Introduction
Glioma is the most common tumor among adults, with
the highest degree of malignancy and the greatest lethality
(1). Due to the heterogeneity and
molecular abnormalities of glioma cells, the efficacy of current
treatment options including surgery, radiotherapy and chemotherapy
for glioma patients is not significant. In the past decade, the
median survival of glioma patients has remained stable at 12 months
or less (2). Previous studies on
glioma mostly have focused on histopathology and molecular biology,
and few have aimed at the systematic investigation of the
correlation between tumor cell phenotype and the function nature in
tumor tissue (3).
With developments in the field of molecular biology,
studies have shown that microRNAs (miRs) play an important role in
tumorigenesis and tumor development. An miR can downregulate the
gene activity of cancer as a tumor suppressor, and can also
downregulate gene activity as a cancer gene; it can regulate the
expression of tumor-associated genes (4). The mutations, deletions,
translocations and mutual regulation abnormalities can also lead to
the abnormal expression levels of related genes. miR abnormalities
are associated with cancer, and it has been found that hundreds of
miRs are cancer-related. Suppression of miR-15b was found to result
in an increase in the cell population of glioma cells in the S
phase and a decrease in cell populations of glioma cells, while
upregulation of miR-15b resulted in cell cycle arrest at the G0/G1
phase (5). Chung et al
(6) reported that upregulation of
miR-15b predicts a low risk of tumor recurrence of hepatocellular
carcinoma.
The metalloproteinase family is a class of proteases
that plays a key role in maintaining the stability of the cell
microenvironment. Matrix metalloproteinases (MMPs) have the ability
to cut each component of the extracellular matrix, such as
collagen, laminin, fibronectin and proteoglycans (7). Matrix metalloproteinase-2 (MMP-2) and
matrix metalloproteinase-9 (MMP-9) belong to the gelatinases, a
class of metalloproteinases that are widely related and researched
in the cancer field, both of which are capable of degrading type IV
collagen, and the latter is the main component of the basement
membrane (8). Both MMP-19 plays a
key role in cancer metastasis and spread. Capsaicin suppresses the
migration of cholangiocarcinoma cells by downregulating the MMP-9
expression signaling pathway (9).
Recently Ma et al (10)
illustrated that SUMO-specific protease 1 regulates the
proliferation and invasion of pancreatic cancer cells through
MMP-9. Park et al (11)
illustrated that an ERK1/2 inhibitor significantly inhibited
proliferation of bladder cancer 5637 cells through suppression of
MMP-9 expression.
Mangiferin, also named as mango element or chinonin,
is a natural polyphenolic compound extracted from the liliaceous
plant, Anemarrhena. Modern pharmacological and clinical studies
have shown that mangiferin has physiological activity and
pharmacological effects in many aspects (12). Mangiferin exhibits a significant
central nervous system stimulant effect and a glucocorticoid-like
anti-inflammatory effect, which can reduce capillary permeability,
and its anti-inflammatory response rate is 38% (13). Mangiferin can block the cell cycle
of human hepatoma cell line BEL-7404 at the G2/M phase, and we
found in our previous study that mangiferin significantly inhibited
the proliferation of the K562 chronic myeloid leukemia cell line
(14). The finding that expression
of miR-15b and MMP-9 were altered following mangiferin treatment
can be the foundation for improved and more effective therapy for
glioma. The aim of the present study was to uncover the molecular
pathways involved in the effect of mangiferin on glioma cells.
Materials and methods
Reagents
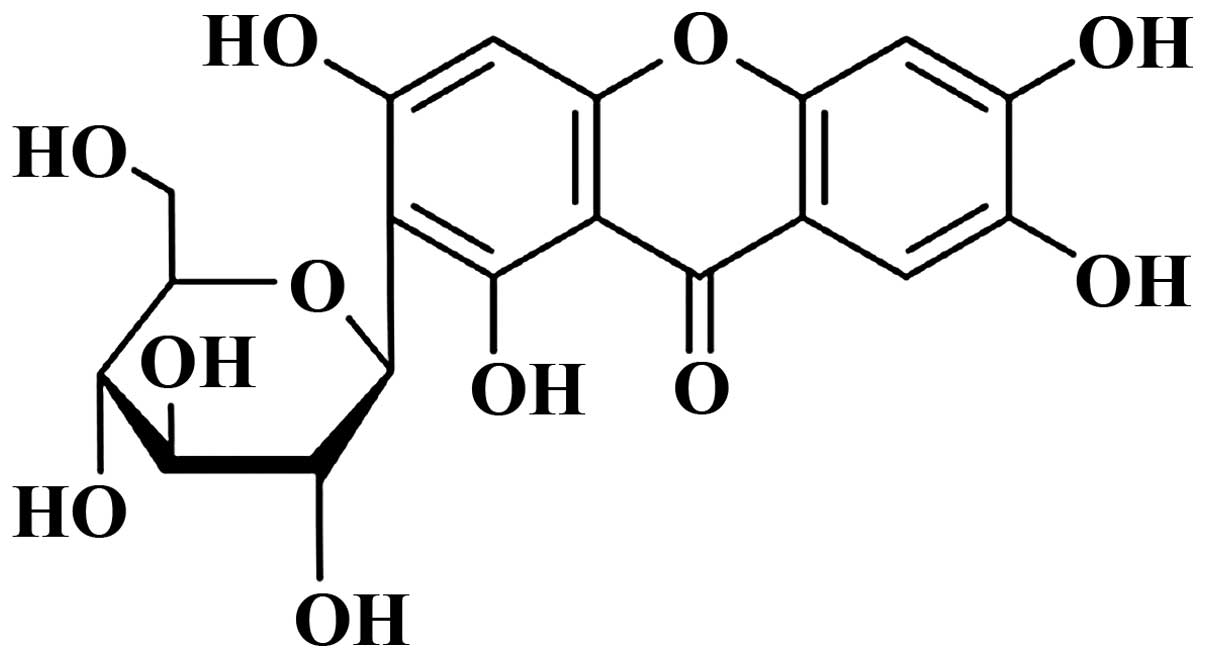
The chemical structure of mangiferin (Sigma; with a
purity >98%) is shown in Fig. 1.
Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Gibco
(Carlsbad, CA, USA). Fetal bovine serum (FBS) was purchased from
Invitrogen (Carlsbad, CA, USA).
3-(4,5-Dimethylthylthiazol-2-yl)-2,5 diphenyltetrazolium bromide
(MTT) was purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing,
China). Annexin V-FITC/propidium iodide (PI) apoptosis detection
kit was purchased from BD Biosciences (San Jose, CA, USA).
Caspase-3 and caspase-9 activity assay kits were purchased from
Tiangen Biotech (Beijing, China). BCA protein assay reagent was
purchased from Shanghai Sangon Biotechnology (Shanghai, China). ABI
7300 HT sequence detection system was purchased from Applied
Biosystems (Foster City, CA, USA).
