently by
two pathologists according to the WHO classification system.
Clinical characteristics of all patients, including the
histological type, FIGO stage, histological differentiation grade
and lymph node statues are described in Table I. Total RNAs and protein were
collected from the fresh CC and CIN tissues after surgical
resection. The project protocol was approved by the Institutional
Ethics Committee of China Medical university prior to the
initiation of the study, and all patients provided written informed
consent.
 | Table ICorrelation of ATAD2 expression and
the clinicopathological characteristics of the 135 cervical cancer
patients. |
Table I
Correlation of ATAD2 expression and
the clinicopathological characteristics of the 135 cervical cancer
patients.
| Characteristics | ATAD2 expression
| P-value |
|---|
| High, n (%) | Low, n (%) |
|---|
| Total cases | 96 (71.1) | 39 (28.9) | 0.493 |
| Age (years) | | |
| ≥45 | 53 (73.6) | 19 (26.4) | |
| <45 | 43 (68.3) | 20 (31.7) | |
| Histological
type | | | 0.578 |
| Squamous | 72 (69.9) | 31 (30.1) | |
| Adenocarcinoma | 24 (80.0) | 8 (20.0) | |
| FIGO stage | | | 0.024a |
| I–IIa | 41 (62.1) | 25 (37.9) | |
| IIb–IV | 55 (79.7) | 14 (20.3) | |
| Differentiation | | | 0.165 |
| Well | 31 (62.0) | 19 (38.0) | |
| Moderate | 27 (73.0) | 10 (27.0) | |
| Poor | 38 (79.2) | 10 (20.8) | |
| Lymph node
status | | | 0.036a |
| Positive | 46 (80.7) | 11 (19.3) | |
| Negative | 50 (64.1) | 28 (35.9) | |
Immunohistochemistry
ATAD2 expression was analyzed on 4-μm
paraffin-embedded specimens. Rabbit anti-ATAD2 (1:200,
Sigma-Aldrich, St. Louis, MO, USA) was used. Sections were stained
with 3,3′-diaminobenzidine (DAB). ATAD2 expression levels were
scored semi-quantitatively according to the percent of positively
stained cells combined with the staining intensity. Samples were
considered positive for ATAD2 if the nucleus/cytoplasm of the
sample cells presented positive staining. The percent positivity
was defined as follows: 0 (0%), 1 (1–10%), 2 (11–50%), 3 (51–80%),
or 4 (>80%). The staining intensity was scored as follows: 0 (no
staining), 1 (weakly stained), 2 (moderately stained) and 3
(strongly stained). Both the staining intensity and the percent
positivity were assessed by two investigators in a double-blinded
manner. The ATAD2 expression score was calculated from the product
of the percent positivity score × the staining intensity score.
Each sample was given a final score ranging from 0 to 12, and the
tumors were classified as follows: negative (−), score 0; low
expression (1+), score 1–4; moderate expression (2+), score 5–8;
and strong expression (3+), score 9–12. The immunohistochemical
ATAD2 staining was grouped into two categories: low expression (0
to 1+) and high expression (2+ to 3+).
Western blot analysis
Cells or tissues were harvested, washed in PBS and
placed in an appropriate volume of lysis buffer (Beyotime
Biotechnology, Beijing, China). Total protein lysates (50
μg) were boiled in SDS-PAGE sample buffer for 10 min, then
loaded onto 12% SDS-PAGE gels and transferred to PVDF membranes
(Millipore, Billerica, MA, USA) after electrophoresis. After
blocking, the primary ATAD2 and GAPDH mouse polyclonal antibodies
(both diluted at 1:1,000; Sigma-Aldrich) were incubated with the
membranes at 4°C overnight. The secondary antibodies were incubated
for 2 h at room temperature. Protein bands were visualized using an
ECL system (Millipore, Bedford, MA, USA).
Cell culture and RNA interference
The CC cell lines, HeLa and SiHa were obtained from
the Chinese Academy of Sciences Cell Bank (Shanghai, China) and
cultured in Dulbecco’s modified Eagle’s medium (DMEM)/RPMI-1640
(Invitrogen, Carlsbad, CA, USA). All media were supplemented with
10% fetal calf serum. ATAD2 siRNAs (purchased from GenePharma Co.,
Ltd., Shanghai, China) were: GGAUCUCUCUUCAAUUAAUT (si-ATAD2#1) and
GUGCGUCGAAGUUGUAGGATT (si-ATAD2#2). For transfections, 24 h after
the cells were seeded in a 24-well plate, they were transfected
with the ATAD2 siRNAs or the negative control siRNA (Neg. siRNA)
using Dharma FECT 1 (Thermo Fisher Scientific, Waltham, MA, USA)
according to the manufacturer’s instructions. The mRNA and protein
levels were detected 48 h later.
Real-time quantitative PCR (qPCR)
Total RNA was extracted from the HeLa and SiHa cells
which were transfected with ATAD2 siRNAs or Neg. siRNA with TRIzol
reagent (Takara Biotechnology, Dalian, China). Real-time
quantitative PCR (qPCR) was performed using a SYBR Premix ExTaq on
a Thermal Cycler Dice Real-Time system (both from Takara
Biotechnology) with the following parameters: 30 sec at 95°C
followed by a two-step PCR for 40 cycles of 95°C for 5 sec and 64°C
for 30 sec. Each reaction was performed as previously described.
The primer sequences were: ATAD2 forward,
5′-GGAATCCCAAACCACTGGACA-3′ and reverse, 5′-GGT
AGCGTCGTCGTAAAGCACA-3′; GAPDH forward,
5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ and reverse,
5′-CACCTTCTACAATGAGCTGCGTGTG-3′. The experiments were repeated in
triplicate. The relative levels of gene expression were represented
as ΔCt = Ct gene − Ct reference, and the fold change of gene
expression was analyzed by the 2−∆∆Ct method.
Cell Counting Kit-8 (CCK-8) and colony
formation assay
For the CCK-8 assay, cells were plated in 96-well
plates in media containing 10% FBS at ~2,000 cells/well, 24 h after
transfection. Then, 10 μl of CCK-8 (KeyGen Bio., Nanjing,
China) solution was added to each well and incubated for 1 h at
37°C. The results were quantitated using a test wavelength of 450
nm. For the colony formation assay, the colony formation was
allowed to proceed for 14 days. Plates were stained with 0.1%
crystal violet. The fixed cell colonies were allowed to air dry.
