Introduction
Non-small cell lung carcinoma is one of the leading
causes of cancer-related mortality worldwide (1). Standard platinum-based chemotherapies
provide marginal improvement in survival at the expense of
substantial toxicity (2). Even with
the addition of targeted therapy, the median survival of metastatic
non-small cell lung cancer patients is approximately one year
(3). Due to the unsatisfactory
results of standard chemotherapy, the identification of new drugs
is crucial.
In recent years, the public has become increasingly
aware of alternative medical therapies through all forms of media
in Western countries, and the use of complementary and alternative
medicine has also increased, particularly among oncology patients.
Curcumin, isolated from turmeric (Curcuma longa L.),
contains curcumin as a major component but also contains
demethoxycurcumin (DMC) and bisdemethoxycurcumin (BDMC),
respectively (4). Extensive
research during the last half century has revealed several
important functions of curcumin such as antioxidant,
anti-inflammatory and anticancer properties (5–7).
Recently, more and more studies have demonstrated the stronger
activity of DMC when compared with curcumin in many aspects
(8–10). Numerous studies have shown that DMC
induces cytotoxic effects in many cancer cell lines such as colon
(11) and renal cell cancer
(12), glioma (9) and leukemic cell lines (13). However, no study exists which shows
the effects of DMC on human lung cancer cells, and the role of DMC
in inducing cell cycle arrest and apoptosis has never been
investigated in detail.
The NCI-H460 cell line is derived from human large
cell lung cancer, which is one of the major types of non-small cell
lung carcinoma. In the present study, we investigated the cytotoxic
effects of DMC on human lung cancer NCI-H460 cells and we found
that DMC induced cell death through the induction of apoptosis
in vitro.
Materials and methods
Chemicals and reagents
DMC, dimethyl sulfoxide (DMSO), propidium iodide
(PI) and Trypsin-EDTA were purchased from Sigma Chemical Co. (St.
Louis, MO, USA). Culture medium RPMI-1640, fetal bovine serum
(FBS), L-glutamine and penicillin-streptomycin were purchased from
Gibco/Invitrogen Life Technologies (Carlsbad, CA, USA). Primary
antibodies (anti-AIF, -Endo G, -GRP78, -GADD153, -IRE1β, -ATF-6α,
-ATF-6β, and -caspase-4) were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell culture
The human lung cancer cell line NCI-H460 was
purchased from the Food Industry Research and Development Institute
(Hsinchu, Taiwan). The cells were cultured in RPMI-1640 medium
supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml
streptomycin and 2 mM glutamine and were incubated in a 5%
CO2 humidified incubator at 37°C in a 75-cm2
tissue culture flask.
Assessment of cell morphological changes
and viability
NCI-H460 cells were plated onto 12-well plates at a
density of 2×105 cells/well, and DMC was added at final
concentrations of 0, 15, 20, 25, 30 or 35 μM. In control
wells, only DMSO (solvent) was added. The cells were exposed for 24
and 48 h. A phase-contrast microscope was used to observe
morphological changes in the examined cells at the end of the time
period. Cell viability was estimated through flow cytometric
methods as described elsewhere (14,15).
Cell cycle distribution and sub-G1
assays
Approximately 2×105 cells/well of
NCI-H460 cells in 12-well plates were incubated with 0, 15, 20, 25,
30, 35 and 40 μM of DMC for 48 h. The cells were harvested
by centrifugation, washed with PBS and fixed in 70% ethanol at
−20°C overnight. The cells were then resuspended in PBS containing
40 μg/ml of PI, 0.1 mg/ml RNase and 0.1% Triton X-100 in a
dark room for 30 min and were subsequently analyzed by a flow
cytometer (FACSCalibur; Becton-Dickinson, San Jose, CA, USA)
(16,17). The cell cycle distribution and
sub-G1 groups (apoptosis) were calculated and analyzed by CellQuest
(Becton-Dickinson) and ModFit LT software (Verity Software House
Inc., Topsham, ME, USA).
Reactive oxygen species (ROS),
intracellular Ca2+ and mitochondrial membrane potential
(ΔΨm) assays
NCI-H460 cells (2×105 cells/well) were
treated with 35 μM of DMC for different time intervals. At
the end of the incubation, cells from each treatment and
time-points were collected, washed, counted and then were
resuspended in 500 μl of DCFH-DA (10 μM) for 30 min
for ROS (H2O2) measurement, resuspended in
500 μl of Fluo-3/AM (2.5 μg/ml) for 30 min for
intracellular Ca2+ concentration measurement and
resuspended in 500 μl of DiOC6 (4 μmol/l)
for 30 min to determine the levels of ΔΨm. All samples were then
individually analyzed by flow cytometry as described previously
(16).
