Introduction
Cervical cancer is associated with high incidence
and mortality. It is the second most common malignancy worldwide
among females, and is the seventh most common cancer overall, with
more than 527,000 new cases diagnosed in 2012 (8% of female cases
and 4% of the total). Standard treatment includes surgical
treatment, radiotherapy and chemotherapy. The side-effects of
chemotherapy are adverse, such as cisplatin, which is associated
with serious side-effects and the development of resistance;
therefore, additional strategies for the treatment of cervical
cancer are urgently required (1).
Tumor angiogenesis plays an important role in the
processes of tumor growth, invasion and metastasis.
Platelet-derived growth factor (PDGF) and other growth factors,
particularly vascular endothelial growth factor (VEGF), promote
tumor angiogenesis and tumor invasion through autocrine and/or
paracrine mechanisms. In pancreatic cancer cell lines, PDGF-B
specifically binds to the receptor, PDGFR-β, to activate the
Notch-1 and NF-κB signaling pathway. This results in increased
expression of VEGF, which promotes tumor angiogenesis (2). Thus, downregulation of PDGF-B
expression may effectively inhibit the proangiogenic effects of
VEGF.
Interleukin (IL)-24, also known as melanoma
differentiation-associated gene-7, has broad-spectrum antitumor
activity in a variety of tumors such as lung (3), ovarian (4) and breast cancers (5). It has been shown to exert significant
growth inhibition and apoptosis induction effects, with no obvious
adverse effects on normal cells (6).
Our preliminary studies showed that delivery of
exogenous IL-24 using the cationic liposome-mediated method for
transfection with the recombinant plasmid pDC316- IL-24
significantly augmented the sensitivity of tumors to cisplatin in a
nude mouse cervical cancer xenograft model (7). However, the mechanism underlying this
effect remains to be elucidated.
In the present study, the synergistic inhibitory
effects of the recombinant plasmid pDC316-hIL-24 and cisplatin on
angiogenesis and lymphangiogenesis were investigated in a cervical
cancer xenograft model established in nude mice. Furthermore, the
effects on the expression of VEGF-C/VEGFR-3 and PDGF-B and
microvessel density in the cervical cancer xenografts were
investigated to clarify the mechanism. This information will be
important in developing improved strategies for the treatment of
cervical cancer.
Materials and methods
Cell culture
Human cervical carcinoma HeLa cells were provided by
the Cell Center of the Union Medical College (Beijing, China).
Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)
high glucose-complete medium at 37°C in a 5% CO2
incubator. Logarithmic growth phase cells were collected, and the
cell number was adjusted to 1×107/ml.
Use and care of experimental animals
Specific pathogen-free (SPF) female nude mice
(BALB/c; 13–15 g, aged 4 weeks) were obtained from the Shanghai
Silaike Experimental Animal Co., Ltd. (Shanghai, China). Mice were
maintained in pathogen-free conditions and used for study in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals. All surgical procedures and
care administered to the animals were approved by the Institutional
Ethics Committee.
Ethics statement
All surgical procedures and care administered to the
animals were approved by the Institutional Ethics Committee.
Evaluation of the benefits for the
experimental animals
i) The experiment, in which the needs of the animals
were fully considered, was approved by the ethics committee,
including physiological (adequate food, water, temperature and
illumination), environmental, psychological and social needs
(socially raised, 4–6 animals per cage, avoid tiredness and
overstimulation). The outcomes of the preliminary experiment and
the literature were taken into consideration to make rational
design of the sample size and operation standard. ii) A daily
observation was preformed to prevent the animals from anger,
comfortlessness, fear, nervousness, pain or damage and to keep them
at normal status. Abuse, excessive or incorrect medication was
avoided. For subcutaneous injection, which was easy to operate,
narcotics were not applicable; for tail vein injection, which was
not easy to operate, intraperitoneal anesthesia was given to
alleviate the pain of the animals. iii) At the time of endpoint,
the animals were sacrificed within 15 sec to avoid the nervousness
of the other animals.
Xenograft animal model and grouping
The xenograft tumor model was established by
subcutaneous (s.c.) injection of ~1×106 HeLa cells (100
μl suspension) into the left axillary lateral of each nude
mouse. The model was deemed to be established successfully
following the appearance of xenografted tumors after 7 days and
with tumor diameters of ~4–5 mm after 14 days. Subsequently,
successfully xenografted mice were randomly divided into six groups
(n=6 per group): i) PBS buffer control group: 100 μl
injected once per 3 days for a total of five times; ii) empty
plasmid group: empty plasmid pDC316 (Benyuan Zhengyang Gene
Technology Co., Beijing, China); 100 mg/l, 100 μl once per 3
days, for a total of five times) and liposome Lipofectamine 2000
(Invitrogen, Shanghai, China; 25 μl for transfection); iii)
half-dose DDP (cisplatin) group: intraperitoneal (i.p.) injection
of cisplatin (Qilu Pharmaceutical Co., Ltd., Jinan, China; 2.5
mg/kg, 100 μl once a day for 3 days); iv) IL-24 group: local
multipoint intratumoral injection of pDC316-hIL-24 (Benyuan
Zhengyang Gene Technology Co.); 100 mg/l, 100 μl once per 3
days for a total of five times) and liposome Lipofectamine 2000 (25
μl for transfection); v) full-dose DDP (cisplatin) group
(DDP group): i.p. injection of cisplatin (5 mg/kg, 100 μl
i.p. once a day for a total of 3 days; and vi) combined treatment
group: cisplatin (2.5 mg/kg, 100 μl i.p. once a day for 3
days) followed by local multipoint intratumoral injection of
pDC316-hIL-24 (100 mg/l, 100 μl once per 3 days for a total
of five times). The tumor size (L) and short diameter (W) were
measured every 3 days with a vernier caliper. Weight and the
overall status of the mice were recorded at the same time. Nude
mice were sacrificed by cervical dislocation at 2 weeks after the
cessation of treatment. Tumor volume was measured and weighed; and
the inhibition of tumor weight was calculated. Tumor size V
(mm3) = L × W2/2.