The clone formation rate was calculated.
Cell cycle analysis
HeLa and SiHa cells in 6-well plates were
transfected with ATAD2 siRNA (si-ATAD2#2) or Neg. siRNA. After 24 h
of transfection, the cells were seeded at a density of
5×105 cells/well, trypsinized, fixed with 70% cold
ethanol at 4°C, and washed in cold PBS. A quantity of 100 μl
RNase A and 400 μl PI staining solution was added, and the
cells were incubated at 4°C in the dark for 30 min. Cell cycle
analysis was performed using a flow cytometer (FACSCalibur; BD
Biosciences, San Jose, CA, USA). A software was then used to detect
and record the red fluorescence upon excitation at a wavelength of
488 nm.
Wound healing/scratch migration and
Transwell assays
For the wound healing/scratch migration assay, the
cells were seeded into 6-well plates at a density that after 24 h
of growth, they reached ~60% confluency as a monolayer. The
monolayer was gently and slowly scratched with a new pipette tip
across the center of the well. After scratching, the well was
washed twice with PBS to remove the detached cells and was then
replenished with fresh medium. The cells were grown for an
additional 24 h. Images were captured of the monolayer using a
microscope. The gap distance was quantitatively evaluated using
software. For the Transwell assay, the HeLa and SiHa cells, with
200 μl serum-free media were added to the upper compartment
which was coated with 30 μl Matrigel (1:2 dilution; Costar,
Corning, NY, USA). RMPI-1640 containing 10% FBS was added to the
lower compartment, and further incubation was carried out for 24 h.
The cells that penetrated the membrane of the chamber were stained
with hematoxylin. The cells on the upper membrane were removed with
a cotton tip. The number of invasive cells was assessed in 10
randomly selected fields under a microscope. The experiments were
tested in triplicate.
Statistical analysis
The statistical data were analyzed by SPSS 17.0 for
Windows. The correlation between ATAD2 expression and cervical
cancer patient clinicopathological features was analyzed using the
χ2 test. Student’s t-test analysis was used to compare
messenger RNA in the various groups. Prognostic significance was
evaluated by the Cox hazards model and Kaplan-Meier survival
method. A difference was considered statistically significant at
P<0.05.
Result
Expression of ATAD2 in the CC tissue
samples and cell lines
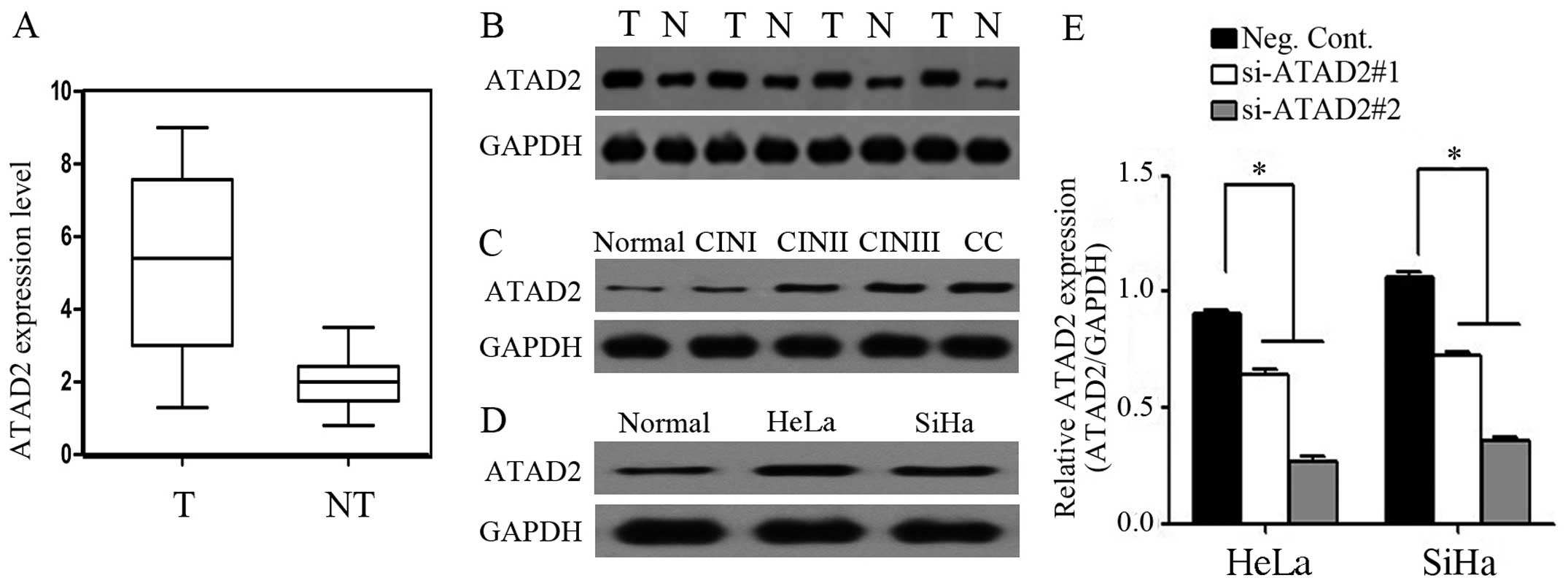
At the protein level, the mean level of ATAD2
expression in the CC tissues was 3.24-fold higher than that in the
non-tumor tissues (median expression, 5.157 and 1.917,
respectively) (Fig. 1A). Western
blot analysis showed that ATAD2 expression was markedly higher in
the CC tissues compared to their adjacent normal cervical tissues
(Fig. 1B), and ATAD2 expression in
the CC and CINII-III tissues was higher than that in the normal
cervical and CINI tissues (Fig.