Caspase-3, -8 and -9 activity assay
NCI-H460 cells were plated onto 12-well plates at a
density of 2×105 cells/well and incubated with or
without 35 μM DMC. The cells were then incubated for 0, 6,
24 and 48 h and harvested, washed and resuspended in 25 μl
of 10 μM substrate solution (PhiPhiLux and CaspaLux kit;
OncoImmunin, Inc., Gaithersburg, MD, USA) before being incubated at
37°C for 60 min. The cells were washed again in PBS and were
analyzed by flow cytometry as described previously (16,18,19).
Effects of DMC on expression of
apoptosis-associated proteins as determined by western blot
analysis
Cells (2×106/dish) were treated with 35
μM DMC and incubated for 0, 6, 24 and 48 h. The abundance of
selective proteins associated with apoptosis was determined by
western blotting. Briefly, at the end of the incubation, the cells
were harvested and lysed as described previously (18,19).
The levels of apoptosis-associated proteins were determined in the
cell lysates using primary antibodies (those associated with the
cell cycle such as anti-p21, p27, CDC25c, CDK2, cyclin A and cyclin
E; those associated with apoptosis such as anti-AIF, Endo G, PARP,
Fas-L and Fas; those associated with ER stress such as ATF6α,
ATF-6β, IRE1β, GRP78, GADD153, caspase-4 and -12). For equal
protein loading, each membrane was stripped and reprobed with the
anti-β-actin antibody (18,19).
Confocal laser scanning microscopy
NCI-H460 cells (3×105 cells/well) were
placed on 6-well chamber slides and incubated with or without 35
μM DMC for 48 h. The cells were then fixed, washed and
permeabilized as described previously (16,17).
Then anti-AIF, Endo G, ATF6β, IRE1α and p-PERK (all in green
fluorescence) were individually used for staining each sample
overnight, followed by washing and then staining with the secondary
antibody (FITC-conjugated goat anti-mouse IgG). The cells were then
stained using PI (red fluorescence) for nuclear examination under a