Cell lines and cell culture
The human U87 glioma cell line was purchased from
the Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai,
China). U87 cells were maintained in a 37°C, 5% CO2
incubator in DMEM (Gibco) supplemented with 10% FBS (Invitrogen)
containing 100 U/ml penicillin and 100 mg/ml streptomycin.
MTT assay
Human U87 glioma cells were seeded (5×103
cells/well) in 96-well plates for 24 h. Cells of each well were
treated with various doses of mangiferin (25, 50 and 100 μM)
(15) for 3 days. Subsequently, 50
μl of MTT was added to each well and incubated at 37°C for
an additional 4 h. The supernatant was discarded, and 200 μl
dimethylsulfoxide was then added into each well to dissolve for 10
min at room temperature while being shaken. Optical density was
measured at the wavelength of 570 nm.
Apoptosis assay
Human U87 glioma cells were seeded (1x106
cells/well) in 6-well plates for 24 h. Cells of each well were
treated with various doses of mangiferin (25, 50 and 100 μM)
for 2 days. According to the manufacturer’s instructions (BD
Biosciences), the apoptotic cells were measured by the Annexin
V-FITC/propidium iodide (PI) apoptosis detection kit. Annexin
V-FITC (5 μl) was added to each well and incubated at 37°C
in the dark for 10 min. Then, 5 μl PI was added to each
well, and cell apoptosis was analyzed by a Cytomics FC 500 flow
cytometer.
DAPI staining assay
Human U87 glioma cells were seeded (1×106
cells/well) in 6-well plates for 24 h. Cells of each well were
treated with various doses of mangiferin (25, 50 and 100 μM)
for 2 days. Cells were then stained with 1 μg/ml DAPI
solution for 10 min at room temperature. Apoptotic cells were
observed using a fluorescence microscope (Olympus Corp., Tokyo,
Japan).
Caspase-3 and caspase-9 activation
assay
Human U87 glioma cells were seeded (5×103
cells/well) in 96-well plates for 24 h. Cells of each well were
treated with various doses of mangiferin (25, 50 and 100 μM)
for 2 days. In accordance with the manufacturer’s instructions
(Tiangen Biotech), caspase-3 and caspase-9 activation was detected
using caspase-3 and caspase-9 activity assay kit. Cells were
harvested and then incubated in ice-cold cell lysis buffer for 30
min. The protein concentration was determined using the BCA protein
assay reagent (Shanghai Sangon Biotechnology, Shanghai, China).
Protein (10 μl) was incubated with the relevant caspase
substrate at 37°C for 4–6 h. Caspase-3 and caspase-9 activation was
measured with a microplate spectrophotometer (Bio-Rad Laboratories,
Hercules, CA, USA) at an absorbance of 405 nm.
Quantitative real-time PCR (qRT-PCR)
Human U87 glioma cells were seeded (1×106
cells/well) in 6-well plates for 24 h. Cells of each well were
treated with various doses of mangiferin (25, 50 and 100 μM)
for 2 days. miR-15b expression of the cells was measured by
qRT-PCR. Firstly, total RNA was extracted from the cells or
transfected cells using TRIzol reagent (Invitrogen). Next, the
relative level of miR-15b was detected by the ABI 7300 HT sequence
detection system (Applied Biosystems, Foster, CA, USA) in cells or
and transfected cells. miR-15b sequences were:
5′-GATACTCGAGCAGAAGTTTGGCTAATTTAATAATC-3′ (forward) and
5′-GCGAATTCGCCAAGGATGACCTTAAGCCTC-3′ (reverse). U6 sequences were:
5′-GCTTGCTTCGGCAGCACATATAC-3′ (forward) and
5′-TGCATGTCATCCTTGCTCAGGG-3′ (reverse). PCR cycles were as follows:
95°C for 10 min, followed by 35 cycles of 95°C for 30 sec, 58°C for
30 sec and 72°C for 45 sec.
Gelatin zymography
Human U87 glioma cells were seeded (1×106
cells/well) in 6-well plates for 24 h. Cells of each well were
treated with various doses of mangiferin (25, 50 and 100 μM)
for 2 days. The culture supernatants were collected, and 20
μl of collected media was added to an equal volume of sodium
dodecylsulfate (SDS) sample buffer. Collected media were subjected
to 10% polyacrylamide gel electrophoresis containing 1 mg/ml
gelatin. After electrophoresis, the gels were washed twice with
2.5% Triton X-100 (37°C for 15 min) and incubated in a reaction
buffer at 37°C for 11–12 h. After incubation, the gels were
subsequently stained with 0.5% (w/v) Coomassie blue R-250 for 2 h.
Finally, Coomassie brilliant blue R-250 (Amresco, Solon, Oh, USA)
was used to stain the gels.
Cell transfection
miR-15b mimics and anti-miR-15b mimics were
synthesized by Wuhan Genesil Biotechnology Co., Ltd. (Wuhan,
China). Human U87 glioma cells were seeded (1×106
cells/well) in 6-well plates for 24 h. In accordance with the
manufacturer’s instructions (Invitrogen), the mimics were allowed
to transfect with Lipofectamine 2000 into the U87 glioma cells.
Statistical analysis
Data analysis was performed with SPSS 17.0 software
and data are presented as means ± SD. Differences were analyzed
using one-way ANOVA. P-values of <0.05 were considered to
indicate statistically significant results.
