Introduction
Immunotherapy is an attractive strategy for the
prevention of tumor recurrence and the control of cancer growth
(1,2). Immunotherapy can induce
antigen-specific T cell responses that can inhibit tumor growth.
Effective activation of naïve T cells depends on TCR engagement and
efficient co-stimulation (3,4). The
4-1BB ligand (L) is expressed on antigen-presenting cells (APC),
and can interact with 4-1BB on activated T cells, co-stimulating
T-cell cytokine production and proliferation (5–8) and
prolonging T-cell survival (7,9).
Therefore, 4-1BB and 4-1BBL are important regulators of T-cell
immunity.
CEACAM6 (also known as CD66c or NCA-90) is a
non-specific cross-reacting glycoprotein antigen that shares some
antigenic determinants with CEACAM5 (also known as CD66e, which
codes for the protein, CEA) (10).
CEACAM6 is also expressed on granulocytes and epithelia from
various organs, and has a broader expression zone in the
proliferating cells of hyperplastic colonic polyps and adenomas,
compared with normal mucosa (11),
as well as by many human cancers (11–13).
Relatively high serum levels of CEACAM6 are found in patients with
lung, pancreatic, breast, colorectal and hepatocellular carcinomas.
The amount of CEACAM6 does not correlate with the amount of CEACAM5
expressed (12). For all the
tumors, the amount of CEACAM6 expressed was greater than that of
CEACAM5 and reflected tumor histotype. CEACAM6 is a more promising
target for antibody-based anti-metastatic and chemosensitizing
therapy than CEACAM5 in all the solid tumors studied. Furthermore,
CEACAM6 is a useful antigen to target in select subtypes of solid
tumors (14).
Previously, we found that vaccination with
recombinant attenuated Salmonella harboring the 4-1BBL gene
efficiently enhanced T-cell immunity and inhibited the development
of carcinogen-induced colorectal cancer in rats (15). In the present study, we investigated
the effects of vaccination with a recombinant Salmonella-based
CEACAM6 and 4-1BBL vaccine on the development of
1,2-dimethylhydrazine (DMH)-induced colorectal tumors in rats. The
results indicated that this recombinant vaccine increased the
number of CD3+CD8+ tumor-infiltrating
lymphocyte (TIL) and CD56+ cells, decreased the number
of FOXP3 TIL cells, enhanced antitumor immune responses and
inhibited the development of DMH-induced colorectal cancer in
rats.
Materials and methods
Animals
A total of 24 male Sprague Dawley (SD) rats, aged
6–8 weeks, were obtained from the Experimental Animal Center of
Soochow university. The rats were housed in a
specific-pathogen-free (SPF) facility in a 12-h light/dark cycle at
50±10% humidity and 21±2°C with free access to water and food. The
body weights of individual rats were measured weekly. The
experimental protocols were approved by the Ethics Committee of the
First Affiliated Hospital of Soochow university. Ether was used for
mouse euthanasia and anesthesia.