RT-PCR analysis of IL-24 expression
Determination of pDC316-hIL-24 expression in the
xenografted tumors was performed using PrimeScript™ First Strand
cDNA synthesis kit (Takara Biotechnology, Dalian, China). Primers
of IL-24 were: forward, 5′-GCCAAGCTTATGAATTTTCAACAGA GG-3′ and
reverse, 5′-GCCGTCGACCTAGAGCTTGTAGAATTT-3′. Expression of GADPH was
analyzed as a control using the following primers: forward,
5′-TGAACGGGAAGCTC ACTGG-3′ and reverse, 5′-TCCACCACCCTGTTGCTGGA-3′.
The amplification was performed using the Applied Biosystems
GeneAmp PCR system 2700 with the following reaction conditions: a
94°C pre-denaturation for 2 min, followed by 32 cycles of a 94°C
denaturation for 20 sec, a 58°C annealing for 20 sec and a 72°C
extension for 20 sec, with a final extension at 72°C for 8 min. PCR
products were separated by 1.5% agarose gel electrophoresis.
Immunohistochemical detection of VEGF,
VEGF-C, VEGFR-3 and P-CK
After dewaxing, hydration and antigen retrieval,
VEGF expression was detected by immunohistochemical staining with
primary and secondary detection antibodies (Boster, Wuhan, China).
Immunoreactivity was visualized by incubation with DAB chromogenic
agent DAB kit (Zhongshan Jinqiao Biological Engineering Co.,
Beijing, China). Staining was evaluated in 10 randomly selected
fields per section, with intensity divided into four levels as
follows: 0, no staining; 1, pale yellow; 2, brown; and 3, tan.
Scores were assigned according to the percentage of positive cells
in each field of vision as follows: 10%, 0 points; 11–20%, 1 point;
21–60%, 2 points; and 61–100%, 3 points. The average staining
intensity score (IS) was calculated for each section based on
Bresalier’s semiquantitative method (8). IS represents the average multiplied
product of the staining intensity score and the positive cell score
per 10 randomly selected fields of vision in each section.
Immunohistochemical (IHC) detection of
CD34 for microvessel density (MVD) enumeration
Solid tumor growth and metastasis require sustained
angiogenesis, the extent of which is indicated by MVD. Microvessel
enumeration can be achieved by IHC labeling of CD34, which is
expressed stably and specifically by tumor capillaries and
endothelial cells (9).
Immunohistochemical detection of CD34, specimen preparation and
immunohistochemical staining procedures were performed using an SP
kit according to the manufacturer’s instructions. Subsequently,
areas rich in vasculature tissue (known as hot spots) were
identified by visualization of the highest density of yellow-brown
areas in whole sections under a light microscope (magnification,
×100). MVD was calculated at higher magnification microscopy (×400)
using the following criteria (10):
any brown-stained endothelial cells or cell clusters were recorded
as a separate vessel, with clear demarcation between all the
vessels. MVD was calculated as the average number of microvessels
in five randomly selected fields of vision.
Western blot analysis
Analysis of PDGF-B protein levels in tumor tissue
was performed using WesternBreeze® Chemiluminescent kit
(Life Technologies, Grand Island, NY, USA) according to the
manufacturer’s instructions. Tumor tissue protein was extracted,
and the concentrations were determined. After SDS-PAGE
electrophoresis, it was transferred to a membrane, and immune
response and exposure were determined (anti-mouse PDGF-B primary
antibody; BioWorld Products, Visalia, CA, USA). The analysis of
experimental results was performed using Quantity One image
analysis software.
Statistical methods
SPSS 16.0 statistical software was used for
statistical analysis. Multi-group comparisons were performed using
ANOVA after the test of normality. t-tests were used for
comparisons between two groups. Bonferroni corrections were used if
necessary. Non-normal data were compared using SNK tests. Data are
presented as means ± SD. A P-value <0.05 was considered to
indicate a statistical significant result.
Results
Inhibition of tumor growth and weight
loss of nude mice
Mice in the cisplatin groups (full-dose and
half-dose) exhibited poor mental state and reduced feeding. The
overall status of mice in the IL-24 and half-dose DDP+IL-24 groups
was better than that in the DDP full-dose group. There were no
adverse changes in the mental status and feeding habits of nude
mice in the IL-24 group (data not shown).
The changes in tumor volumes after the different
interventions are shown in Table I.
The tumor volumes were subjected to two-factor analysis of
variance. Results of Mauchly’s test of sphericity did not support
the ‘spherical symmetry’ hypothesis (P<0.001); therefore, the
coefficient was corrected using Greenhouse-Geisser, Huynh-Feldt and
Lower-bound calibrations, and the test results showed statistical
significant P<0.01 after calibration. Compared to the control
groups (PBS and empty vector), tumor growth was significantly
reduced following treatment with cisplatin and/or recombinant IL-24
plasmid. These effects were more marked following treatment with
the IL-24 recombinant plasmid and the combined therapy, which
mediated almost complete inhibition of tumor growth. The difference
was statistically significant at different time-points within the
same group (P<0.001) as well as among different groups
(P<0.001), and there were interaction effects between time and
treatment. There were significant differences between groups (all
P-values <0.05) except between the control group and empty
vector group, between the IL-24 group and half-dose DDP+IL-24, and
between the full-dose and half-dose DDP groups (P>0.05).
 | Table IWeight change of nude mice (g) and
tumor volume change (mm3) after intervention (mean ±
SD). |
Table I
Weight change of nude mice (g) and
tumor volume change (mm3) after intervention (mean ±
SD).