Results
Mangiferin suppresses the proliferation
of U87 glioma cells
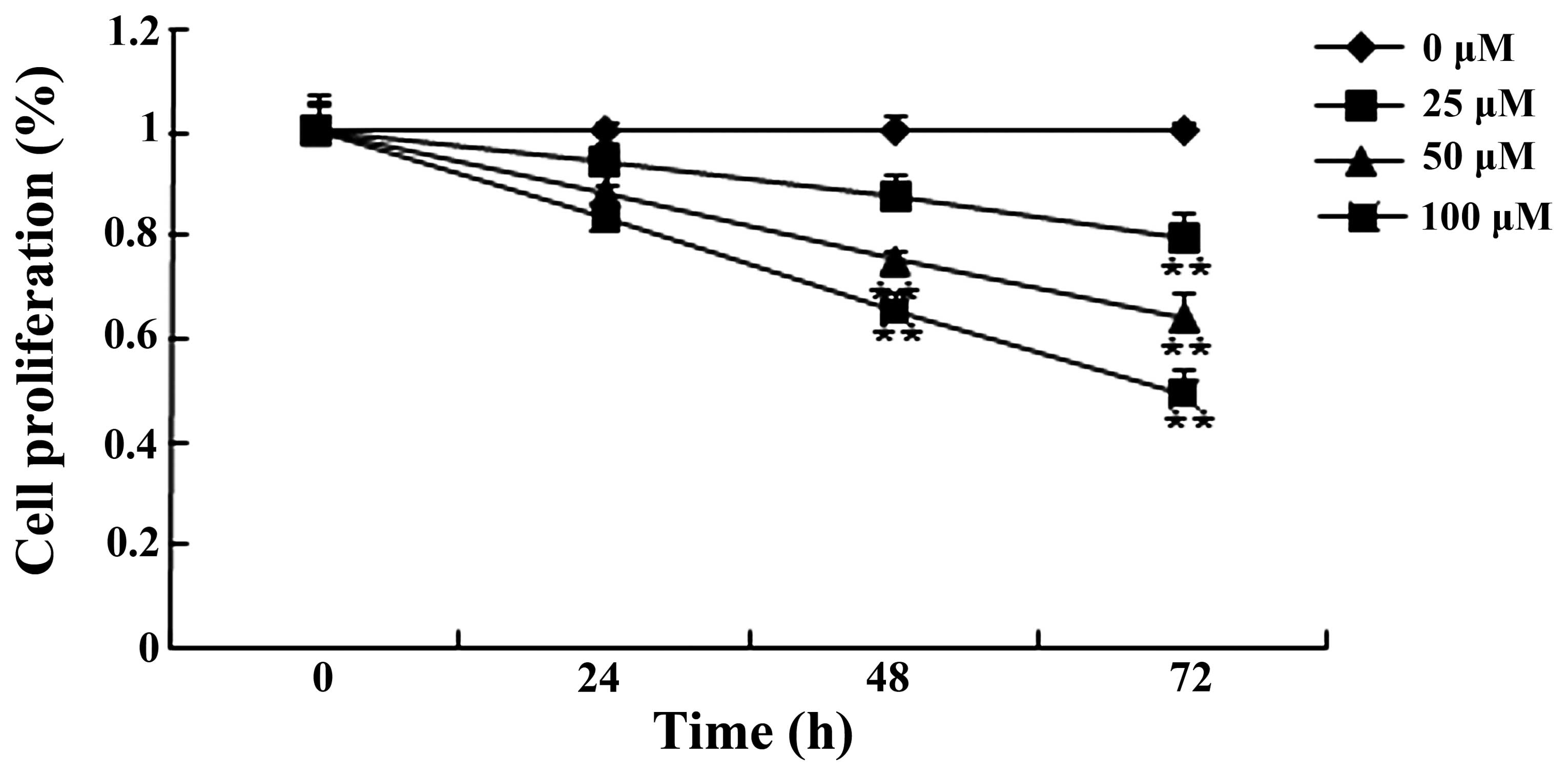
To explore the effect of mangiferin on the
proliferation of U87 glioma cells, the cell growth viability was
examined by MTT assay. As shown in Fig.
2, mangiferin (25, 50 and 100 μM) inhibited the
proliferation of the U87 glioma cells in a time- and dose-dependent
manner. Following treatment with mangiferin (50 and 100 μM)
for 48 and 72 h and mangiferin (25 μM) for 72 h, the
mangiferin-treated U87 glioma cells showed a significant decrease
in proliferation relative to that of the 0 μM mangiferin
treatment group (Fig. 2).
Mangiferin induces apoptosis in the U87
glioma cells
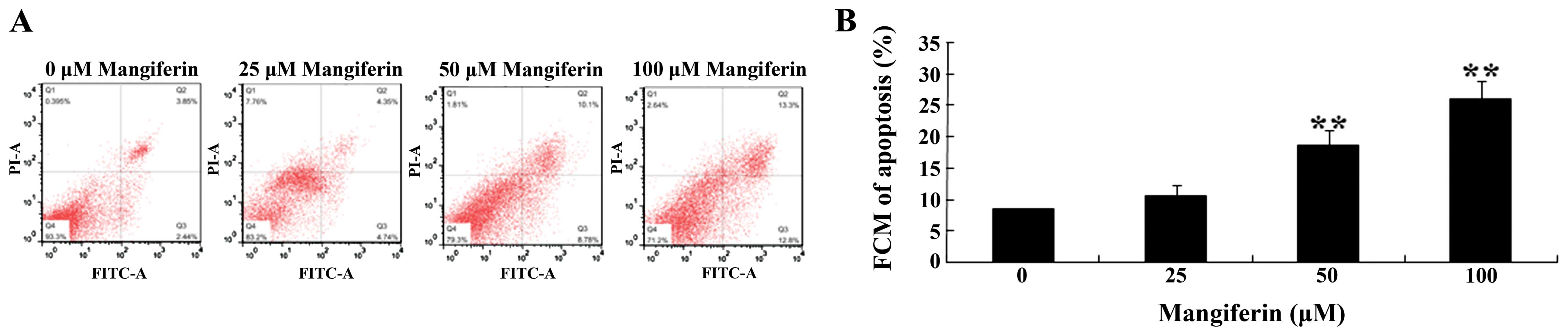
To determine the effects of mangiferin on the
apoptosis of U87 glioma cells, Annexin V-FITC/propidium iodide (PI)
apoptosis detection kit and DAPI staining assay were employed to
evaluate the cell apoptosis rate and caspase-3 and caspase-9
activation in the U87 glioma cells, respectively. As shown in
Fig. 3A and B, mangiferin (25, 50
and 100 μM) increased the cell apoptosis rate in a
dose-dependent manner. Following treatment with mangiferin (50 and
100 μM) for 48 h, the cell apoptosis rate was markedly
increased, compared to that of the 0 μM mangiferin treatment
group (Fig. 3A and B). Meanwhile,
the cell apoptosis was observed using DAPI staining assay. We found
that the cell apoptosis rate was significantly increased following
mangiferin treatment (25, 50 and 100 μM) (Fig. 3C).