Leica TCS SP2 confocal spectral microscope as described previously
(16,17).
Statistical analysis
All data are expressed as the mean ± SD of 3
experiments. Statistical analysis was performed using the Student’s
t-test, with a value of P<0.05 considered to indicate a
statistically significant difference between the DMC-treated and
untreated (control) group.
Results
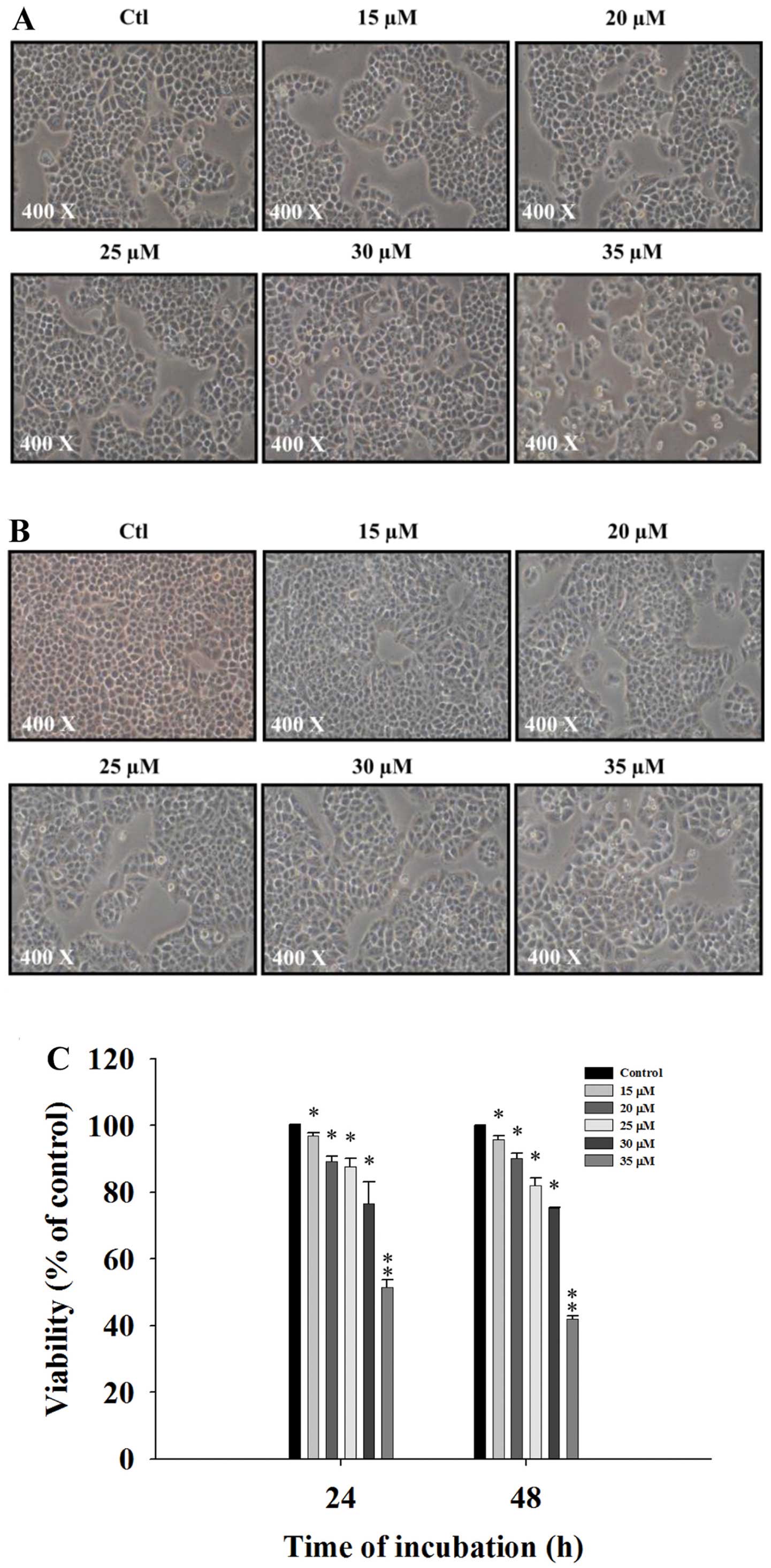
DMC induces cell morphological changes
and decreases the cell viability of NCI-H460 cells
The NCI-H460 cells were treated with various
concentrations of DMC for 24 and 48 h and then were photographed to
examine morphological changes. The percentage of total viable cells
was then determined. DMC significantly induced cell morphological
changes in a concentration-dependent manner and these changes were
based on an increase in cell death and debris (Fig. 1A and B). The flow cytometric assay
indicated that DMC decreased the percentage of viable cells in a
concentration-dependent manner (Fig.
1C).
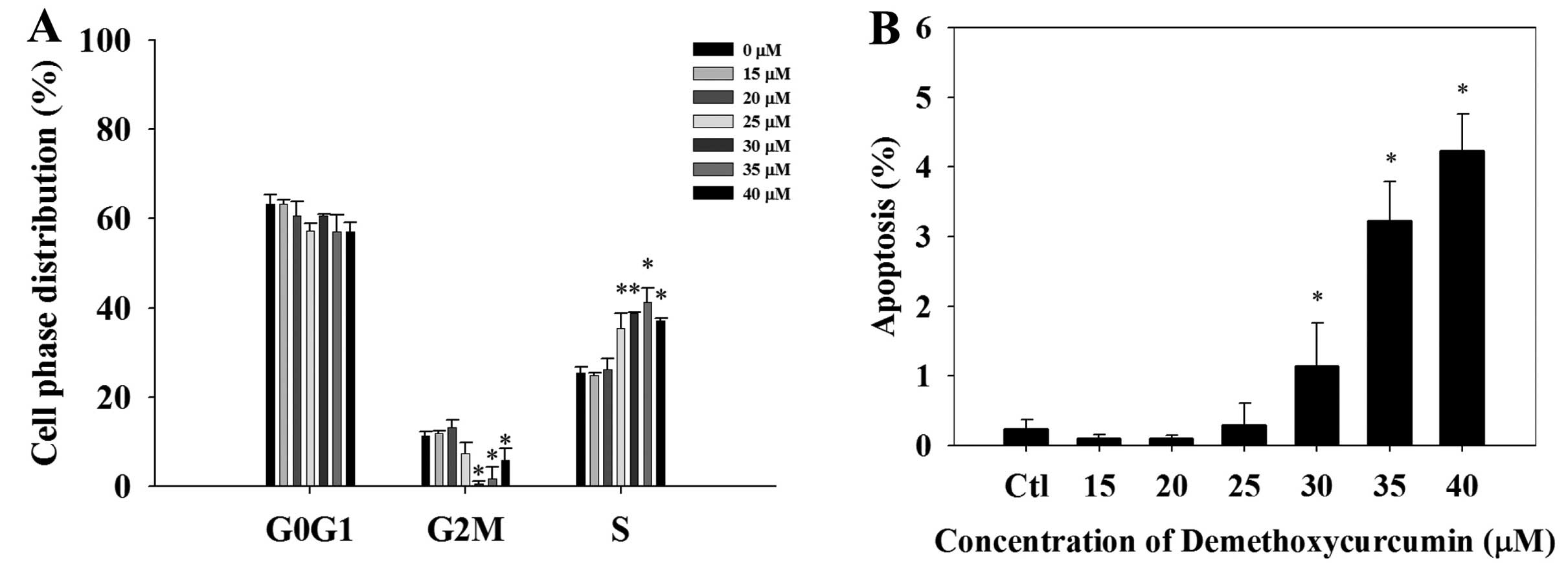
Cell cycle arrest and apoptosis of the
NCI-H460 cells after treatment with DMC
The cell cycle distribution of the NCI-H460 cells
after treatment with 0, 15, 20, 25, 30, 35 and 40 μM of DMC
for 48 h is shown in Fig. 2A. Cell
cycle arrest appeared to occur at the S stage after exposure to
DMC. The sub-G1 peak, indicating the proportion of apoptosis,
increased in a dose-dependent manner when the concentration of DMC
was increased (Fig. 2B).
Effects of DMC on ROS production, ΔΨm and
intracellular Ca2+ levels in the NCI-H460 cells
Following DMC treatment for different time
intervals, ROS (Fig. 3A) and
intracellular Ca2+ (Fig.
3C) were significantly increased as compared with the control
group. In addition, there was a significant loss of ΔΨm after
treatment with 35 μM of DMC (Fig. 3B).
DMC promotes the activity of caspase-3,