| After therapy
(days) | Control group | Empty plasmid
group | Half-dose DDP
group | IL-24 group | Full-dose DDP
group | Half-dose DDP+IL-24
group |
|---|
| Weight change of nude
mice (g) |
| 0 | 20.2±0.26 | 20.1±0.19 | 20.0±0.31 | 20.1±0.34 | 20.0±0.36 | 20.0±0.44 |
| 3 | 20.6±0.23 | 20.5±0.15 | 19.8±0.30 | 21.2±0.28 | 19.6±0.36 | 19.7±0.29 |
| 6 | 21.4±0.29 | 21.4±0.22 | 19.4±0.57 | 20.7±0.32 | 18.1±1.20 | 19.2±0.46 |
| 9 | 21.9±0.43 | 22.2±0.26 | 19.2±0.73 | 20.7±0.27 | 16.7±1.53 | 19.0±0.68 |
| 12 | 22.5±0.48 | 22.8±0.31 | 18.8±0.48 | 20.5±0.20 | 15.9±1.02 | 18.6±0.72 |
| 15 | 23.3±0.55 | 23.7±0.42 | 18.4±0.47 | 21.1±0.39 | 15.6±0.93 | 18.2±0.79 |
| 18 | 24.0±0.74 | 24.4±0.34 | 18.4±0.52 | 21.2±0.27 | 15.3±0.88 | 18.6±0.72 |
| 21 | 24.6±0.63 | 24.9±0.27 | 18.3±0.66 | 21.3±0.31 | 15.6±1.03 | 18.5±0.59 |
| 24 | 24.9±0.73 | 25.1±0.98 | 18.1±0.35 | 22.0±0.35 | 17.1±0.78 | 18.6±0.41 |
| 27 | 25.3±0.40 | 24.9±1.13 | 18.2±1.04 | 20.9±0.20 | 16.9±0.68 | 18.8±0.32 |
| Tumor volume change
(mm3) |
| 0 | 189.5±47.8 | 210.5±95.9 | 176.9±100.6 | 200.1±79.4 | 236.1±135.6 | 216.1±54.0 |
| 3 | 446.3±73.8 | 453.8±125.3 | 319.9±73.8 | 172.9±63.0 | 354.0±105.8 | 216.3±74.6 |
| 6 | 684.1±95.8 | 710.7±139.4 | 559.2±104.8 | 175.0±47.4 | 462.3±103.8 | 298.2±42.7 |
| 9 | 914.6±131.7 | 1,015.8±191.4 | 581.6±90.0 | 199.1±50.6 | 573.3±125.8 | 319.2±37.8 |
| 12 | 1,353.3±324.9 | 1,335.0±347.1 | 540.8±108.3 | 328.8±45.3 | 702.2±148.5 | 355.8±47.2 |
| 15 | 1,532.0±230.6 | 1,738.3±164.0 | 710.6±241.1 | 452.2±41.4 | 698.7±190.3 | 367.3±125.0 |
| 18 | 2,020.0±213.2 | 1,940.5±336.2 | 1,075.1±402.9 | 529.2±56.0 | 941.3±271.1 | 360.1±116.2 |
| 21 | 2,185.5±262.3 | 2,147.1±214.2 | 1,131.6±422.3 | 582.7±52.8 | 1,031.5±161.9 | 335.6±117.3 |
| 24 | 2,609.1±144.7 | 2,655.0±171.1 | 1,277.9±455.4 | 616.9±28.5 | 1,201.3±253.1 | 381.4±114.9 |
| 27 | 2,978.3±68.6 | 3,007±235.4 | 1,906.3±514.3 | 613.1±44.5 | 1,446.1±675.9 | 281.4±114.9 |
Tumor growth was faster in the two control groups,
leading to overall weight gain. Cisplatin is associated with
serious side-effects. The weight changes of nude mice after
intervention are also shown in Table
I. Mauchly’s test of sphericity was performed and weight
changes in the nude mice also refused the‘spherical symmetry’
hypothesis. The difference was statistically significant at
different time-points within the same group (P<0.001) as well as
among different groups (P<0.001), and there were interaction
effects between time and treatment. Results of t-test with
Bonferroni corrections revealed that, there were significant
differences between groups (all P-values <0.05) except between
the control group and empty vector group, and between the half-dose
DDP and half-dose DDP+IL-24 groups (P>0.05).
IL-24 overexpression increases the
expression of VEGF and CD34 in the tumor tissues
Fig. 1A shows
expression of IL-24 using RT-PCR in xenograft tumors of the control
group, empty plasmid group, half-dose DDP group, IL-24 group,
full-dose DDP group, and half-dose DDP+IL-24 group. IL-24 was
expressed in the IL-24 group and half-dose DDP+IL-24 group. IHC
assays were performed to detect VEGF and CD34 expression in
xenograft tumors of the control, empty plasmid and IL-24 groups
(Fig. 1B and C). Fig. 1D revealed that there were
significant differences in VEGF and CD34 expression between the
control group and IL-24 group (P<0.001 and P=0.001,
respectively).