1C). Compared to the normal cell line, HeLa and SiHa cells
showed relatively higher levels of ATAD2 expression by western
blotting (Fig. 1D). In addition,
ATAD2 expression in the HeLa and SiHa cells transfected with the
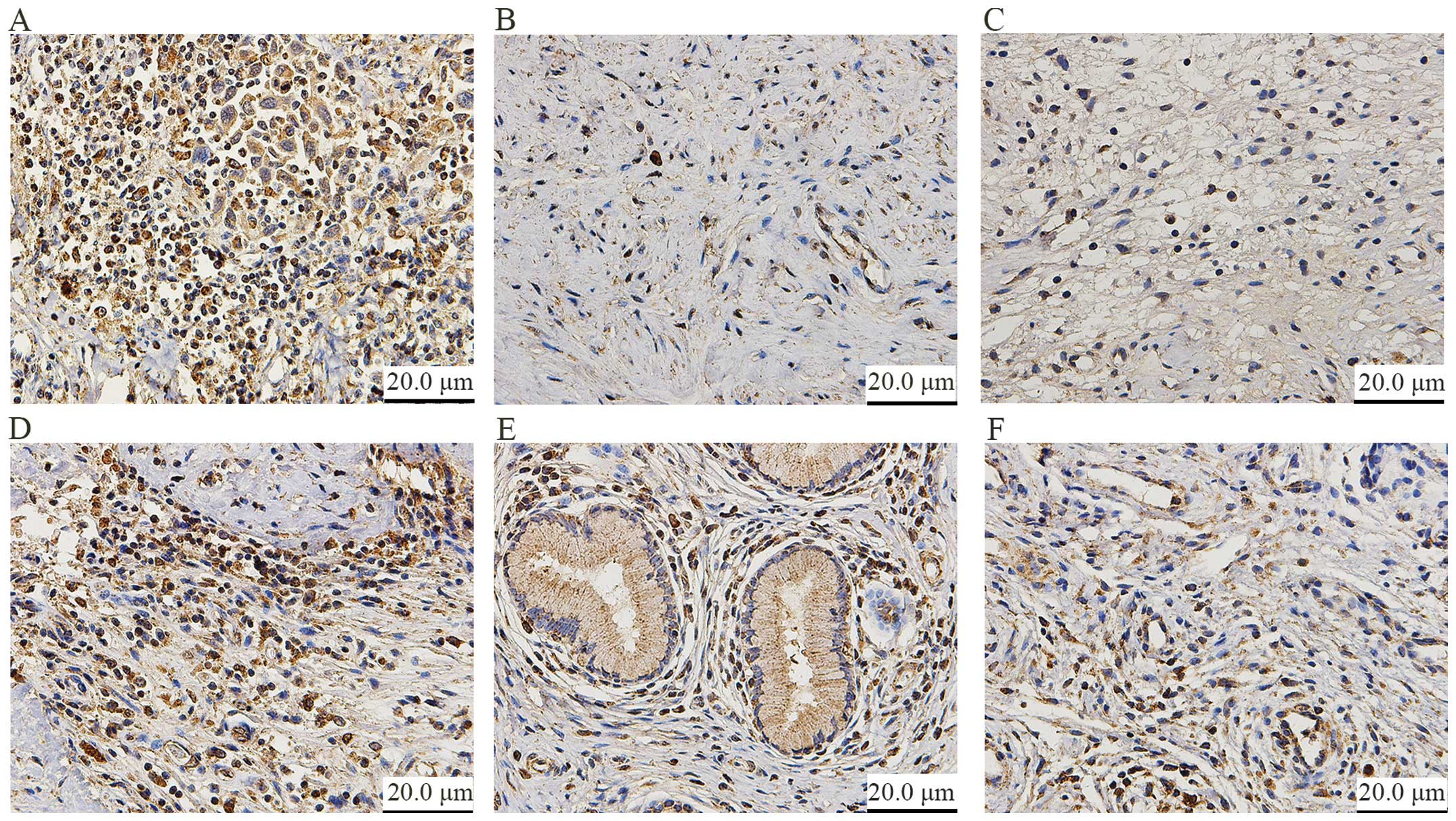
ATAD2 siRNA showed efficient depletion (Fig. 1E). According to the immunohisto
chemical staining, ATAD2 was highly expressed in the CC tissues
(96/135, 71.1%) and CINIII tissues (16/30, 53.3%), whereas little
or no staining was observed in the tumor-adjacent and normal
cervical tissues (Fig. 2).
Expression of ATAD2 in the CC, CINII-III, CINI, and normal cervical
tissues is summarized in Table
II.
 | Table IIExpression of ATAD2 in CC, CIN and
normal cervical tissues. |
Table II
Expression of ATAD2 in CC, CIN and
normal cervical tissues.
| Tissues | n | High, n (%) | P-value |
|---|
| Normal cervical | 30 | 6 (20.0) | |
| CINI | 30 | 8 (26.7) | 0.542 |
| CINII-III | 30 | 16 (53.3) | 0.007a |
| CC | 135 | 96 (71.1) | <0.001a |
Correlation of ATAD2 expression with
cervical cancer patient clinical outcome
High levels of ATAD2 expression were significantly
associated with FIGO stage and lymph node status. Association of
ATAD2 expression and the clinicopathological characteristics of the
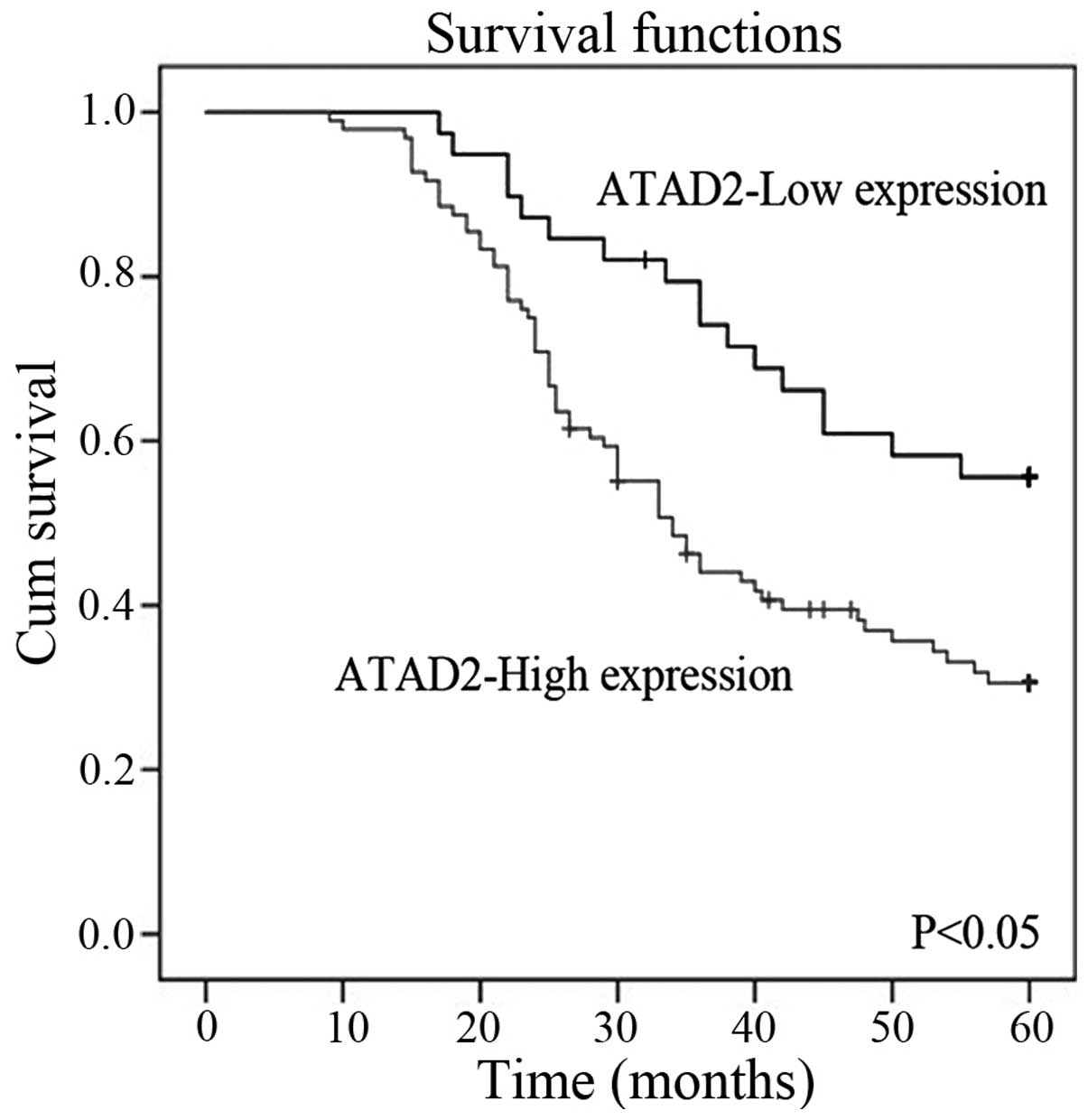
135 cervical cancer patients are summarized in Table I. Overall survival was significantly
reduced in patients with high ATAD2 expression compared to patients
with low ATAD2 expression (P<0.05, Fig. 3). In addition, a multivariate
analysis demonstrated that ATAD2 status, FIGO stage and lymph node