-8 and -9 in the NCI-H460 cells
After treatment with DMC for different durations,
the NCI-H460 cells exhibited increased caspase-3 activity. The
caspase-3 activity reached its maximum when the duration of
treatment was 48 h. The caspase-8 and -9 activities in the NCI-H460
cells were also increased following treatment of DMC at 35
μM (Fig. 4). These effects
were time-dependent with the exception of the 24-h incubation,
which showed a reduction in activity compared with the 6-h
treatment.
DMC affects cell cycle arrest and levels
of apoptosis-associated proteins and protein translocation in the
NCI-H460 cells
In order to investigate whether DMC induces
apoptosis in NCI-H460 cells via changes in the cell cycle and
levels of apoptosis-associated proteins, the NCI-H460 cells were
treated with 35 μM of DMC for 0, 6, 24 and 48 h and then
levels of total proteins from the samples were quantitated and the
levels of apoptosis-associated proteins were measured by western
blotting. DMC significantly promoted the expression of p21 and p27
but reduced the expression of CDC25A, cyclin E, cyclin A and CDK2
(Fig. 5A). AIF, Endo G, PARP, Fas
ligand (Fas L) and Fas were also upregulated (Fig. 5B). Furthermore, DMC promoted
expression of ER stress-associated proteins GRP78, GADD153, IRE1β,
ATF6α, ATF6β, caspase-12 and -4 (Fig.
5C). Moreover, DMC promoted the expression of calpain 1
(Fig. 5C), which is associated with
apoptotic pathways. The results revealed that DMC induced apoptosis
in the NCI-H460 cells through caspase-, ER stress- and
mitochondrial-dependent pathways. The results from the confocal
laser microscopy also revealed that DMC promoted the release of AIF
(Fig. 6A), Endo G (Fig. 6B), ATF6β (Fig. 6C), IRE1α (Fig. 6D) and p-PERK (Fig. 6E) from the mitochondria to the
cytosol and nuclei, respectively.
 | Figure 5DMC affects the cell cycle and
expression of apoptosis-associated proteins in the NCI-H460 cells.