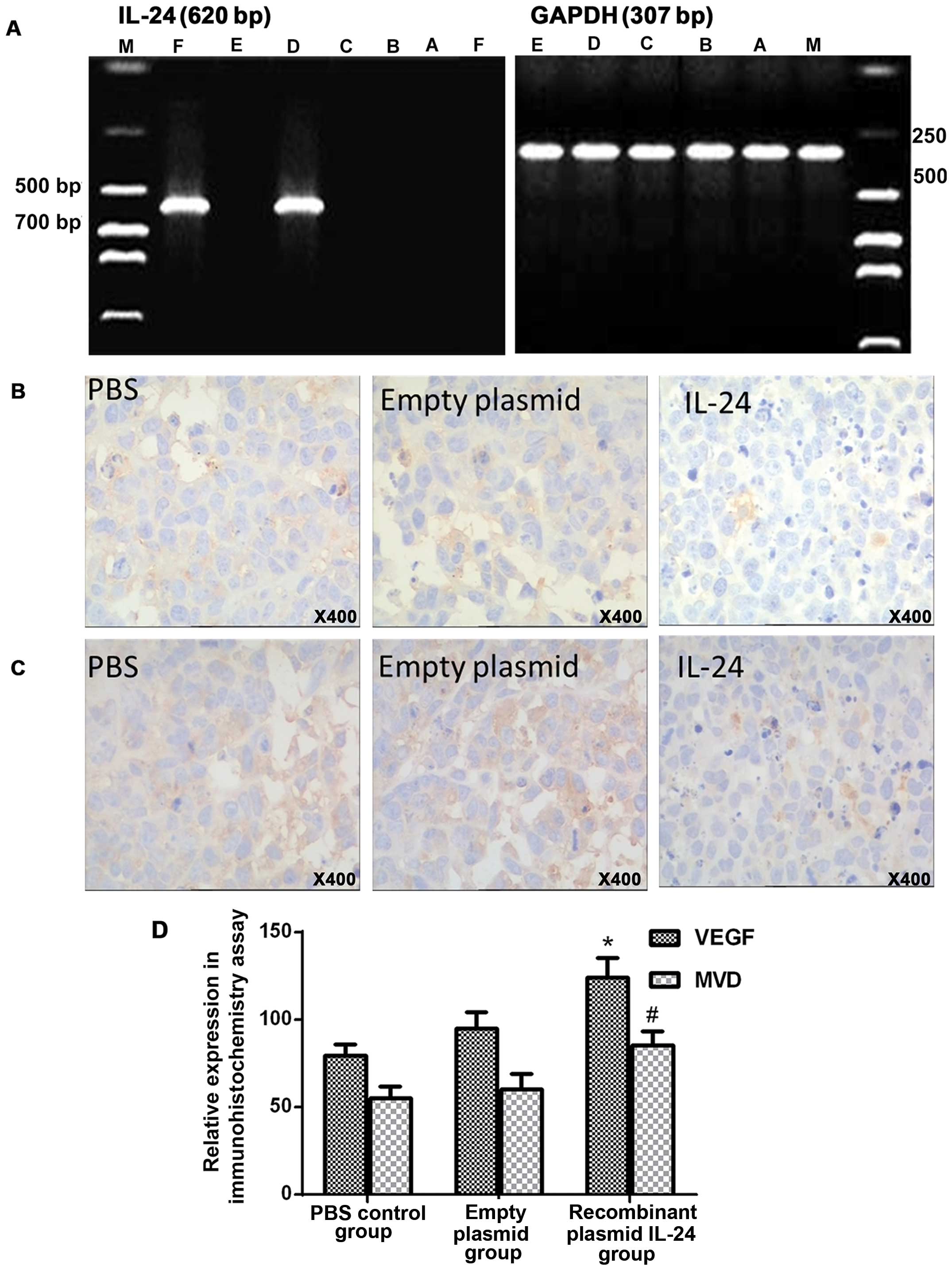 | Figure 1(A) Expression of IL-24 in the
xenografts. Lane M, DNA marker; lane A, control group; lane B,
empty plasmid group; lane C, half-dose DDP group; lane D, IL-24
group; lane E, full-dose DDP group; lane F, half-dose DDP+IL-24
group. Immunohistochemical detection of expression of (B) VEGF
(magnification, ×400) and (C) CD34 (magnification, ×400). (D)
Comparison of intensity scores of panel B and C.
*,#P<0.05 compared with corresponding control
groups. |
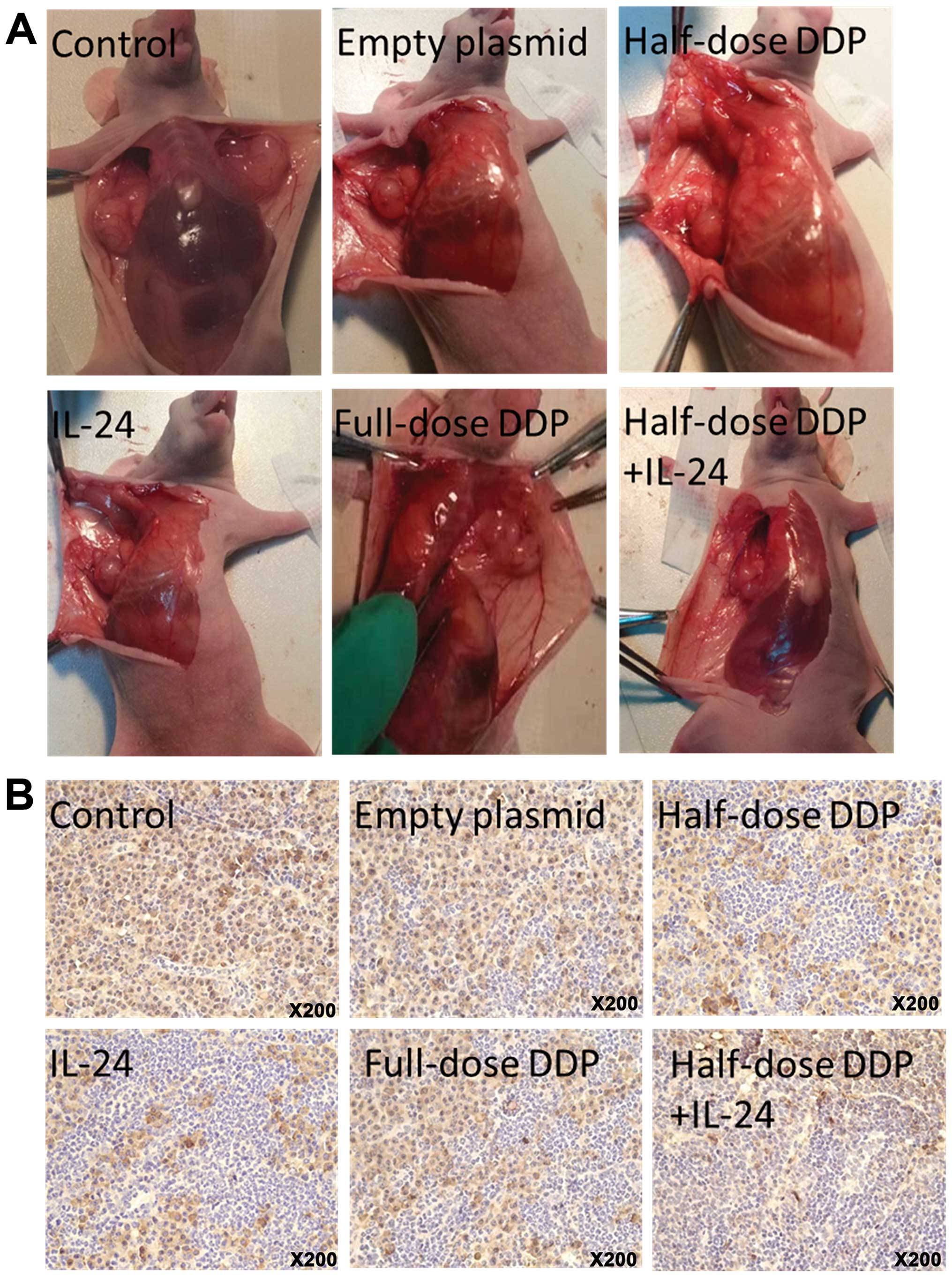
Tumor lymph node metastasis
Visible tumor metastatic lymph node was found near
the axilla, beside abdominal aortas and in the neck and groin
(Fig. 2A). Lymph node metastasis
results in the IL-24, cisplatin, combined therapy, empty plasmid
and control group mice were 1/6, 4/6, 1/6, 5/6 and 5/6,
respectively. Lymph node metastasis was significantly different
between the five groups (χ2=11.25, P=0.024). Lymph node
metastasis was significantly reduced in the IL-24 and half-dose
DDP+IL-24 groups compared with the other groups (P<0.05).