Mangiferin induces caspase-3 and
caspase-9 activation in the U87 glioma cells
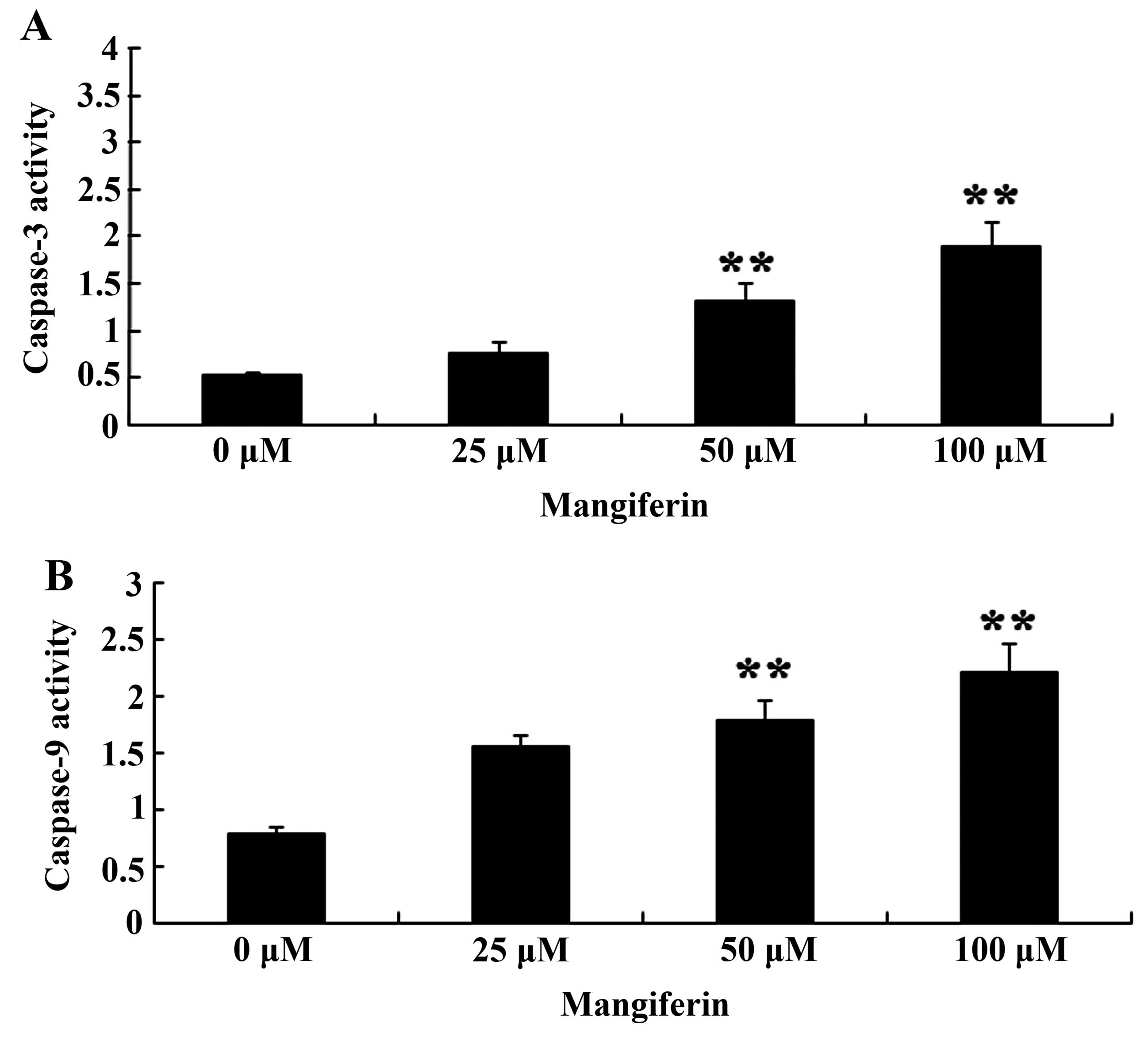
To further elucidate the effects of mangiferin on
caspase-3 and caspase-9 activation in the U87 glioma cells,
caspase-3 and caspase-9 activation was estimated using activity
assay kits. Caspase-3 and caspase-9 activation was also markedly
increased following the treatment of mangiferin (50 and 100
μM) for 48 h in comparison to that of the 0 μM
mangiferin treatment group (Fig.
4).
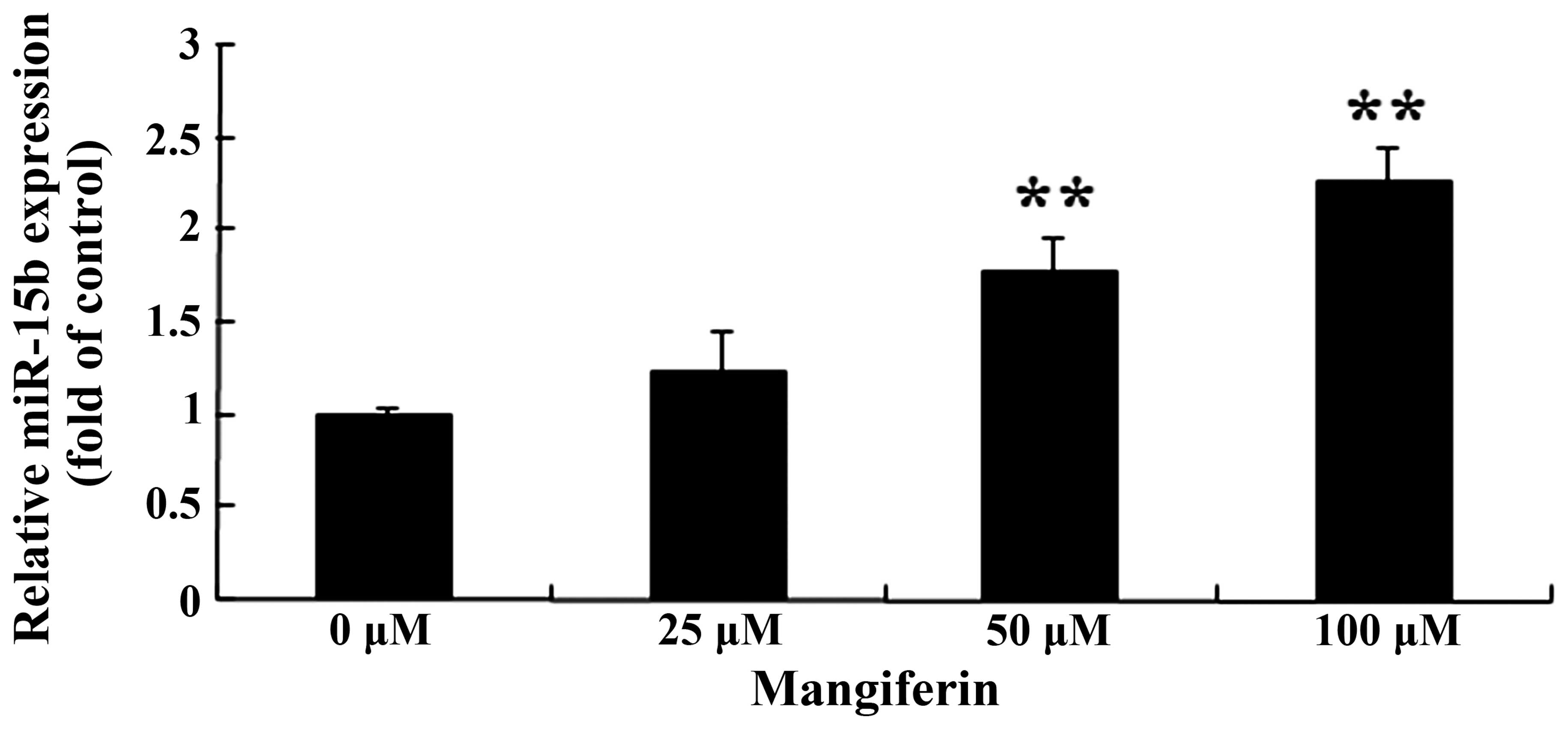
Mangiferin promotes miR-15b expression in
the U87 glioma cells
To evaluate whether mangiferin promotes miR-15b
expression, we used qRT-PCR to analyze miR-15b expression in the
U87 glioma cells. The expression of miR-15b in the U87 glioma cells
was markedly increased, following the treatment of mangiferin (50
and 100 μM) for 48 h (Fig.