Cells were exposed to either vehicle or DMC (35 μM) for
various time periods. Cells were harvested and total proteins were
determined and then protein expression was determined by western
blotting as described in Materials and methods. (A) p21, p27,
CDC25A, cyclin A, cyclin E and CDK2. (B) AIF, Endo G, PARP, Fas-L
and Fas. (C) Calpain 1, ATF6α, ATF6β, IRE1β, GRP78, GADD153,
caspase-12 and -4. DMC, demethoxycurcumin; Fas L, Fas ligand. |
Discussion
Much evidence has shown that stimulating or inducing
tumor cell apoptosis is one possibility for tumor treatment in
patients with cancer. Although a few studies have shown that DMC
induces cell death and apoptosis in human cancer cells as described
previously, there is no study to show that DMC affects human lung
cancer cells. The results of the present study revealed that DMC
induced cell morphological changes (Fig. 1A and B) and decreased the percentage
of viable cells (Fig. 1C) via the
induction of the sub-G1 phase (apoptosis) and cell cycle arrest
(Fig. 2A). DMC-induced apoptosis in
the NCI-H460 cells was dose-dependent (Fig. 2B).
Dysregulation of the cell cycle is associated with
tumorigenesis (18). We found that
NCI-H460 cells were arrested at the S phase after treatment with
DMC (Fig. 2A). Downregulation of
CDK2, CDC25A, cyclin A and cyclin E as shown by western blotting
(Fig. 5A), may be involved in the
mechanism of this arrest. Based on the findings, DMC may exert its
anticancer effects on NCI-H460 cells through both cell cycle arrest
and apoptotic induction.
To further examine the molecular mechanism of DMC in
NCI-H460 cells, we used flow cytometry and found that DMC
significantly decreased the levels of ΔΨm (Fig. 3B) following a 24-h treatment and
increased ROS and Ca2+ levels (Fig. 3A and C) at all treatment time
periods. DMC also promoted the activities of caspase-3, -9 and -8
(Fig. 4).
Numerous studies have demonstrated that apoptotic
cell death can occur through the extrinsic or the intrinsic
apoptotic pathway (20–22). The agents (Fas L) connected with Fas
(CD95) then trigger the extrinsic pathway followed by the
activation of caspase-8 and then activation of effector caspase-3
to cause cell apoptosis (23).
Thus, we hypothesized that the extrinsic apoptotic pathway in
NCI-H460 cells was activated following exposure to DMC. Thus, we
used western blotting to examine the expression of FAS/CD95 in the
NCI-H460 cells. The results revealed that DMC increased the
expression of FAS/CD95 and Fas L (Fig.
5B) accompanied by increased caspase-8 activity (Fig. 4B).
It is currently known that cancer cell survival or
death following exposure to anticancer drugs is associated with
mitochondrial function. Thus, anticancer drugs may induce cancer
cell apoptosis through mitochondrial-dependent and -independent
pathways. Anticancer drugs may induce mitochondrial dysfunction in
cells via dissipation of ΔΨm leading to liberation of numerous cell
death proteins from the mitochondria (24). Therefore, it was reported that the
intrinsic pathway depends on the dysfunction of the mitochondria
resulting from an increase in the ratio of Bak:Bcl-2 which is
caused by anticancer drugs thus leading to AIF and Endo G release
from the mitochondria before inducing apoptosis (25–27)
which is termed caspase-independent pathways or alternatively
causing cytochrome c release, activation of caspase-9 and -3
resulting in apoptosis termed the caspase-dependent pathway
(28). Our results showed that DMC
induced mitochondrial dysfunction (decreased levels of ΔΨm)
(Fig. 3B) and increased caspase-8,
-9 and -3 activity (Fig. 4) and
increased the protein levels of AIF and Endo G (Fig. 5B) in the NCI-H460 cells. We also
used confocal laser microscopy to confirm that DMC increased the
expression of AIF and Endo G (Fig. 6A
and B). Based on the findings, we suggest that DMC induced
apoptosis in NCI-H460 cells through the mitochondrial-dependent and
-independent pathways.
DMC induced ROS production in the NCI-H460 cells
(Fig. 3A) and ROS have been shown
to be involved in cell growth and apoptosis. An appropriate level
of intracellular ROS promotes cellular proliferation (29), while excessive production of ROS may
cause oxidative stress leading to cell apoptosis (29,30).