As a marker of tumor cell infiltration, P-CK
expression was detected using IHC as brown or tan granules in the
cytoplasm of cancer cells (Fig.
2B). Cancer cells in the control and empty plasmid groups were
typically observed as hyperchromatic cells with a large nucleus.
Tumor cells were arranged in clusters or cords; the volume of tumor
cells was large with visible mitotic phase, while the lymphocytes
and neutrophils were less. HeLa cell invasion was significantly
lower in the IL-24 group compared with that of the control groups.
The majority of the infiltrate in the half-dose DDP+IL-24 group
comprised lymphocytes and neutrophils, while only minor
infiltration and necrosis, larger cells, loose cytoplasm and marked
nuclear staining were observed. HeLa cell infiltration was less
marked in the cisplatin group, although large nuclei were still
observed.
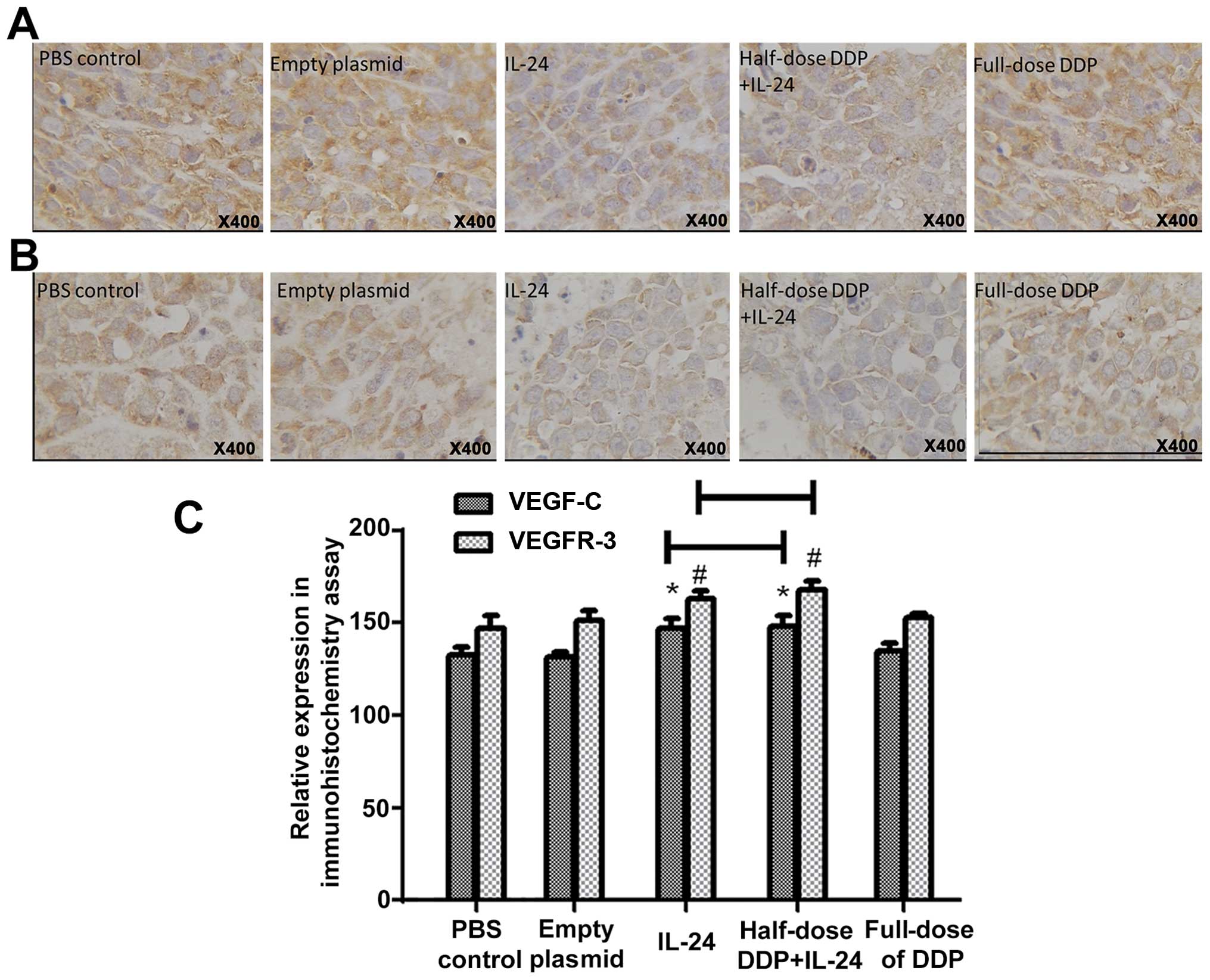
VEGF-C and VEGFR-3 protein expression as
markers of lymphangiogenesis
Expression of VEGF-C and VEGFR-3 was predominantly
detected in the cytoplasm of the cancer cells as brownish yellow
granules (Fig. 3A and B).