5).
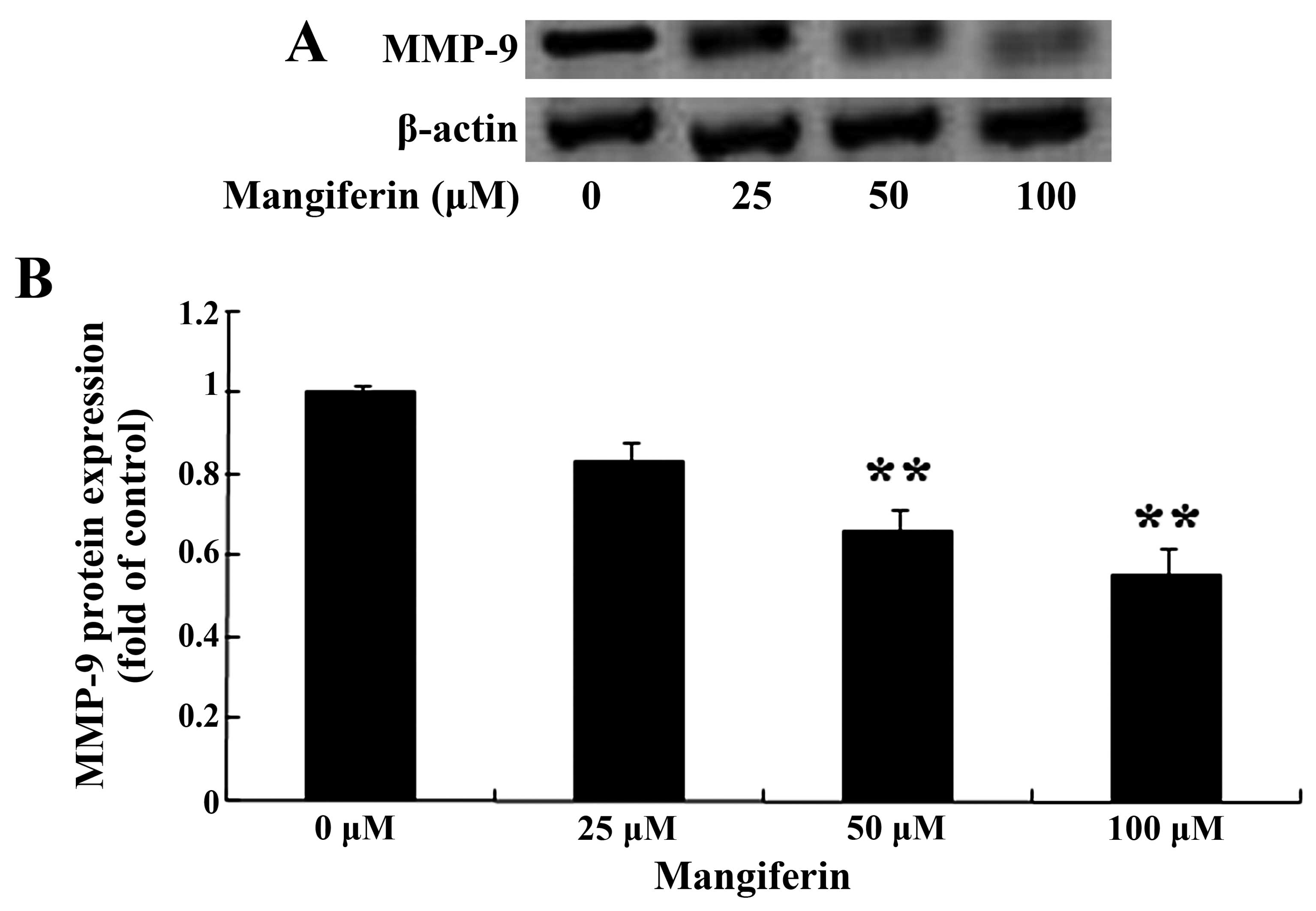
Mangiferin suppresses MMP-9 expression in
the U87 glioma cells
We estimated the expression of MMP-9 following the
treatment of mangiferin in the U87 glioma cells. Following
treatment with mangiferin (50 and 100 μM) for 48 h, the
results of the gelatin zymography assay revealed that the
expression of MMP-9 in the U87 glioma cells was markedly suppressed
in comparison to that of the 0 μM mangiferin treatment group
(Fig. 6).