It has been reported that ROS may induce ER stress
which leads to the release of stored Ca2+ from the ER
leading to mitochondrial Ca2+ loading from ER stores
(31). Both ROS and ER
Ca2+ are required to initiate mitochondrial dysfunction
(31). Our results revealed that
DMC increased the ROS and Ca2+ production (Fig. 3A and C). Furthermore, it was
reported that mitochondria take up Ca2+ and initiate
apoptosis through opening of their permeability transition pores
(32). This then results in the
release of cytochrome c from the mitochondrial membrane
which then activates caspase-9 and triggers the effector caspase-3
for causing apoptosis (33). It was
reported that markers of ER stress such as transcriptionally
induced GRP78 and GADD153 are produced (31). We found that DMC increased the
protein levels of GRP78, GADD153, IRE1β, ATF6α, ATF6β, caspase-12
and -4 and the expression of calpain 1 (Fig. 5C), that are associated with
apoptosis pathways.
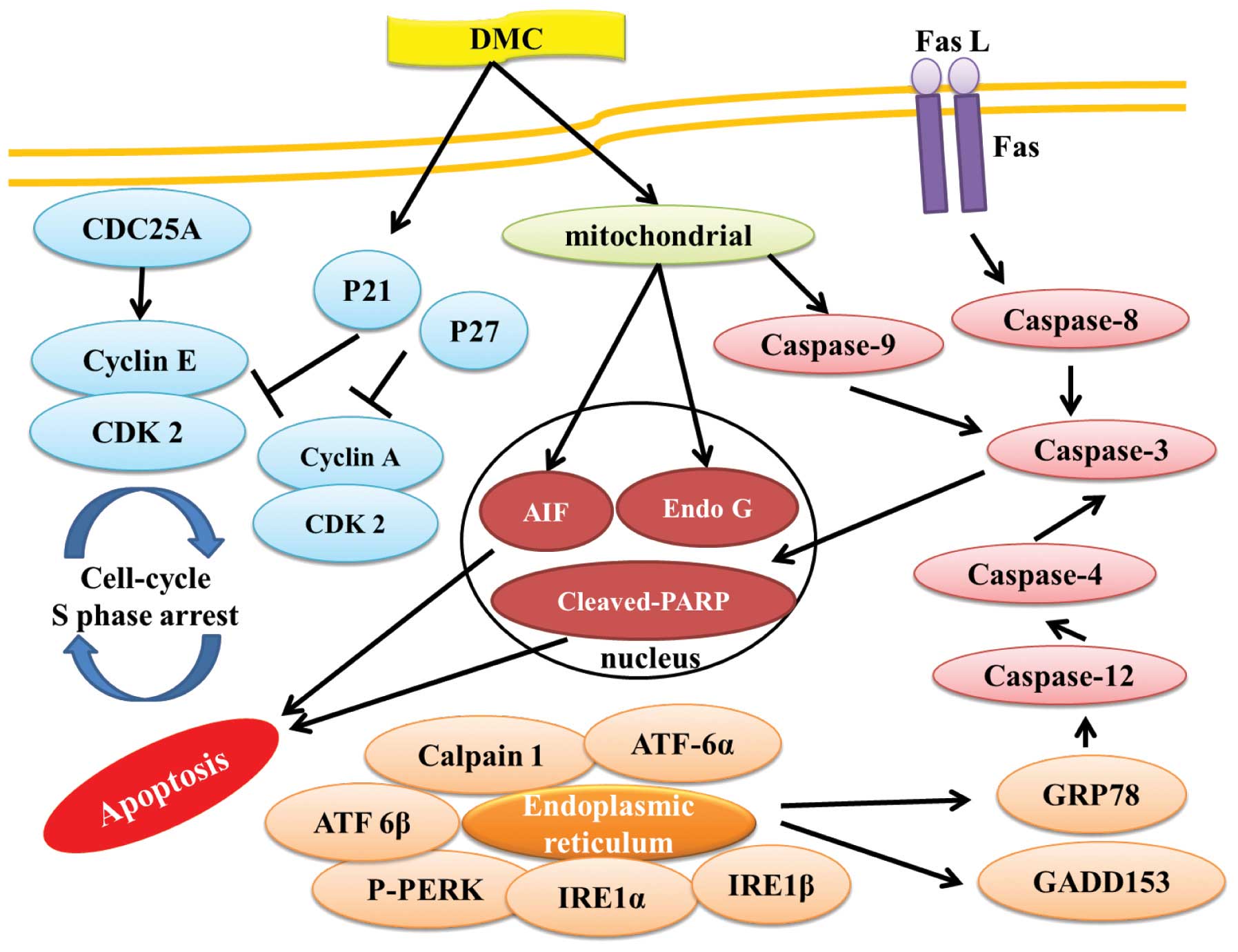
In conclusion, DMC induced cell death (cytotoxic
effect) in human lung cancer NCI-H460 cells, mediated through the
induction of phase S cell cycle arrest by inhibition of the
check-point proteins CDC25A, cyclin A, cyclin E and CDK2 and
induced cell apoptosis associated with caspase-or
mitochondrial-dependent pathways. Furthermore, DMC induced cell
apoptosis also through cross-talk between the extrinsic and the
intrinsic pathway as summarized in Fig.