status were significant prognostic factors for cervical cancer
patients. A high level of ATAD2 expression was associated with
increased risk of a poor prognosis (hazard ratio, 1.803, P=0.006)
(Table III).
 | Table IIIUnivariate and multivariate analyses
of the individual parameters correlated with overall survival of
the cervical cancer patients. |
Table III
Univariate and multivariate analyses
of the individual parameters correlated with overall survival of
the cervical cancer patients.
| Variables | Univariate analysis
| Multivariate
analysis
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| ATAD2 | 2.509 | 1.530–4.115 | <0.001a | 1.803 | 1.180–2.756 | 0.006a |
| Age | 1.019 | 0.993–1.046 | 0.156 | | | |
| FIGO stage | 3.651 | 2.360–5.648 | <0.001a | 1.852 | 1.214–2.285 | 0.004a |
|
Differentiation | 1.118 | 0.881–1.419 | 0.357 | | | |
| Histological
type | 1.194 | 0.680–1.759 | 0.711 | | | |
| Lymph node
status | 3.985 | 2.256–5.309 | <0.001a | 2.533 | 1.665–3.852 | 0.001a |
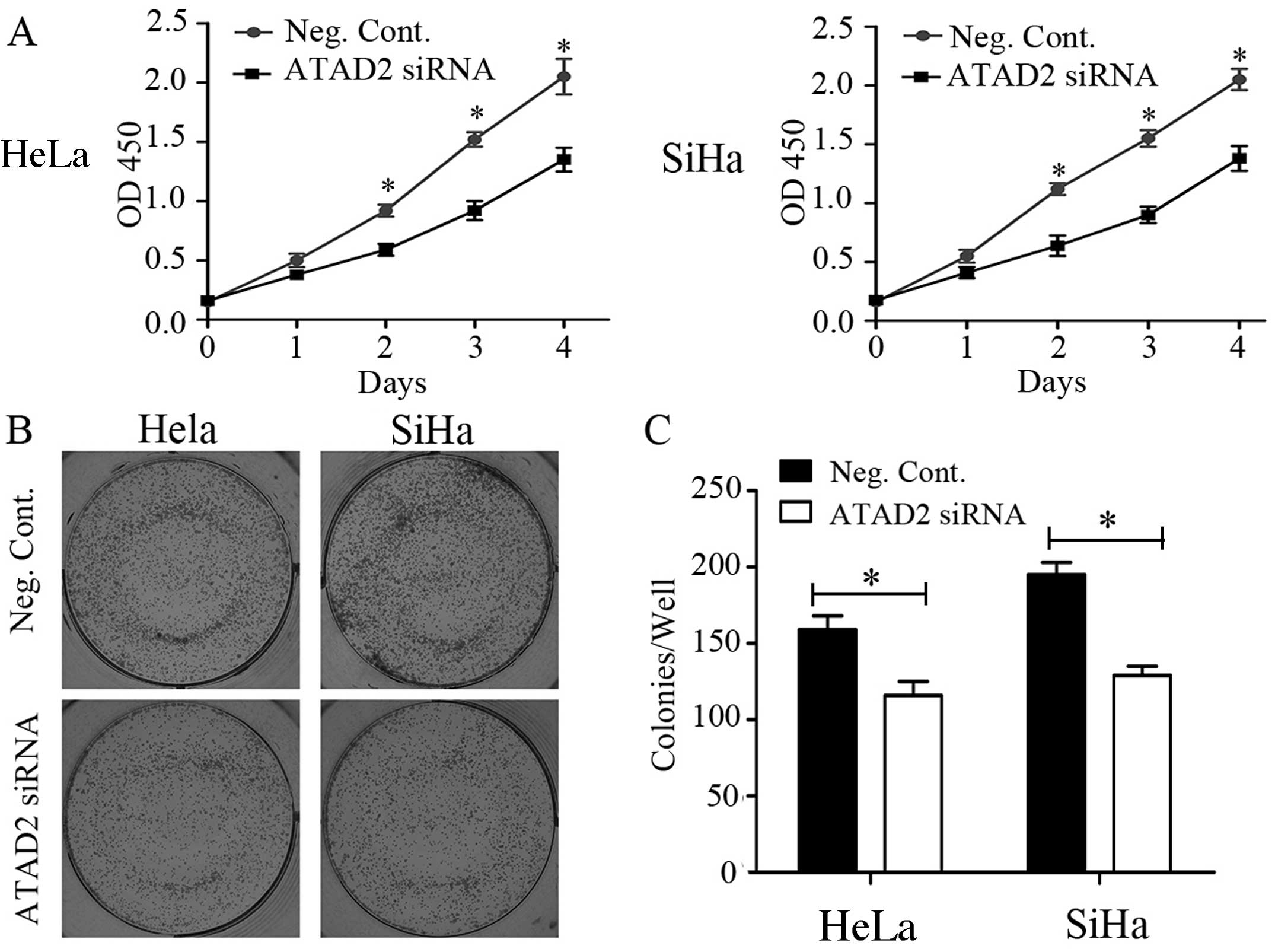
Depletion of ATAD2 inhibits tumor cell
proliferation in the CC cell lines
A significant reduction in the proliferation rate of
the CC cell lines was observed with the CCK-8 assay after
transfection with ATAD2 siRNA compared to Neg. siRNA (Fig. 4A). Consistent with the CCK-8 assay,
the depletion of ATAD2 in the HeLa (Neg. siRNA vs. ATAD2 siRNA:
159±9 vs. 116±9, P<0.05) and SiHa (Neg. siRNA vs. ATAD2 siRNA:
195±8 vs. 129±6, P<0.05) cells led to a significant reduction in
the number and size of foci (Fig.