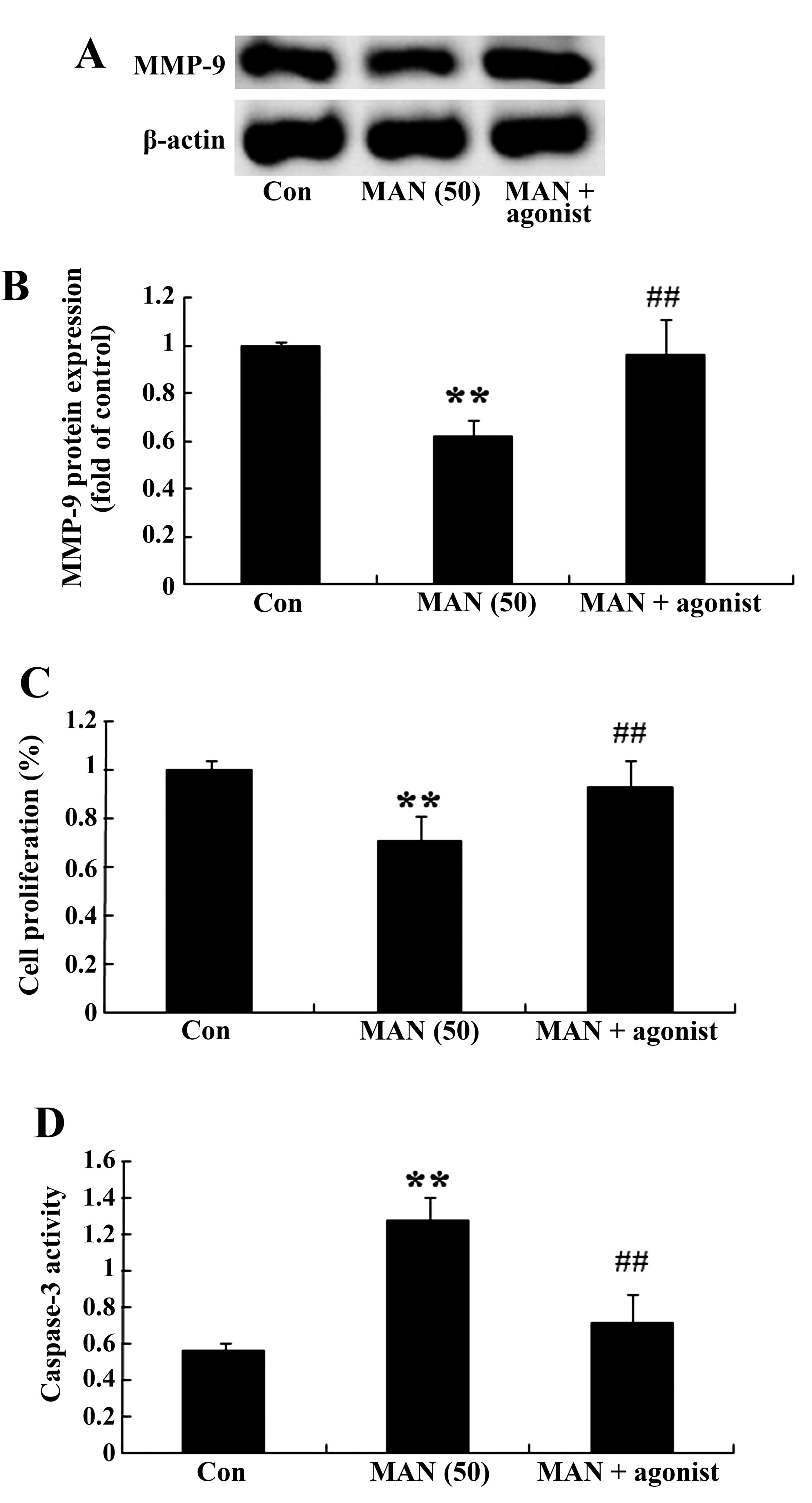
MMP-9 agonist restrains the effect of
mangiferin on U87 glioma cells
The relationship of MMP-9 expression and the effect
of mangiferin on U87 glioma cells was detected. Following treatment
with mangiferin (50 μM) for 48 h, the MMP-9 agonist
(P-aminophenylmercuric acetate, 1 mM) significantly increased the
MMP-9 expression in the U87 glioma cells in comparison to that of
the 0 μM mangiferin group (Fig.
7A and B). Next, the MMP-9 agonist reversed the effect of
mangiferin on cell proliferation and the caspase-3 activation in
the U87 glioma cells (Fig. 7C and
D).
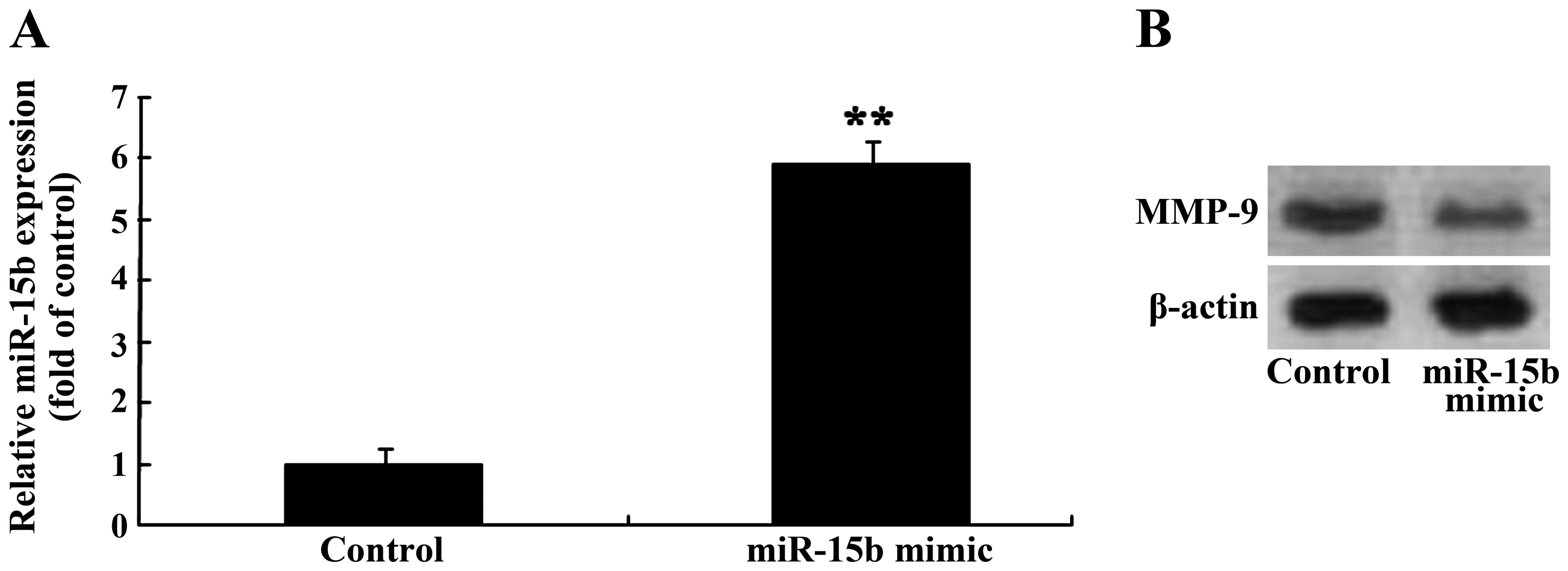
Overexpression of miR-15b and expression
of MMP-9 in the U87 glioma cells
To investigate the association between the
overexpression of miR-15b and expression of MMP-9 in the U87 glioma
cells, miR-15b mimics were transfected into the U87 glioma cells
and the expression levels of miR-15b and MMP-9 were measured with
qRT-PCR and gelatin zymography assay, respectively. Firstly,
miR-15b mimics markedly increased the expression of miR-15b in the
U87 glioma cells, compared to that in the control group (Fig. 8A). Next, miR-15b mimics also reduced
the expression MMP-9 in the U87 glioma cells, compared to that of
the control group (Fig. 8B).