Construction and identification of
expression plasmids
Total RNA was isolated from testis of rats and
reverse transcribed into cDNA using a specific kit (Takara, Dalian,
China). The primers used were: 5-CAGAGCCAAACAACAGAT (forward) and
5-CATTATTACTTATGCTGACCT (reverse) according to an open reading
frame of full-length CEACAM6 (GenBank: BC078962.1). The
PCR-amplified DNA was cloned into the pMDT-19 vector, which was a
kind gift from Professor Cen (Soochow university), to yield
pMDT-19-CEACAM6. CEACAM6 cDNA was then excised from the plasmid by
restriction enzymes NheI and MluI and ligated into
the pIRES (Palo Alto, CA, USA) to yield pIRES-CEACAM6. Recombinant
vectors were identified and the 4-1BBL cDNA fragment was excised
from the plasmid pMDT-18-4-1BBL, which was constructed as
previously described (16), by
restriction enzymes EagI and XbalI, blunt-ended, and
ligated into the pIRES2 to yield pIRES2-4-1BBL. The plasmid
pIRES2-CEACAM6-4-1BBL contained the CEACAM6 fused to the C terminus
of murine 4-1BBL, thereby generating a dual-function chimeric
construct. The plasmids were digested by restriction enzymes
NheI, MluI, EagI and XbalI, and
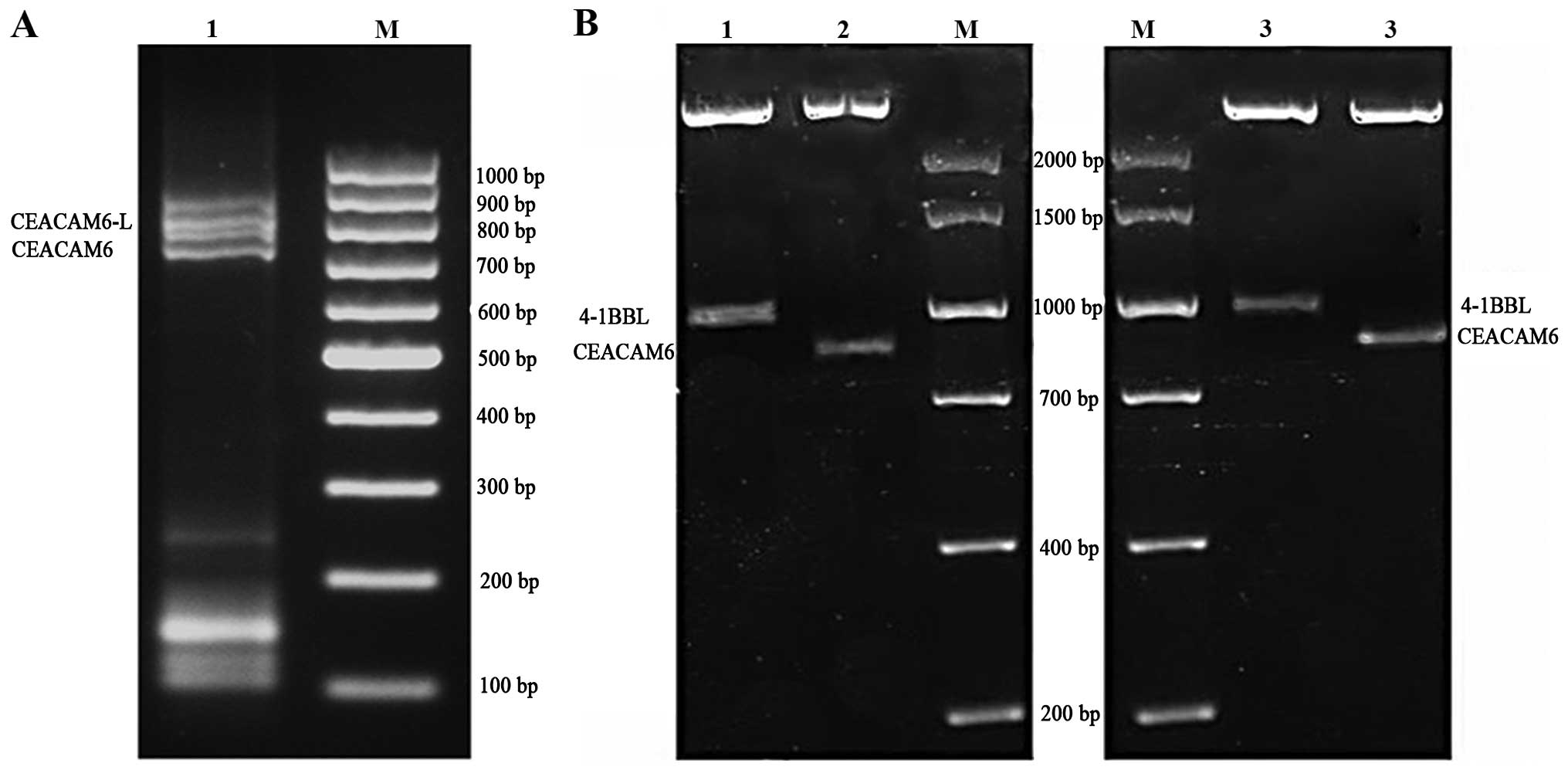
measured by 1% agarose gel electrophoresis. The results showed the
products of RT-PCR of pIRES-CEACAM6, pIRES-4-1BBL and
pIRES-CEACAM6-4-1BBL were ~801 and 930 bp, which corresponded with
CEACAM6 cDNA and 4-1 BBL cDNA (Fig.