4B). The DNA content determined using flow cytometry revealed
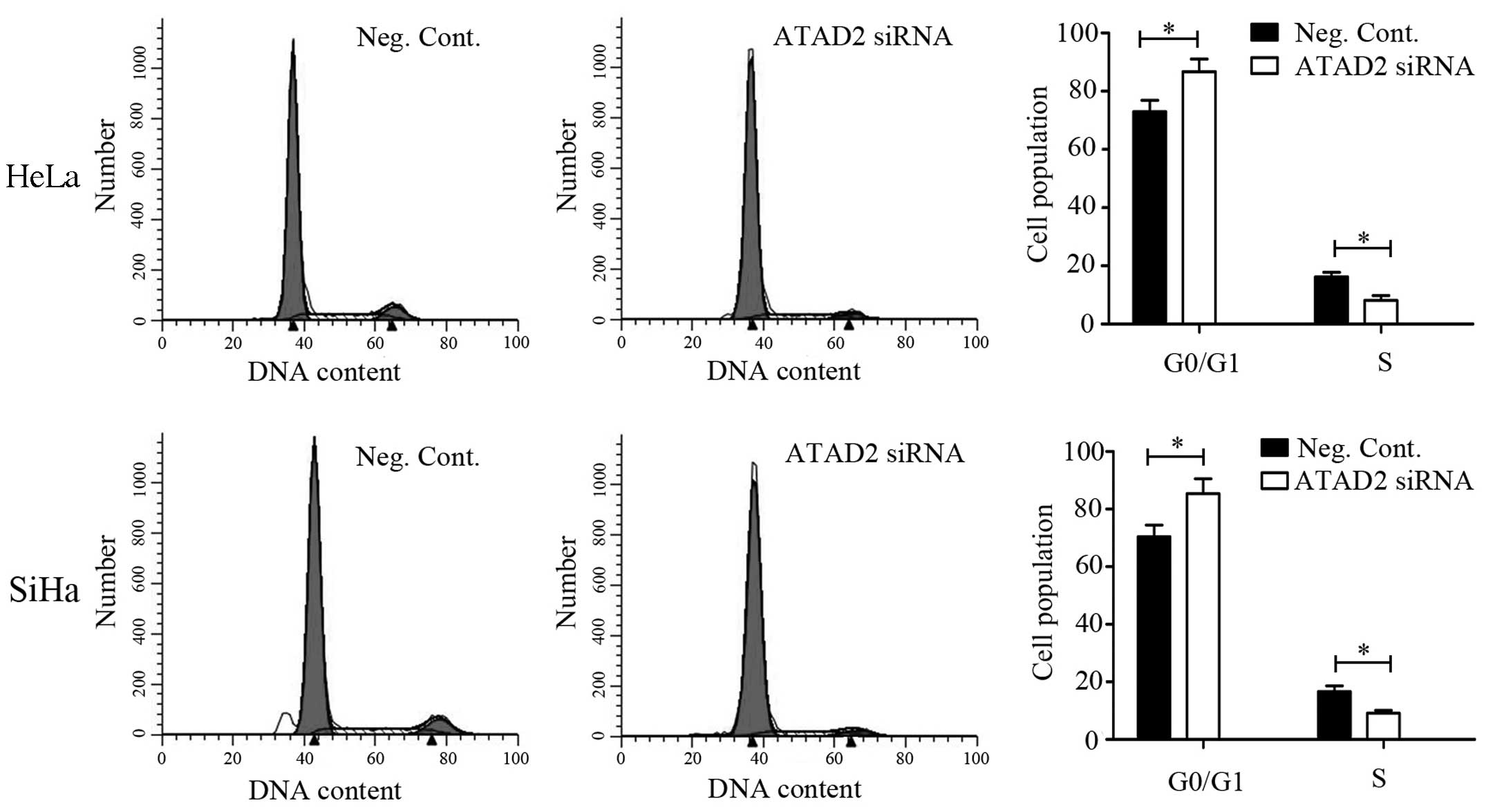
that ATAD2 siRNA transfection increased the percentage of cells in
the G1 phase and decreased those in the S phase in both the HeLa
and SiHa cell lines (P<0.05, Fig.
5).