Univariate analysis of variance indicated statistically significant
differences in the average grayscale values of VEGF-C-positive
cells (F=17.49, P<0.001) and of VEGFR-3-positive cells (F=19.23,
P<0.001) between the five groups. The average grayscale values
of VEGF-C and VEGFR-3 in the IL-24 and half-dose DDP+IL-24 groups
were significantly higher than those in the full-dose DDP, control
and empty plasmid groups (P<0.001), while there were no
statistically significant differences between the latter three
groups (P>0.05) (Fig. 3C).
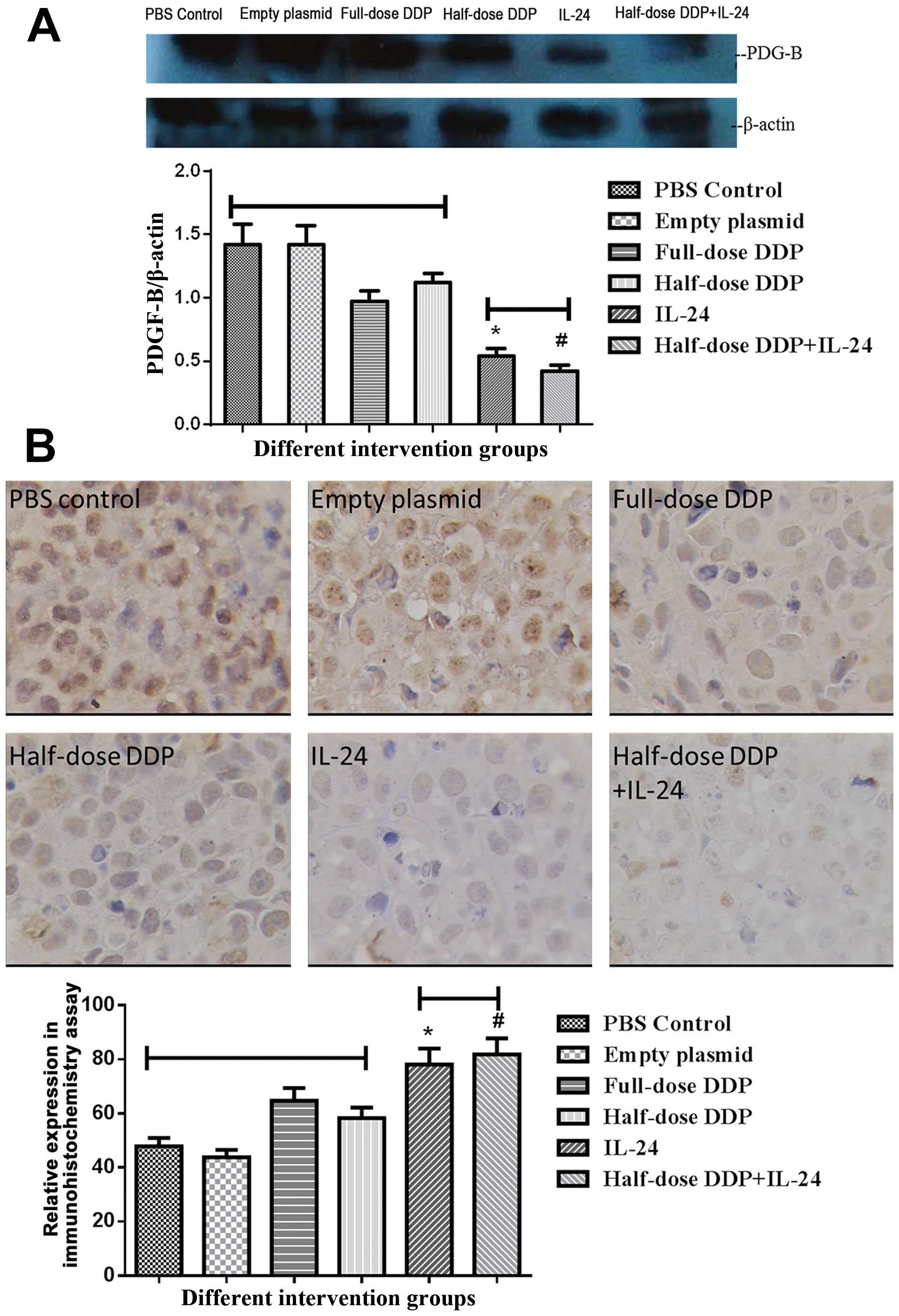
Western blotting and immunohistochemical
detection of PDGF-B protein expression
PDGF-B protein was detected in the xenografts of
each group by western blotting. Immunohistochemical analysis showed
high expression of PDGF-B in the tumor cells in the PBS group,
empty plasmid group, DDP group and half-dose DDP group, with no
significant differences between the four groups (P>0.05). The
levels were significantly reduced in the IL-24 and half-dose
DDP+IL-24 groups, compared with that in the other four groups
(P<0.001) (Fig. 4).
Discussion
Cisplatin is a commonly used chemotherapeutic agent
for cervical cancer. However, the development of resistance and
serious side-effects limit the efficacy of this approach.
Consequently, the identification of viable adjunct therapies and
dosing regimens that increase the sensitivity of tumor cells to the
antitumor effects of cisplatin are a current focus of research,
with the aim of reducing the effective dose of cisplatin and
avoiding its side-effects.
The antitumor activity of IL-24 has been
demonstrated in a number of cancers and has shown efficacy in
clinical trials for metastatic melanoma (11). Our previous study showed that
recombinant IL-24 expressed from the pDC316-hIL-24 vector in
combination with cisplatin inhibits the growth of xenografted
cervical cancer cell growth in a nude mouse model (7). In the present study, our results
obtained using the same animal model showed that recombinant IL-24
acts synergistically with cisplatin to inhibit tumor growth and
angiogenesis by downregulation of VEGF, VEGF-C and PDGF-B
expression.