7. DMC appears to have multiple molecular targets, and its
application in the treatment of lung cancer patients warrants
further investigation.
Acknowledgments
This study was supported in part by a research grant
from China Medical University (no. CMU102-ASIA-20). Experiments and
data analysis were performed in part through the use of the Medical
Research Core Facilities Center, Office of Research and Development
at China Medical University, Taichung, Taiwan, R.O.C.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Non-small Cell Lung Cancer Collaborative
Group: Chemotherapy in non-small cell lung cancer: a meta-analysis
using updated data on individual patients from 52 randomised
clinical trials. BMJ. 311:899–909. 1995. View Article : Google Scholar
|
|
3
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jayaprakasha GK, Jagan Mohan Rao L and
Sakariah KK: Improved HPLC method for the determination of
curcumin, demethoxycurcumin, and bisdemethoxycurcumin. J Agric Food
Chem. 50:3668–3672. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Balasubramanyam M, Koteswari AA, Kumar RS,
Monickaraj SF, Maheswari JU and Mohan V: Curcumin-induced
inhibition of cellular reactive oxygen species generation: novel
therapeutic implications. J Biosci. 28:715–721. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan WH, Wu HJ and Hsuuw YD: Curcumin
inhibits ROS formation and apoptosis in methylglyoxal-treated human
hepatoma G2 cells. Ann NY Acad Sci. 1042:372–378. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Priyadarsini KI, Maity DK, Naik GH, Kumar
MS, Unnikrishnan MK, Satav JG and Mohan H: Role of phenolic O-H and
methylene hydrogen on the free radical reactions and antioxidant
activity of curcumin. Free Radic Biol Med. 35:475–484. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahmed T and Gilani AH: Inhibitory effect
of curcuminoids on acetylcholinesterase activity and attenuation of
scopolamine-induced amnesia may explain medicinal use of turmeric
in Alzheimer’s disease. Pharmacol Biochem Behav. 91:554–559. 2009.
View Article : Google Scholar
|
|
9
|
Luthra PM, Kumar R and Prakash A:
Demethoxycurcumin induces Bcl-2 mediated G2/M arrest and apoptosis
in human glioma U87 cells. Biochem Biophys Res Commun. 384:420–425.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yodkeeree S, Chaiwangyen W, Garbisa S and
Limtrakul P: Curcumin, demethoxycurcumin and bisdemethoxycurcumin
differentially inhibit cancer cell invasion through the
down-regulation of MMPs and uPA. J Nutr Biochem. 20:87–95. 2009.
View Article : Google Scholar
|
|
11
|
Tamvakopoulos C, Dimas K, Sofianos ZD,
Hatziantoniou S, Han Z, Liu ZL, Wyche JH and Pantazis P: Metabolism
and anticancer activity of the curcumin analogue,
dimethoxycurcumin. Clin Cancer Res. 13:1269–1277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JW, Hong HM, Kwon DD, Pae HO and Jeong
HJ: Dimethoxycurcumin, a structural analogue of curcumin, induces
apoptosis in human renal carcinoma caki cells through the
production of reactive oxygen species, the release of cytochrome c,
and the activation of caspase-3. Korean J Urol. 51:870–878. 2010.
View Article : Google Scholar
|
|
13
|
Anuchapreeda S, Tima S, Duangrat C and
Limtrakul P: Effect of pure curcumin, demethoxycurcumin, and
bisdemethoxycurcumin on WT1 gene expression in leukemic cell lines.