1).
Transfection of CEACAM6 and 4-1BBL
Each plasmid DNA was transfected into COS-7 cells
using Lipofectamine™ 2000 reagent (Invitrogen) according to the
manufacturer’s instructions. After 48 h of culture, the transfected
cells were fixed with 3% paraformaldehyde in PBS for 15 min,
treated with 50 mm NH4Cl for 10 min, permeabilized in
0.1% Triton X-100 for 5 min and washed with PBS. Following exposure
to PBS containing 5% non-fat milk, the transfected cells were
subjected to double immunostaining by polyclonal anti-CEACAM6
antibody (Abcam, Cambridge, UK) and polyclonal anti-4-1BBL antibody
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) in the
blocking buffer, followed by incubation with Cy3 conjugated with
goat anti-rabbit IgG (Biyuntian Biotechnology Research Institute,
Shanghai, China) and donkey anti-goat IgG conjugated with FITC. To
stain the nuclei of COS-7 cells, immunostained cells were exposed
for 30 min to PBS containing Hoechst 33342. The samples were washed
with PBS and examined using a confocal laser scanning
microscope.
Construction and identification of
attenuated Salmonella-containing plasmids
Plasmids pIRES, pIRES-4-1BBL, pIRES-CEACAM6 and
pIRES-CEACAM6-4-1BBL were electrotransfected into Salmonella
LB5000. After modification, the plasmids were extracted and
electrotransfected into attenuated S. serovar typhimurium
SL3261. The recombinant bacteria were designated as pIRES/SL3261
(SL3261 with pIRES plasmid), pIRES-4-1BBL/SL3261 (SL3261 with
pIRES-4-1BBL plasmid), pIRES-CEACAM6/SL3261 (SL3261 with
pIRES-CEACAM6 plasmid), and pIRES-CEACAM6-4-1BBL/SL3261 (SL3261
with pIRES-CEACAM6-4-1BBL plasmid). The bacteria were cultivated in
LB-medium (antibiotics free) for 5 days and subsequently harvested.
Specimens were seeded in LB plates containing kanamycin for the
screening of positive clones. PCR was applied to identify plasmids
extracted from the positive clones.
Establishment of rat model of colorectal
tumor
Individual animals were administered subcutaneously
with 20 mg/kg DMH (Sigma, St. Louis, MO, USA) weekly for 18
consecutive weeks. Eight weeks after the first DMH administration,
the rats were randomized and gavaged with 2 ml PBS, PBS containing
2×109 pIRES-transformed SL3261A,
pIRES-4-1BBL-transformed SL3261B, pIRES-CEACAM6-transformed SL3261C
or pIRES-CEACAM6-4-1BBL-transformed SL3261D four times every two
weeks as the pIRES/SL3261, pIRES-4-1BBL/SL3261,
pIRES-CEACAM6/SL3261 or pIRES-CEACAM6-4-1BBL/SL3261 group. At 18
weeks after the first DMH injection, the rats were anesthetized,
blood samples were collected, and the animals were sacrificed. The
colons were dissected for immunohistochemical staining.
Gross and histological examination
Tissues from the entire colon (from the cecum to the
anus) of individual rats were removed, washed thoroughly with
ice-cold saline, cut longitudinally, and laid flat on a board. The
number of tumors was counted in a blinded manner. The disease stage
of the colorectal cancers were evaluated using the Duke’s stage
system (17), with minor
modifications. The stages were classified as: stage A, tumors only
in the mucosa; stage B and C, tumors invading into muscularis
propria, albeit without distant metastasis; and stage D, tumor with
distant metastasis.
Immunohistochemistry and cell count
analysis
Adult rat tumor specimens were immunohistochemically
analyzed for their infiltration with CD3-, CD4-, CD8-, CD56-,
FOXP3- and CEACAM6-positive T cells. Tissue sections were prepared
from formalin-fixed, paraffin-embedded tissue. After
deparaf-finization and rehydration, the slides were boiled in 10
mmol/l citrate buffer (pH 6.0) for 15 min to retrieve the antigens.