Tumor angiogenesis plays an important role in the
processes of tumor growth, invasion and metastasis. Angiogenesis
depends mainly on the equilibrium between vascular endothelial cell
proliferation and inhibitory factors. VEGF, which has a direct
effect on vascular endothelial cell growth and vascular
permeability, is expressed at high levels in tumor tissue (12). Lymph node metastasis is an important
mechanism of cervical cancer invasion and is also an important
factor affecting the prognosis of patients. Recent evidence has
shown that PDGF, VEGF-C and its receptor VEGFR-3 are closely
correlated with angiogenesis, lymphangiogenesis and metastasis in a
variety of human malignancies. PDGF-B and its receptor are
overexpressed in many human malignant tumors, and its expression
level is correlated with tumor malignancy (13). Donnem et al (14) showed that suppression of PDGF-B
expression significantly reduced growth and promoted the apoptosis
of lung cancer cells in vitro. Expression of PDGF-B and
VEGFR3 is significantly correlated with lymph node metastasis and
prognosis of small cell lung cancer (15). Cao (16) reported that PDGF-B transfection
caused mouse fibroblast cells to induce lymphangiogenesis and
promote regional lymph node metastasis. Furthermore, PDGFR-B has
been shown to be specifically expressed at high levels in cervical
lesions (17) and correlated with
vascular density. Sano et al (18) reported that PDGF-B induces
endothelial cell proliferation, migration and formation of
tube-like structure blood vessels. Overexpression of PDFG is
related to tumor microvessel density in many tumor cells including
oral squamous cell carcinoma (19),
pancreatic carcinoma (20) or early
colon cancer. Furthermore, in the presence of low VEGF expression,
the activity of PDGF in infiltrating cells is strongly correlated
with MVD (21).
Studies have shown that the interaction between
VEGF-C and VEGFR-3 plays a critical role in the growth and survival
of lymphatic endothelial cells (22) and promotes tumor lymphangiogenesis
and lymphatic metastasis (23,24).
Mandriota et al (25)
confirmed that VEGF-C and VEGFR-3 promote lymphangiogenesis in a
tumor animal model, causing lymphatic invasion and lymph node
metastasis. Many studies in human solid tumors such as breast,
lung, pancreatic, colon, gastric, esophageal and oral cancers have
also identified the importance of the VEGF-C/VEGFR-3 interaction in
lymph node metastasis of malignant tumors (26–31).
Furthermore, Skobe et al (32) reported that VEGF-C induces the
formation of new lymphatic vessels and enhances lymphatic
metastasis. In a study of VEGF-C expression in cervical cancer and
its relationship with the clinical pathological characteristics of
cervical cancer, Harshimoto et al (33) reported that VEGF-C expression was
significantly higher in cancers with deeper stromal invasion and
lymph node metastasis.
In the present study, the expression of VEGF and MVD
was lower in the recombinant IL-24 group compared with that in the
control and empty plasmid groups, indicating a reduction in tumor
angiogenesis. This resulted in tumor blood supply decrease, which
further inhibited tumor growth. Furthermore, VEGF-C and VEGFR-3
expression was increased in the recombinant IL-24 and combined
treatment groups compared with the other groups, indicating that
IL-24 downregulates the expression of these molecules. Lymph node
metastasis was reduced in the IL-24 and combined treatment groups
compared with the other groups, suggesting that IL-24 plays a role
in inhibiting lymphatic metastasis of transplanted tumor by
downregulating the expression of VEGF-C. PDGF-B was highly
expressed in the PBS control and empty plasmid groups, whereas
after the IL-24 intervention, and especially after intervention
with cisplatin, PDGF-B expression was significantly decreased.
Tumor volume and tumor inhibition were also significantly reduced
in the intervention group. These observations suggest that
increased PDGF-B has a positive correlation with tumor invasion and
metastasis. Thus, inhibition of the expression of PDGF-B family
factors and other angiogenic factors is implicated as one of the
mechanisms by which IL-24 inhibits tumor growth and metastasis.
IL-24 had no obvious side-effects in terms of animal
weight loss in this experimental animal model, which is consistent
with the absence of reports of IL-24 toxicity to normal cells
(34). It can also reduce the
chemotherapy drug dosage and improve the quality of life of
patients.
In summary, the results of the present study showed
that recombinant IL-24 acts synergistically with cisplatin to
inhibit tumor growth and angiogenesis. Our data indicate that these
effects are mediated by downregulation of VEGF, VEGF-C and PDGF-B
expression. Thus, IL-24 is implicated as a potential adjunct
therapy to enhance tumor chemosensitivity to cisplatin
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, et
al: Cancer incidence and mortality worldwide: Sources, methods and
major patterns in GLOBOCAN 2012. Int J Cancer. 36:E359–E386.
2014.
|
|
2
|
Wang Z, Kong D, Banerjee S, et al:
Down-regulation of platelet-derived growth factor-D inhibits cell
growth and angiogenesis through inactivation of Notch-1 and nuclear
factor-kappaB signaling. Cancer Res. 67:11377–11385. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramesh R, Ito I, Gopalan B, Saito Y,
Mhashilkar AM and Chada S: Ectopic production of MDA-7/IL-24
inhibits invasion and migration of human lung cancer cells. Mol
Ther. 9:510–518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gopalan B, Shanker M, Chada S and Ramesh
R: MDA-7/IL-24 suppresses human ovarian carcinoma growth in vitro
and in vivo. Mol Cancer. 6:112007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sarkar D, Su ZZ, Vozhilla N, Park ES,
Gupta P and Fisher PB: Dual cancer-specific targeting strategy
cures primary and distant breast carcinomas in nude mice. Proc Natl
Acad Sci USA. 102:14034–14039. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gupta P, Su ZZ, Lebedeva IV, et al:
mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing
cytokine. Pharmacol Ther. 111:596–628. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L, Wang ZX and Wang ZH: Combination of
IL-24 and cisplatin inhibits cervical cancer growth in a xenograft
nude mice model. Asian Pac J Cancer Prev. 12:3293–3298.