Invasive and migratory capacities of the
HeLa and SiHa cells are decreased by ATAD2 knockdown
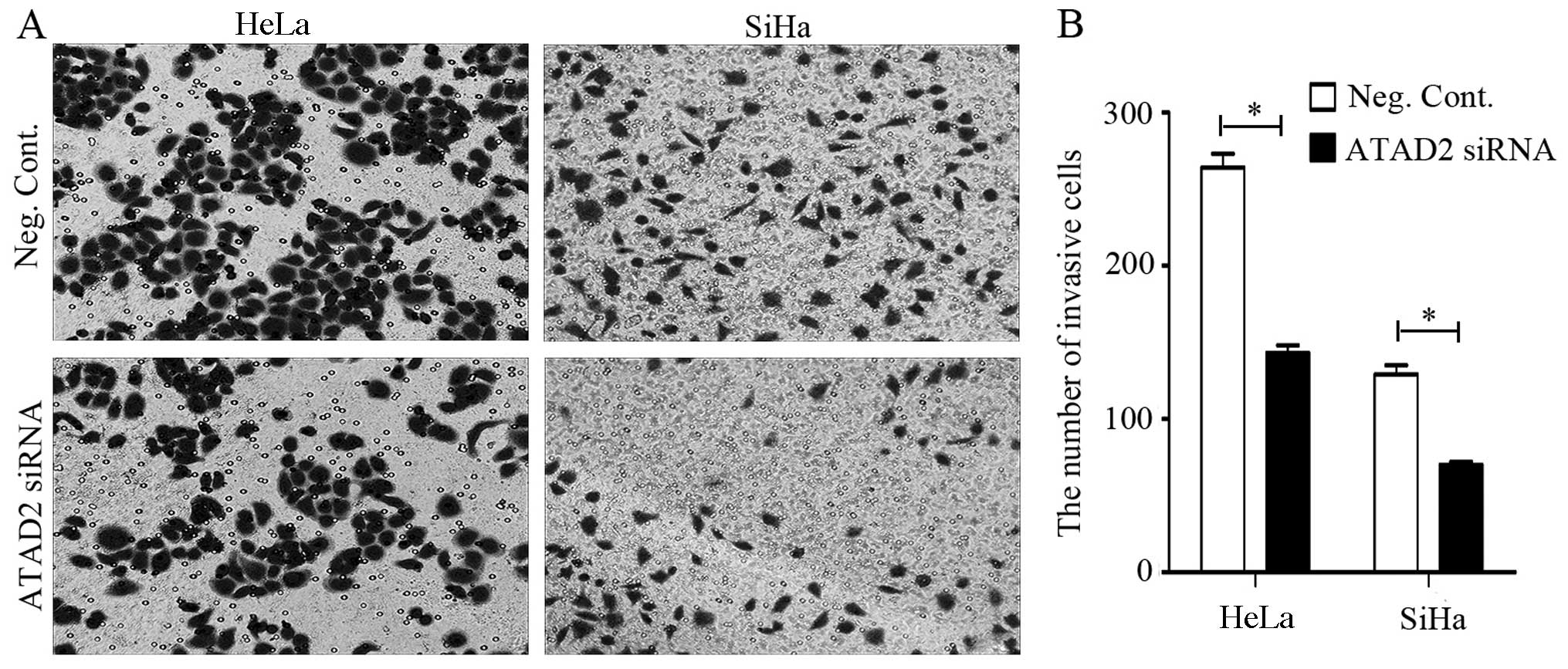
Cell invasion and migration assays demonstrated that
the HeLa and SiHa CC cell lines that were transfected with ATAD2
siRNA displayed attenuated invasive and migratory capacities than
these capacities in the negative controls (Figs. 6 and 7). The depletion of ATAD2 in the HeLa
(Neg. siRNA vs.ATAD2 siRNA: 263.7±9.5 vs. 142.7±5.0, P<0.05) and
SiHa (Neg. siRNA vs. ATAD2 siRNA: 128.7±5.5 vs. 70.0±2.0,
P<0.05) cells led to a significant reduction in invasive cells
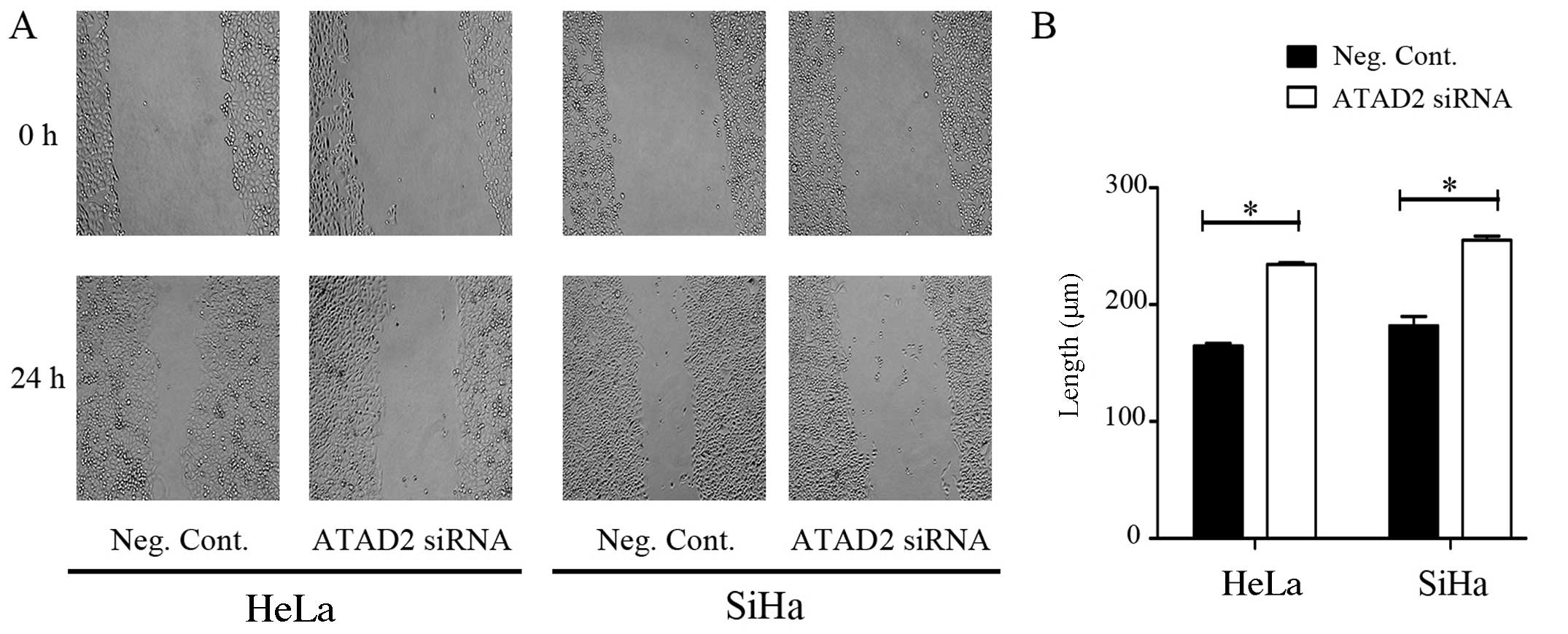
(Fig. 6). The migratory ability of
the HeLa and SiHa cells was assessed by a wound healing/scratch
migration assay. Migration of the HeLa (Neg. siRNA vs. ATAD2 siRNA:
164.57±2.31 vs. 234.31±1.65, P<0.05) and SiHa (Neg. siRNA vs.