The endogenous peroxidase activity was blocked by incubation with
0.6% H2O2 in methanol for 20 min. The
sections were then blocked with 10% normal horse serum. Mouse
monoclonal antibodies recognizing rat CD3 (Santa Cruz
Biotechnology, Inc.), rabbit polyclonal antibodies recognizing rat
CD4 (Santa Cruz Biotechnology, Inc.), CD8 (Santa Cruz
Biotechnology, Inc.), rabbit monoclonal antibodies recognizing rat
CEACAM6 (Abcam), mouse monoclonal antibodies recognizing rat CD56
(Abcam) and FOXP3 (Abcam) were applied as primary antibodies at
room temperature for 2 h. The slides were incubated with a
biotinylated secondary antibody (horse-anti-mouse IgG,
horse-anti-rabbit IgG; Santa Cruz Biotechnology) for 30 min at room
temperature and AB reagent was applied according to the
manufacturer’s instructions. The antigen detection was conducted by
a color reaction with 3,3′-diaminobenzidine (DAB + chromogen;
DakoCytomation Corp., Carpinteria, CA, USA). The sections were
counterstained with hematoxylin (AppliChem, Darmstadt, Germany) and
mounted with Aquatex (Merck, Darmstadt, Germany). The number of
positive cells/filed was estimated and assigned a number: 0, none;
1, 1/100 cells; 2, 1/100–1/10 cells; 3, 1/10–1/3 cells; 4, 1/3–2/3
cells; and 5, >2/3 cells. The intensity of staining was then
determined as: 0, none; 1, weak; 2, intermediate; and 3, strong.
The first and second scores were then added together resulting in a
maximum staining score of 8 for any tissue core (18). Two independent blinded investigators
(C.J. and Y.L.) performed immunohistochemical analysis and the
results were strongly consistent between the two readings. The
results were recorded as the mean ± standard deviation for each