2011.PubMed/NCBI
|
|
8
|
Bresalier RS, Ho SB, Schoeppner HL, et al:
Enhanced sialylation of mucin-associated carbohydrate structures in
human colon cancer metastasis. Gastroenterology. 110:1354–1367.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Traweek ST, Kandalaft PL, Mehta P and
Battifora H: The human hematopoietic progenitor cell antigen (CD34)
in vascular neoplasia. Am J Clin Pathol. 96:25–31. 1991.PubMed/NCBI
|
|
10
|
Weidner N: Current pathologic methods for
measuring intratumoral microvessel density within breast carcinoma
and other solid tumors. Breast Cancer Res Treat. 36:169–180. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee HN, Lee KH, Lee DW, Lee YS, Park EK
and Park JS: Weekly cisplatin therapy compared with triweekly
combination chemotherapy as concurrent adjuvant chemoradiation
therapy after radical hysterectomy for cervical cancer. Int J
Gynecol Cancer. 21:128–136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishikawa M, Kitayama J, Kazama S and
Nagawa H: Expression of vascular endothelial growth factor C and D
(VEGF-C and -D) is an important risk factor for lymphatic
metastasis in undifferentiated early gastric carcinoma. Jpn J Clin
Oncol. 33:21–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakamura Y, Tanaka F, Yoshikawa Y, et al:
PDGF-BB is a novel prognostic factor in colorectal cancer. Ann Surg
Oncol. 15:2129–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Donnem T, Al-Saad S, Al-Shibli K, Andersen
S, Busund LT and Bremnes RM: Prognostic impact of platelet-derived
growth factors in non-small cell lung cancer tumor and stromal
cells. J Thorac Oncol. 3:963–970. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Donnem T, Al-Saad S, Al-Shibli K, Busund
LT and Bremnes RM: Co-expression of PDGF-B and VEGFR-3 strongly
correlates with lymph node metastasis and poor survival in
non-small-cell lung cancer. Ann Oncol. 21:223–231. 2010. View Article : Google Scholar
|
|
16
|
Cao Y: Direct role of PDGF-BB in
lymphangiogenesis and lymphatic metastasis. Cell Cycle. 4:228–230.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mayer TJ, Frauenhoffer EE and Meyers AC:
Expression of epidermal growth factor and platelet-derived growth
factor receptors during cervical carcinogenesis. In Vitro Cell Dev
Biol Anim. 36:667–676. 2000. View Article : Google Scholar
|
|
18
|
Sano H, Ueda Y, Takakura N, et al:
Blockade of platelet-derived growth factor receptor-beta pathway
induces apoptosis of vascular endothelial cells and disrupts
glomerular capillary formation in neonatal mice. Am J Pathol.
161:135–143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li C, Shintani S, Terakado N, et al:
Microvessel density and expression of vascular endothelial growth
factor, basic fibroblast growth factor, and platelet-derived
endothelial growth factor in oral squamous cell carcinomas. Int J
Oral Maxillofac Surg. 34:559–565. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujimoto K, Hosotani R, Wada M, et al:
Expression of two angiogenic factors, vascular endothelial growth
factor and platelet-derived endothelial cell growth factor in human
pancreatic cancer, and its relationship to angiogenesis. Eur J
Cancer. 34:1439–1447. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takahashi Y, Bucana CD, Liu W, et al:
Platelet-derived endothelial cell growth factor in human colon
cancer angiogenesis: role of infiltrating cells. J Natl Cancer
Inst. 88:1146–1151. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Makinen T, Veikkola T, Mustjoki S, et al:
Isolated lymphatic endothelial cells transduce growth, survival and
migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J.
20:4762–4773. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zu X, Tang Z, Li Y, Gao N, Ding J and Qi
L: Vascular endothelial growth factor-C expression in bladder
transitional cell cancer and its relationship to lymph node
metastasis. BJU Int. 98:1090–1093. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsumoto M, Natsugoe S, Okumura H, et al:
Overexpression of vascular endothelial growth factor-C correlates
with lymph node micrometastasis in submucosal esophageal cancer. J
Gastrointest Surg. 10:1016–1022. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mandriota SJ, Jussila L, Jeltsch M, et al:
Vascular endothelial growth factor-C-mediated lymphangiogenesis
promotes tumour metastasis. EMBO J. 20:672–682. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsurusaki T, Kanda S, Sakai H, et al:
Vascular endothelial growth factor-C expression in human prostatic
carcinoma and its relationship to lymph node metastasis. Br J
Cancer. 80:309–313. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Valtola R, Salven P, Heikkila P, et al:
VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in
breast cancer. Am J Pathol. 154:1381–1390. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kajita T, Ohta Y, Kimura K, et al: The
expression of vascular endothelial growth factor C and its
receptors in non-small cell lung cancer. Br J Cancer. 85:255–260.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yonemura Y, Fushida S, Bando E, et al:
Lymphangiogenesis and the vascular endothelial growth factor
receptor (VEGFR)-3 in gastric cancer. Eur J Cancer. 37:918–923.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kitadai Y, Amioka T, Haruma K, et al:
Clinicopathological significance of vascular endothelial growth
factor (VEGF)-C in human esophageal squamous cell carcinomas. Int J
Cancer. 93:662–666. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saaristo A, Partanen TA, Arola J, et al:
Vascular endothelial growth factor-C and its receptor VEGFR-3 in
the nasal mucosa and in nasopharyngeal tumors. Am J Pathol.
157:7–14. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Skobe M, Hawighorst T, Jackson DG, et al:
Induction of tumor lymphangiogenesis by VEGF-C promotes breast
cancer metastasis. Nat Med. 7:192–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hashimoto I, Kodama J, Seki N, et al:
Vascular endothelial growth factor-C expression and its
relationship to pelvic lymph node status in invasive cervical
cancer. Br J Cancer. 85:93–97. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Su Z, Emdad L, Sauane M, et al: Unique
aspects of mda-7/IL-24 antitumor bystander activity: establishing a
role for secretion of MDA-7/IL-24 protein by normal cells.
Oncogene. 24:7552–7566. 2005. View Article : Google Scholar : PubMed/NCBI
|