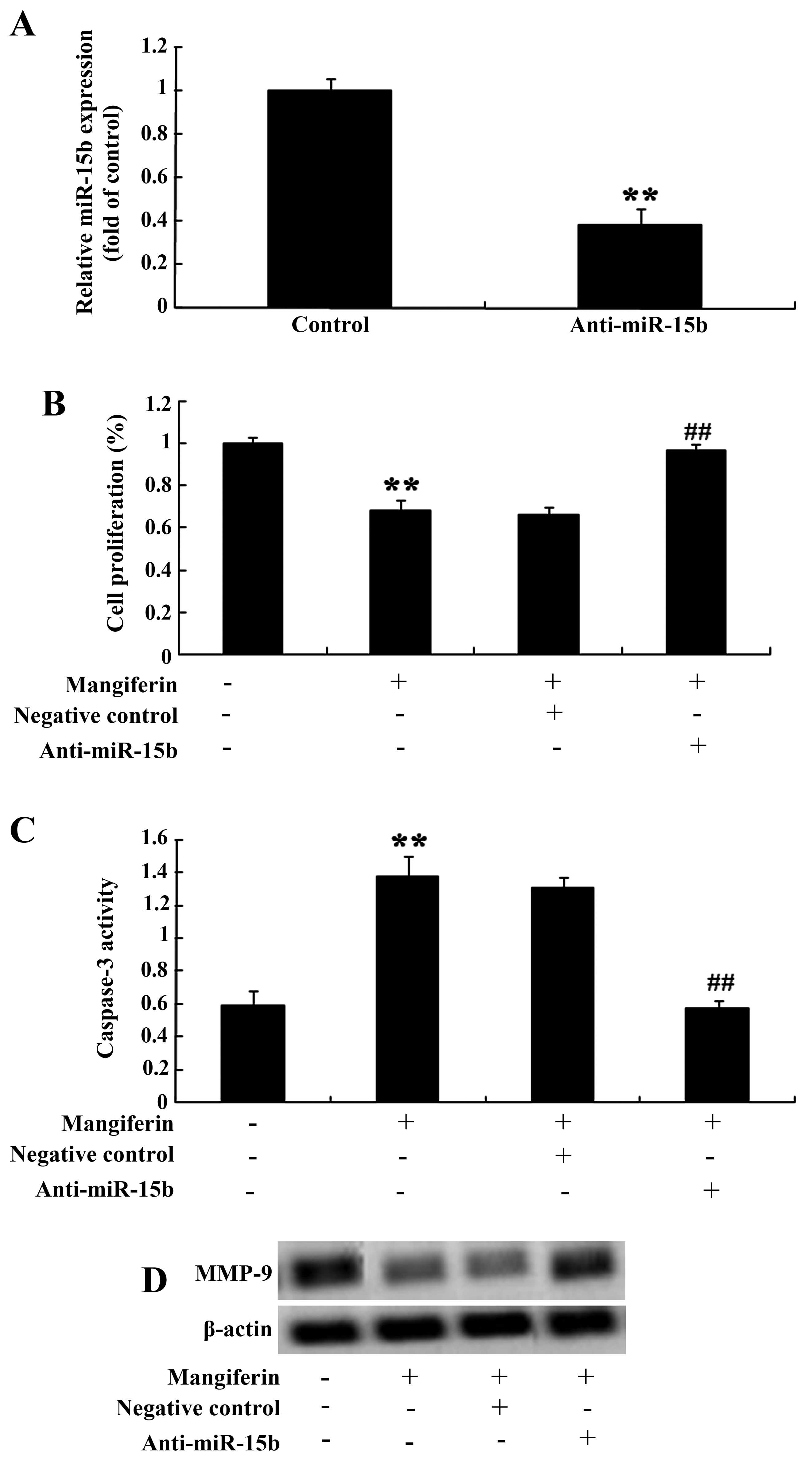
Anti-miR-15b and the effect of
mangiferin
We further determine the correlations of miR-15b
expression level and the effect of mangiferin on glioma cells.
Firstly, anti-miR-15b mimics were transfected into the U87 glioma
cells. Lower expression of miR-15b was observed in the transfected
cells (Fig. 9A). Similarly,
anti-miR-15b mimics meaningfully reversed the effect of mangiferin
(50 μM) on cell proliferation (Fig. 9B) and apoptosis of U87 glioma cells
(Fig. 9C) at 48 h. Meanwhile,
anti-miR-15b mimics also accelerated the levels of MMP-9 in the U87
glioma cells (Fig. 9D).
Discussion
Uncontrolled proliferation and high invasive
ability, the most typical malignant phenotypes of malignant glioma,
limit the ability to cure this cancer. The main reason for the
failure of the clinical treatment of malignant glioma is the
uncontrolled proliferation and invasive growth (16). In the present study, our data
revealed that mangiferin suppressed the proliferation of U87 glioma
cells. Mangiferin was previously found to inhibit human
nasopharyngeal carcinoma cell proliferation by Bcl-2 and Bax
expression (15). Meanwhile, we
found that mangiferin induced the cell apoptosis rate and increased
the activation of caspase-3 and caspase-9 in U87 glioma cells.
García-Rivera et al (17)
indicated that the antitumor effects of mangiferin were through
increased apoptosis in MDA-MB231 breast cancer cells. du
Plessis-Stoman et al (18)
illustrated that combination treatment with oxaliplatin and
mangiferin accelerated apoptosis through inhibition of NF-κB in
cancer cell lines.
miRNAs play an important role in normal growth and
development, proliferation, differentiation and apoptosis of cells
(19). miRNAs are mainly involved
in the regulation of genes related to cell fate, especially in the
development-related genes; however, almost no relevant genes that
maintain the basic activities of cells have miRNA target sites
(20). In the present study, we
found that mangiferin specifically increased the expression of
miR-15b in the U87 glioma cells.
Among the metalloproteinase family members, the
gelatinases MMP-2 and MMP-9 have been ‘hot topics’ of research
(21). According to reports, MMP2
expression is increased in a variety of cancers, including lung
cancer and the prognosis is poor. In addition, our findings showed
that the expression of MMP-9 in U87 glioma cells was controlled by
the treatment of mangiferin. Meanwhile, Kim et al (22) reported that mangiferin exerts
anti-photoaging activity by regulating MMP-9 expression through
inhibition of mitogen-activated protein kinase kinase 1 and
extracellular signal-regulated kinase. In PMA-stimulated human
astroglioma cells, mangiferin inhibited MMP-9 gene expression
through the PI3K/Akt and MAPK signaling pathways (23). However, we found that the MMP-9
agonist reversed the effect of mangiferin on U87 glioma cells.
In the present study, we demonstrated that
overexpression of miR-15b in U87 glioma cells suppressed the
expression level of MMP-9. In contrast, downregulation of miR-15b
reversed the effect of mangiferin in the U87 glioma cells. Zheng
et al (24) reported that
miR-15b deactivated the MEK-ERK pathway through MMP-3 in 9L glioma
cells. Sun et al (25)
suggested that treatment of gastric cancer cells increased miR-15b
expression corresponding with downregulation of MMP-9 and
MMP-2.
In summary, this is the first report that mangiferin
regulates proliferation and apoptosis in glioma cells by inhibiting
the expression of MMP-9 and by inactivating the expression of
miR-15b. The different signaling pathways indicated in the present
study may help us elucidate the anticancer effects of mangiferin,
also aiding in the knowledge of possible drug resistance
mechanisms.