group.
Statistical analysis
Data were presented as the means ± SD. Discrepancies
among different groups were assessed by analysis of variance
(ANOVA) and the difference between two groups was assessed by the
Student’s t-test, while differences in the number of TILs were
investigated by using the non-parametric Wilcoxon rank-sum test.
P<0.05 was considered statistically significant. Statistical
analyses were performed using PASW Statistics software, version
18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Expression of CEACAM6 and 4-1BBL protein
in COS-7 cell and identification of recombinant attenuated
Salmonella
The control, empty plasmid and recombinant gene
transfection COS7 cell groups were identified by RT-PCR.
Amplification products were measured by 1% agarose gel
electrophoresis and recombinant gene transfection cells showed 801
and ~930-bp specific bands under UV light. The control and empty
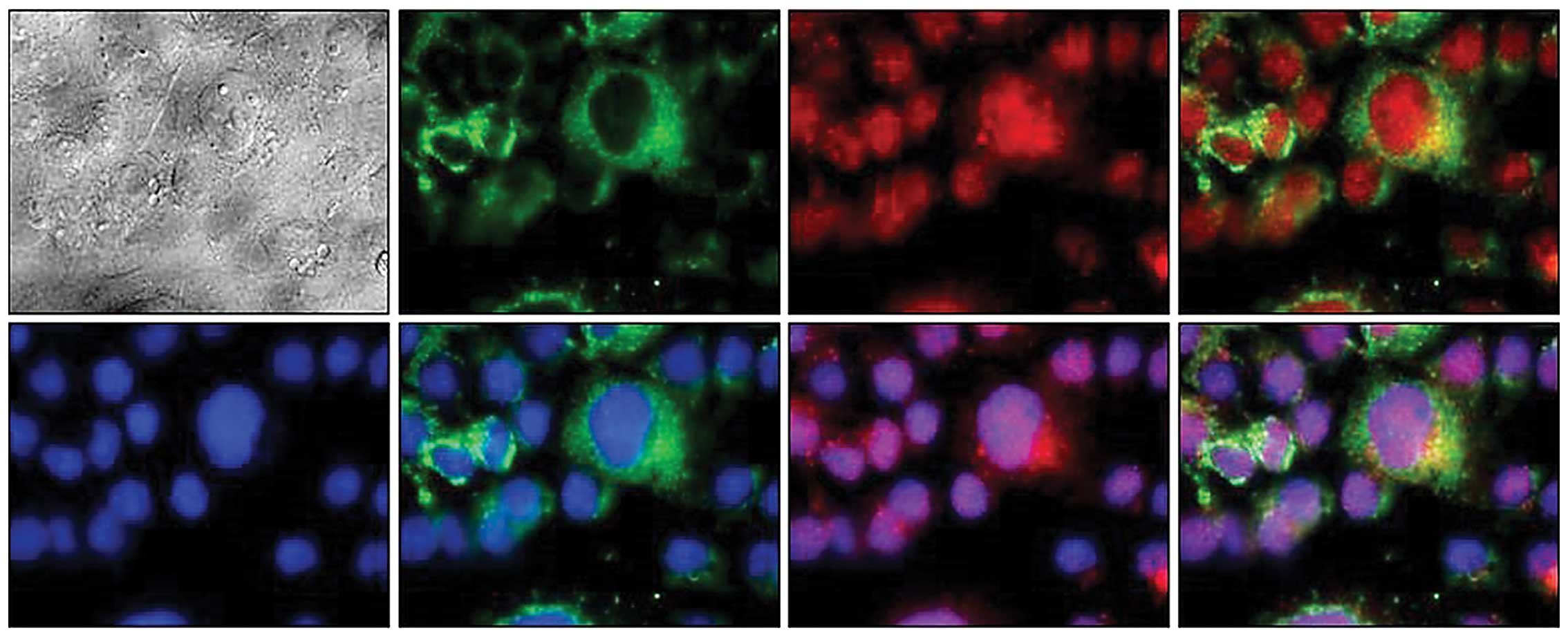
vector groups had no specificity band (data not shown). Confocal
microscopic analysis showed efficient delivery of CEACAM6 and
4-1BBL antigen. As shown in Fig. 2,
4-1BBL protein is a transmembrane protein, mainly located on both
sides of the cell membrane, while CEACAM6 was mainly expressed in
the cytoplasm. When stained with anti-4-1BBL-FITC or
anti-CEACAM6-CY3, 4-1 BBL green fluorescence protein was evident
while CEACAM6 appeared red under laser confocal microscopy. The
attenuated Salmonella-containing plasmids also identified by
RT-PCR, i.e., pIRES-4-1BBL/SL3261 pIRES-CEACAM6/SL3261 and
pIRES-CEACAM6-4-1BBL/SL3261, showed the bands corresponded with
CEACAM6 cDNA and 4-1 BBL cDNA whereas the empty vector group had no