ATAD2 siRNA: 181.87±8.08 vs. 255.05±3.53, P<0.05) cells was also
significantly reduced (Fig. 7).
Discussion
Discovery of the ATAD2 gene is a new breakthrough in
oncology research. The AAA+ ATPase structure domain is a molecular
chaperone using ATP activity to participate in the activities of
cells, cell cycle control, signal transduction, and gene expression
regulation (15). The bromine
structure domain and acetylation of the lysine model adjust
chromosome remodeling and transcriptional control of the
interaction between proteins (16–19).
ATAD2 maps to chromosome 8q24 in a region that is frequently
amplified in cancer (20). It was
demonstrated that ATAD2 is a new type of candidate oncogene and
perhaps a therapeutic target for several types of human tumors,
such as breast, ovarian, endometrial and lung cancer and
hepatocellular carcinoma (9–13).
However, the abnormal expression of ATAD2 and its possible role in
the carcinogenesis of cervical cancer have not been studied to
date.
In the present study, immunohistochemical analysis
showed that the expression of ATAD2 in cervical cancer tissues was
significantly higher than that in the normal/tumor-adjacent
tissues. ATAD2 expression in the CINII-III tissues was
significantly higher than that in the CINI and normal tissues. The
same results were demonstrated by western blotting. A total of 135
cervical cancer tissue samples were analyzed by χ2 test.
The results indicated that ATAD2 is one type of cervical cancer
gene and is related to cervical cancer proliferation and
transference. We found that high expression of ATAD2 in cervical
cancer patients was an independent predictor of overall survival.
Overexpression of the ATAD2 gene may play an important role in the
occurrence and development of cervical cancer, but the specific
mechanism and genes for cervical cancer cell proliferation warrant
further research.
Previous studies showed that ATAD2 was highly
expressed in a variety of human tumor cell lines, including those
derived from leukemia (HL60, Molt4 and Jurkat), lymphoma (Daudi),
lung adenocarcinoma (A549 and A1299), and breast cancer (MCF-7)
(21). ATAD2 in human cancer cells
is associated with a variety of key regulatory mechanisms, such as
regulation of cell proliferation and tumor metastasis (22,23).
ATAD2 can regulate downstream genes such as cyclin D1, c-myc and
E2F, which contribute indirectly or directly to cancer cell
proliferation (24). High
expression of ATAD2 can promote cell proliferation in breast cancer
and is closely related to tumor stage (9). Through small RNA interference, tumor
cell proliferation ability was decreased, and apoptosis was induced
in breast cancer cells (21). In
prostate cancer, ATAD2 also plays an important role in tumor cell
proliferation (25). At present,
how ATAD2 affects cell proliferation and tumor molecular mechanisms
is unclear.
A recent study showed that ATAD2 and some important
mitotic drive protein subtype genes and cell survival genes (IRS2,
VEGF and AKtl) controlled by c-myc and EZH2 are associated with
proliferation of Rb-E2F (26).
ATAD2 exists in the E2F sensitive gene promoter, and when the cells
change from the G1 to the S phase, ATAD2 was increased
significantly (27). In our
research, the HeLa and SiHa cells had relatively higher expression
of ATAD2, and the depletion of ATAD2 by transfection with siRNA in
these two cell lines led to G1 phase cell cycle arrest and reduced
proliferation and colony-forming ability. This result was in
agreement with previous studies, and siRNA-mediated ATAD2 knockdown
significantly inhibited cervical cancer cell invasion and
migration. In agreement with the literature, through activation of
inflammatory factors such as nuclear factor-κB (NF-κB), ATAD2 plays
a main role in tumor progression. Histone methyltransferase NSD2
(also known as MMSET and WHSC1), an NF-κB activator, is a
construction of bromine ANCCA/ATAD2 gene of target genes. It
activates downstream target genes, including IL-6, IL-8, cyclin D
and Bcl-2. A previous study suggests that the downstream target
genes may play an important role in cervical cancer cell metastasis
(28). Our results provide evidence
that ATAD2 is not only important in cervical cancer cell
proliferation, but is also involved in cervical cancer cell
invasion and migration. Further research is necessary to understand
the specific mechanisms.
In summary, the present study identified high
expression of the oncogene ATAD2 in cervical cancer, which may be
important in the acquisition of an aggressive phenotype and may
indicate a poor prognosis for cervical cancer patients. In
addition, the functional studies of ATAD2 suggest that it has a
potential role in regulating cell proliferation, invasion, and
migration. ATAD2 is a candidate target protein for future cancer
therapeutics.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 81171649 awarded to
Y.G.).
References
|
1
|
Khenchouche A, Sadouki N, Boudriche A,
Houali K, Graba A, Ooka T and Bouguermouh A: Human papillomavirus
and Epstein-Barr virus co-infection in cervical carcinoma in
Algerian women. Virol J. 10:3402013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomison J III, Thomas LK and Shroyer KR:
Human papillomavirus: molecular and cytologic/histologic aspects
related to cervical intraepithelial neoplasia and carcinoma. Hum
Pathol. 39:154–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiao S, Liao S, Zhou Y, Jiang B, Li Y and
Xue M: High expression of octamer transcription factor 1 in
cervical cancer. Oncol Lett. 7:1889–1894. 2014.PubMed/NCBI
|
|
4
|
Zou JX, Revenko AS, Li LB, Gemo AT and
Chen HW: ANCCA, an estrogen-regulated AAA+ ATPase coactivator for
ERalpha, is required for coregulator occupancy and chromatin
modification. Proc Natl Acad Sci USA. 104:18067–18072. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boussouar F, Jamshidikia M and Morozumi Y:
Malignant genome reprogramming by ATAD2. Biochim Biophys Acta.