Cancer Chemother Pharmacol. 62:585–594. 2008. View Article : Google Scholar
|
|
14
|
Ji BC, Hsu WH, Yang JS, et al: Gallic acid
induces apoptosis via caspase-3 and mitochondrion-dependent
pathways in vitro and suppresses lung xenograft tumor growth in
vivo. J Agric Food Chem. 57:7596–7604. 2009. View Article : Google Scholar
|
|
15
|
Lin SY, Lai WW, Ho CC, et al: Emodin
induces apoptosis of human tongue squamous cancer SCC-4 cells
through reactive oxygen species and mitochondria-dependent
pathways. Anticancer Res. 29:327–335. 2009.PubMed/NCBI
|
|
16
|
Gorczyca W, Melamed MR and Darzynkiewicz
Z: Laser scanning cytometer (LSC) analysis of fraction of labelled
mitoses (FLM). Cell Prolif. 29:539–547. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsia TC, Yang JS, Chen GW, et al: The
roles of endoplasmic reticulum stress and Ca2+ on
rhein-induced apoptosis in A-549 human lung cancer cells.
Anticancer Res. 29:309–318. 2009.PubMed/NCBI
|
|
18
|
Diehl JA: Cycling to cancer with cyclin
D1. Cancer Biol Ther. 1:226–231. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu DD, Ye YL, Zhang J, Xu JN, Qian XD and
Zhang Q: Distinct pro-apoptotic properties of Zhejiang saffron
against human lung cancer via a caspase-8-9-3 cascade. Asian Pac J
Cancer Prev. 15:6075–6080. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar S: Caspase function in programmed
cell death. Cell Death Differ. 14:32–43. 2007. View Article : Google Scholar
|
|
21
|
Wilson MR: Apoptotic signal transduction:
Emerging pathways. Biochem Cell Biol. 76:573–582. 1998. View Article : Google Scholar
|
|
22
|
Xu G and Shi Y: Apoptosis signaling
pathways and lymphocyte homeostasis. Cell Res. 17:759–771. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HJ, Yang KM, Park YS, Choi YJ, Yun JH,
Son CH, Suh HS, Jeong MH and Jo WS: The novel resveratrol analogue
HS-1793 induces apoptosis via the mitochondrial pathway in murine
breast cancer cells. Int J Oncol. 41:1628–1634. 2012.PubMed/NCBI
|
|
24
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baek SH, Bae ON, Kim EK and Yu SW:
Induction of mitochondrial dysfunction by poly(ADP-ribose) polymer:
implication for neuronal cell death. Mol Cells. 36:258–266. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu KC, Huang YT, Wu PP, Ji BC, Yang JS,
Yang JL, Chiu TH, Chueh FS and Chung JG: The roles of AIF and Endo
G in the apoptotic effects of benzyl isothiocyanate on DU 145 human
prostate cancer cells via the mitochondrial signaling pathway. Int
J Oncol. 38:787–796. 2011.PubMed/NCBI
|
|
28
|
Yoo JO, Lim YC, Kim YM and Ha KS:
Transglutaminase 2 promotes both caspase-dependent and
caspase-independent apoptotic cell death via the calpain/Bax
protein signaling pathway. J Biol Chem. 287:14377–14388. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hajnóczky G, Csordás G, Das S,
Garcia-Perez C, Saotome M, Sinha Roy S and Yi M: Mitochondrial
calcium signalling and cell death: approaches for assessing the
role of mitochondrial Ca2+ uptake in apoptosis. Cell
Calcium. 40:553–560. 2006. View Article : Google Scholar
|
|
31
|
Jacobson J and Duchen MR: Mitochondrial
oxidative stress and cell death in astrocytes - requirement for
stored Ca2+ and sustained opening of the permeability
transition pore. J Cell Sci. 115:1175–1188. 2002.PubMed/NCBI
|
|
32
|
Garrido C, Galluzzi L, Brunet M, Puig PE,
Didelot C and Kroemer G: Mechanisms of cytochrome c release from
mitochondria. Cell Death Differ. 13:1423–1433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rao RV, Ellerby HM and Bredesen DE:
Coupling endoplasmic reticulum stress to the cell death program.
Cell Death Differ. 11:372–380. 2004. View Article : Google Scholar : PubMed/NCBI
|