specific band (data not shown).
Vaccination with the SL3261D reduces the
number of colorectal tumors in rats
Following the induction of colorectal tumors, two
rats from the SL3261A group had bloody stools at 18 weeks after the
initial DMH treatment while the other groups did not exhibit such a
characteristic. Characterization of animals indicated that a large
amount of bloody ascites was found in the rats. A great number of
tumor nodules varying in size were observed in the mesentery and
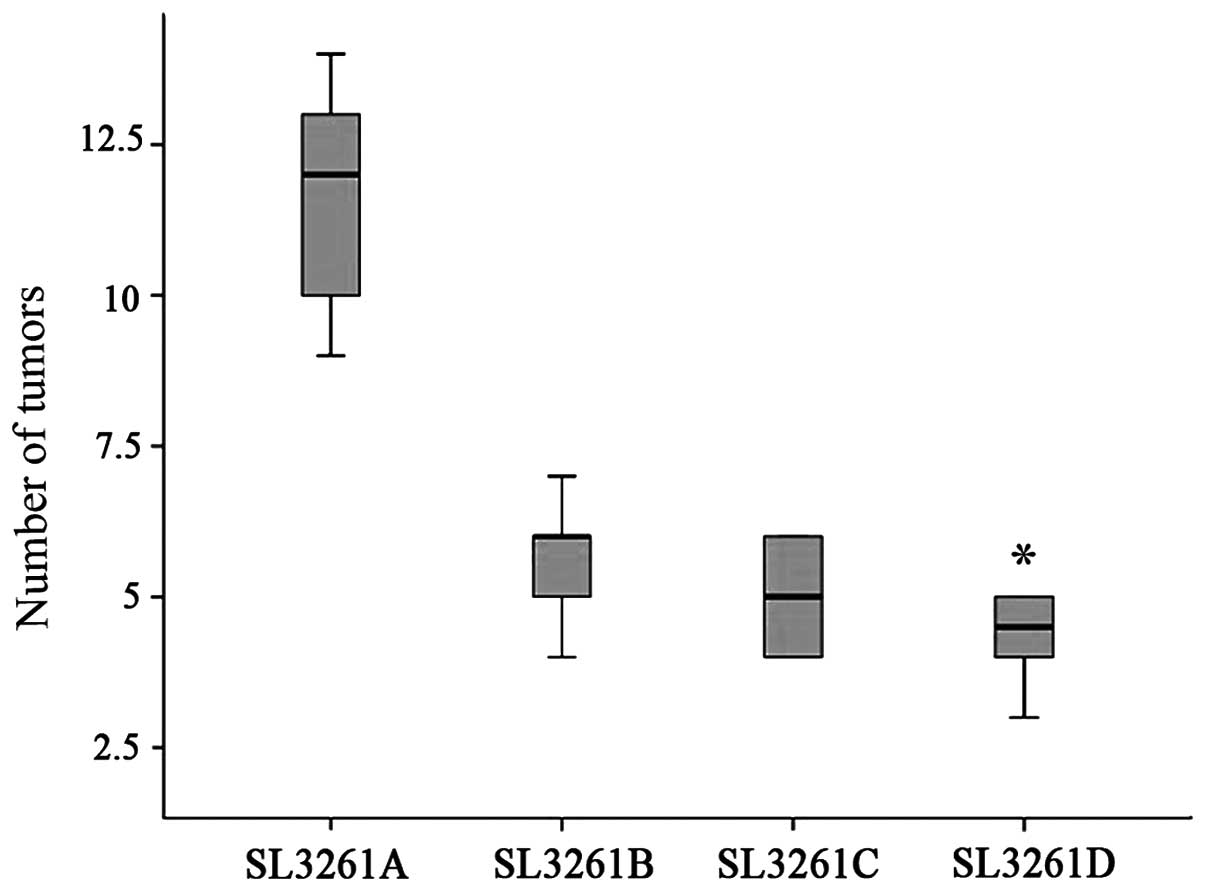
posterior peritoneum. The number of tumors at 18 weeks after DMH
treatment were 11.7±2.1 in the pIRES/SL3261 group, which was
significantly higher than that in the pIRES-4-1BBL/SL3261 group of
5.7±1.2, pIRES-CEACAM6/SL3261 group of 5.0±1.4 and
pIRES-CEACAM6-4-1BBL/SL3261 group of 4.3±1.4 (P<0.05) (Fig. 3). In addition, we found that most of
the tumors in the pIRES/SL3261 group of rats were at an advanced
stage (stage B-C, 4; stage D, 2), while most tumors in the
pIRES-4-1BBL/SL3261 and pIRES-CEACAM6/SL3261 groups were at stage
B-C (SL3261B: stage A, 3; stage B-C, 3; SL3261C: stage A, 1; stage
B-C, 5). However, the tumors in the pIRES-CEACAM6-4-1BBL/SL3261
group of rats were at stage A (stage A, 5; stage B-C, 1) (Table I). Moreover, there was no
significant difference in the rat body weights among the four
groups, although slightly increased body weights were observed in
the pIRES-CEACAM6-4-1BBL/SL3261-vaccinated group of rats 16 weeks
after DMH application.
 | Table IAnalysis of tumor staging in rats
from different experimental groups. |
Table I
Analysis of tumor staging in rats
from different experimental groups.