References
|
1
|
Pan WR, Li G and Guan JH: Polymorphisms in
DNA repair genes and susceptibility to glioma in a Chinese
population. Int J Mol Sci. 14:3314–3324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu X, Wang L, Chen J, Ling Q, Wang H, Li
S, Li L, Yang S, Xia M and Jing L: Estrogen receptor beta agonist
enhances temozolomide sensitivity of glioma cells by inhibiting
PI3K/AKT/mTOR pathway. Mol Med Rep. 11:1516–1522. 2014.
|
|
3
|
Jovcevska I, Kocevar N and Komel R: Glioma
and glioblastoma - how much do we (not) know? Mol Clin Oncol.
1:935–941. 2013.
|
|
4
|
Guo X, Xia J and Yan J: Promoter
methylated microRNAs: Potential therapeutic targets in gastric
cancer (Review). Mol Med Rep. 1:759–765. 2014.
|
|
5
|
Xia H, Qi Y, Ng SS, Chen X, Chen S, Fang
M, Li D, Zhao Y, Ge R, Li G, et al: MicroRNA-15b regulates cell
cycle progression by targeting cyclins in glioma cells. Biochem
Biophys Res Commun. 380:205–210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung GE, Yoon JH, Myung SJ, Lee JH, Lee
SH, Lee SM, Kim SJ, Hwang SY, Lee HS and Kim CY: High expression of
microRNA-15b predicts a low risk of tumor recurrence following
curative resection of hepatocellular carcinoma. Oncol Rep.
23:113–119. 2010.
|
|
7
|
Heo W, Lee YS, Son CH, Yang K, Park YS and
Bae J: Radiation-induced matrix metalloproteinases limit natural
killer cell-mediated anticancer immunity in NCI-h23 lung cancer
cells. Mol Med Rep. 11:1800–1806. 2014.PubMed/NCBI
|
|
8
|
Cheng X, Gu J, Zhang M, Yuan J, Zhao B,
Jiang J and Jia X: Astragaloside IV inhibits migration and invasion
in human lung cancer A549 cells via regulating
PKC-alpha-ERK1/2-NF-kappaB pathway. Int Immunopharmacol.
23:304–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee GR, Jang SH, Kim CJ, Kim AR, Yoon DJ,
Park NH and Han IS: Capsaicin suppresses the migration of
cholangiocarcinoma cells by down-regulating matrix
metalloproteinase-9 expression via the AMPK-NF-kappaB signaling
pathway. Clin Exp Metastasis. 31:897–907. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma C, Wu B, Huang X, Yuan Z, Nong K, Dong
B, Bai Y, Zhu H, Wang W and Ai K: SUMO-specific protease 1
regulates pancreatic cancer cell proliferation and invasion by
targeting MMP-9. Tumour Biol. 35:12729–12735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park SL, Won SY, Song JH, Kim WJ and Moon
SK: EPO gene expression induces the proliferation, migration and
invasion of bladder cancer cells through the p21WAF1-mediated
ERK1/2/NF-κ/MMP-9 pathway. Oncol Rep. 32:2207–2214. 2014.PubMed/NCBI
|
|
12
|
Zhang B, Zhao J, Li S, Zeng L, Chen Y and
Fang J: Mangiferin activates the Nrf2-ARE pathway and reduces
etoposide-induced DNA damage in human umbilical cord mononuclear
blood cells. Pharm Biol. 53:503–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao W, Hou J, Ma J, Yu B, Ren J, Jin W,
Wu J, Zheng D and Fan K: Mangiferin loaded magnetic PCEC
microspheres: preparation, characterization and antitumor activity
studies in vitro. Arch Pharm Res. Sep 30–2014.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu XY, Deng JG, Wang L and Yuan YF:
Synthesis and anti-tumor activity evaluation of gallic
acid-mangiferin hybrid molecule. Med Chem. 9:1058–1062. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan LL, Wang AY, Huang YQ, Luo Y and Ling
M: Mangiferin induces apoptosis by regulating Bcl-2 and Bax
expression in the CNE2 nasopharyngeal carcinoma cell line. Asian
Pac J Cancer Prev. 15:7065–7068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma H, Zhang Y, Wang H, Han C, Lei R, Zhang
L, Yang Z, Rao L, Qing H and Xiang J: Effect and mechanism of
mitomycin C combined with recombinant adeno-associated virus type
II against glioma. Int J Mol Sci. 15:1–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
García-Rivera D, Delgado R, Bougarne N,
Haegeman G and Berghe WV: Gallic acid indanone and mangiferin
xanthone are strong determinants of immunosuppressive anti-tumour
effects of Mangifera indica L. bark in MDA-MB231 breast cancer
cells. Cancer Lett. 305:21–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
du Plessis-Stoman D, du Preez J and van de
Venter M: Combination treatment with oxaliplatin and mangiferin
causes increased apoptosis and downregulation of NFkappaB in cancer
cell lines. Afr J Tradit Complement Altern Med. 8:177–184.
2011.
|
|
19
|
Yang O, Huang J and Lin S: Regulatory
effects of miRNA on gastric cancer cells. Oncol Lett. 8:651–656.
2014.PubMed/NCBI
|
|
20
|
Kavitha N, Vijayarathna S, Jothy SL, Oon
CE, Chen Y, Kanwar JR and Sasidharan S: MicroRNAs: biogenesis,
roles for carcinogenesis and as potential biomarkers for cancer
diagnosis and prognosis. Asian Pac J Cancer Prev. 15:7489–7497.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu Y, Zhu L, Lu L, Zhang L, Zhang G, Wang
Q and Yang P: Role and mechanism of the alkylglycerone phosphate
synthase in suppressing the invasion potential of human glioma and
hepatic carcinoma cells in vitro. Oncol Rep. 32:431–436.
2014.PubMed/NCBI
|
|
22
|
Kim HS, Song JH, Youn UJ, Hyun JW, Jeong
WS, Lee MY, Choi HJ, Lee HK and Chae S: Inhibition of UVB-induced
wrinkle formation and MMP-9 expression by mangiferin isolated from
Anemarrhena asphodeloides. Eur J Pharmacol. 689:38–44. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung JS, Jung K, Kim DH and Kim HS:
Selective inhibition of MMP-9 gene expression by mangiferin in
PMA-stimulated human astroglioma cells: involvement of PI3K/Akt and
MAPK signaling pathways. Pharmacol Res. 66:95–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng X, Chopp M, Lu Y, Buller B and Jiang
F: MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis
via NRP-2 and MMP-3. Cancer Lett. 329:146–154. 2013. View Article : Google Scholar :
|
|
25
|
Sun H, Meng X, Han J, Zhang Z, Wang B, Bai
X and Zhang X: Anti-cancer activity of DHA on gastric cancer - an
in vitro and in vivo study. Tumour Biol. 34:3791–3800. 2013.
View Article : Google Scholar : PubMed/NCBI
|