10:1010–1014. 2013. View Article : Google Scholar
|
|
6
|
Ciró M, Prosperini E, Quarto M, Grazini U,
Walfridsson J, McBlane F, Nucifero P, Pacchiana G, Capra M,
Christensen J, et al: ATAD2 is a novel cofactor for MYC,
overexpressed and amplified in aggressive tumors. Cancer Res.
69:8491–8498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu G, Lu X, Wang Y, He H, Meng X, Xia S,
Zhen K and Liu Y: Epigenetic high regulation of ATAD2 regulates the
Hh pathway in human hepatocellular carcinoma. Int J Oncol.
45:351–361. 2014.PubMed/NCBI
|
|
8
|
Revenko AS, Kalashnikova EV, Gemo AT, Zou
JX and Chen HW: Chromatin loading of E2F-MLL complex by
cancer-associated coregulator ANCCA via reading a specific histone
mark. Mol Cell Biol. 30:5260–5272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsia EY, Kalashnikova EV, Revenko AS, Zou
JX, Borowsky AD and Chen HW: Deregulated E2F and the AAA+
coregulator ANCCA drive proto-oncogene ACTR/AIB1 overexpression in
breast cancer. Mol Cancer Res. 8:183–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wan WN, Zhang YX, Wang XM, Liu YJ, Zhang
YQ, Que YH and Zhao WJ: ATAD2 is highly expressed in ovarian
carcinomas and indicates poor prognosis. Asian Pac J Cancer Prev.
15:2777–2783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raeder MB, Birkeland E, Trovik J, Krakstad
C, Shehata S, Schumacher S, Zack TI, Krohn A, Werner HM, Moody SE,
et al: Integrated genomic analysis of the 8q24 amplification in
endometrial cancers identifies ATAD2 as essential to MYC-dependent
cancers. PLoS One. 8:e548732013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Sun Y, Li Y, Fang Z, Wang R, Pan
Y, Hu H, Luo X, Ye T, Li H, et al: ANCCA protein expression is a
novel independent poor prognostic marker in surgically resected
lung adenocarcinoma. Ann Surg Oncol. 20(Suppl 3): S577–S582. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu G, Liu H, He H, Wang Y, Lu X, Yu Y, Xia
S, Meng X and Liu Y: miR-372 down-regulates the oncogene ATAD2 to
influence hepatocellular carcinoma proliferation and metastasis.
BMC Cancer. 14:1072014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alizadeh AA, Eisen MB, Davis RE, Ma C,
Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al:
Distinct types of diffuse large B-cell lymphoma identified by gene
expression profiling. Nature. 403:503–511. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Creech GS, Paresi C, Li YM and Danishefsky
SJ: Chemical synthesis of the ATAD2 bromodomain. Proc Natl Acad Sci
USA. 111:2891–2896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang P, Guo L, Duan ZJ, Tepper CG, Xue L,
Chen X, Kung HJ, Gao AC, Zou JX and Chen HW: Histone
methyltransferase NSD2/MMSET mediates constitutive NF-κB signaling
for cancer cell proliferation, survival, and tumor growth via a
feed-forward loop. Mol Cell Biol. 32:3121–3131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dhalluin C, Carlson JE, Zeng L, He C,
Aggarwal AK and Zhou MM: Structure and ligand of a histone
acetyltransferase bromodomain. Nature. 399:491–496. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jacobson RH, Ladurner AG, King DS and
Tjian R: Structure and function of a human TAFII250 double
bromodomain module. Science. 288:1422–1425. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shahbazian MD and Grunstein M: Functions
of site-specific histone acetylation and deacetylation. Annu Rev
Biochem. 76:75–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zou JX, Guo L, Revenko AS, Tepper CG, Gemo
AT, Kung HJ and Chen HW: Androgen-induced coactivator ANCCA
mediates specific androgen receptor signaling in prostate cancer.
Cancer Res. 69:3339–3346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caron C, Lestrat C, Marsal S, Escoffer E,
Curtet S, Virolle V, Barbry P, Debernardi A, Brambilla C, Brambilla
E, et al: Functional characterization of ATAD2 as a new
cancer/testis factor and a predictor of poor prognosis in breast
and lung cancers. Oncogene. 29:5171–5181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalashnikova EV, Revenko AS, Gemo AT,
Andrews NP, Tepper CG, Zou JX, Cardiff RD, Borowsky AD and Chen HW:
ANCCA/ATAD2 overexpression identifies breast cancer patients with
poor prognosis, acting to drive proliferation and survival of
triple-negative cells through control of B-Myb and EZH2. Cancer
Res. 70:9402–9412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fouret R, Laffaire J, Hofman P,
Beau-Faller M, Mazieres J, Validire P, Girard P, Camilleri-Bröet S,
Vaylet F, Leroy-Ladurie F, et al: A comparative and integrative
approach identifies ATPase family, AAA domain containing 2 as a
likely driver of cell proliferation in lung adenocarcinoma. Clin
Cancer Res. 18:5606–5616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsia EY, Zou JX and Chen HW: The roles and
action mechanisms of p160/SRC coactivators and the ANCCA
coregulator in cancer. Prog Mol Biol Transl Sci. 87:261–298. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duan Z, Zou JX, Yang P, Wang Y, Borowsky
AD, Gao AC and Chen HW: Developmental and androgenic regulation of
chromatin regulators EZH2 and ANCCA/ATAD2 in the prostate via MLL
histone methylase complex. Prostate. 73:455–466. 2013. View Article : Google Scholar
|
|
26
|
Zhou Y, Sui C, Li B, Yin Z, Tan Y, Yang J
and Liu Z: Repeat hepatectomy for recurrent hepatocellular
carcinoma: a local experience and a systematic review. World J Surg
Oncol. 8:552010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
De Angelis PM, Svendsrud DH, Kravik KL and
Stokke T: Cellular response to 5-fuorouracil (5-FU) in
5-FU-resistant colon cancer cell lines during treatment and
recovery. Mol Cancer. 5:202006. View Article : Google Scholar
|
|
28
|
Zhang L, Hao Q, Bao L, Liu W, Fu X, Chen Y
and Wu H: Phenethyl issothiocyanate suppresses cervical carcinoma
metastasis potential and its molecular mechanism. Mol Med Rep.
10:2675–2680. 2014.PubMed/NCBI
|