| Groups | Stage A | Stage B–C | Stage D |
|---|
| pIRES/SL3261 | 0 | 4 | 2 |
|
pIRES-4-1BBL/SL3261 | 3 | 3 | 0 |
|
pIRES-CEACAM6/SL3261 | 1 | 5 | 1 |
|
pIRES-CEACAM6-4-1BBL/SL3261 | 5 | 1 | 0 |
Detection of TIL, CD56+ and
CEACAM6 cells
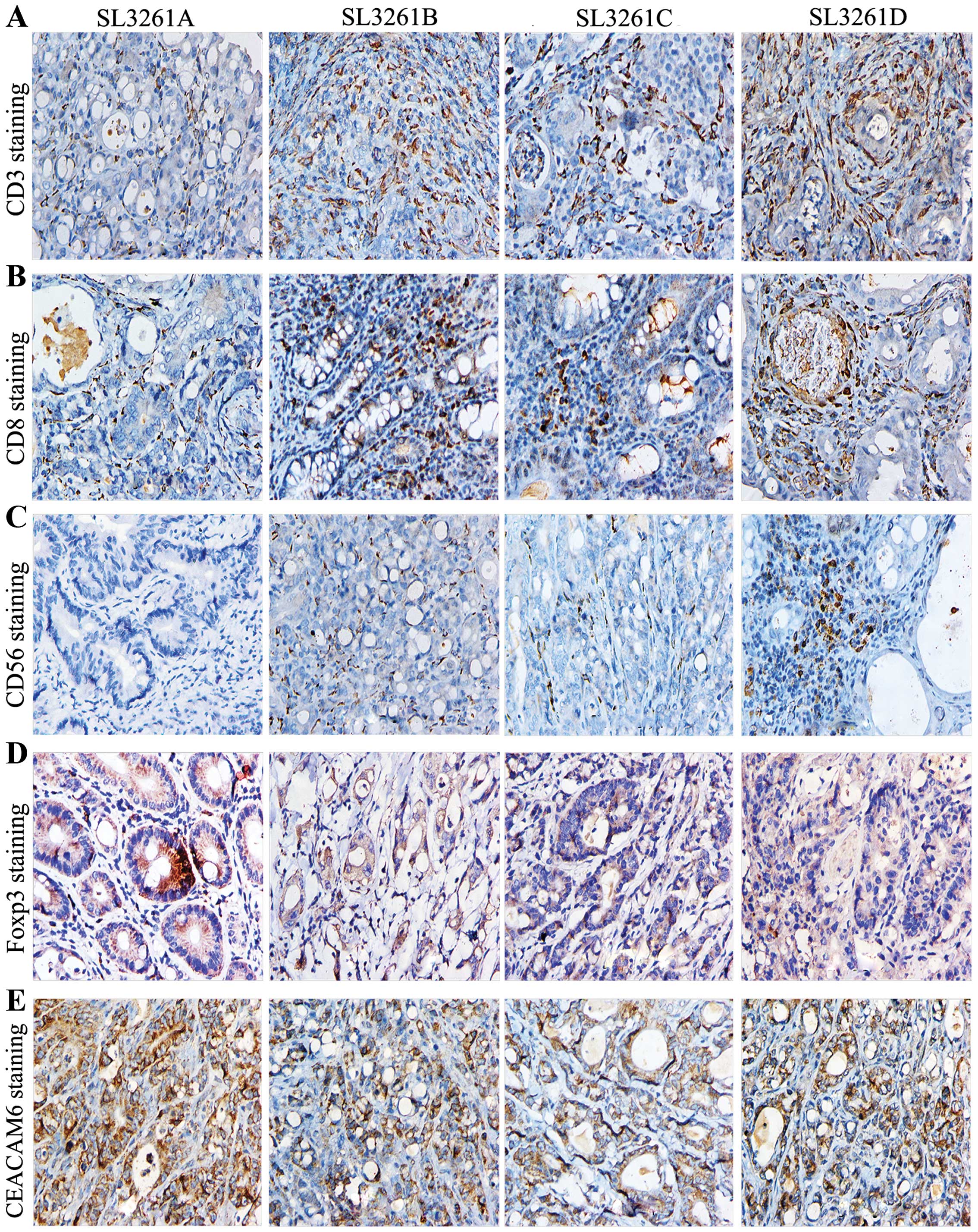
To determine whether TIL numbers correlated with the
number and staging of colorectal tumors in rats, we observed TIL
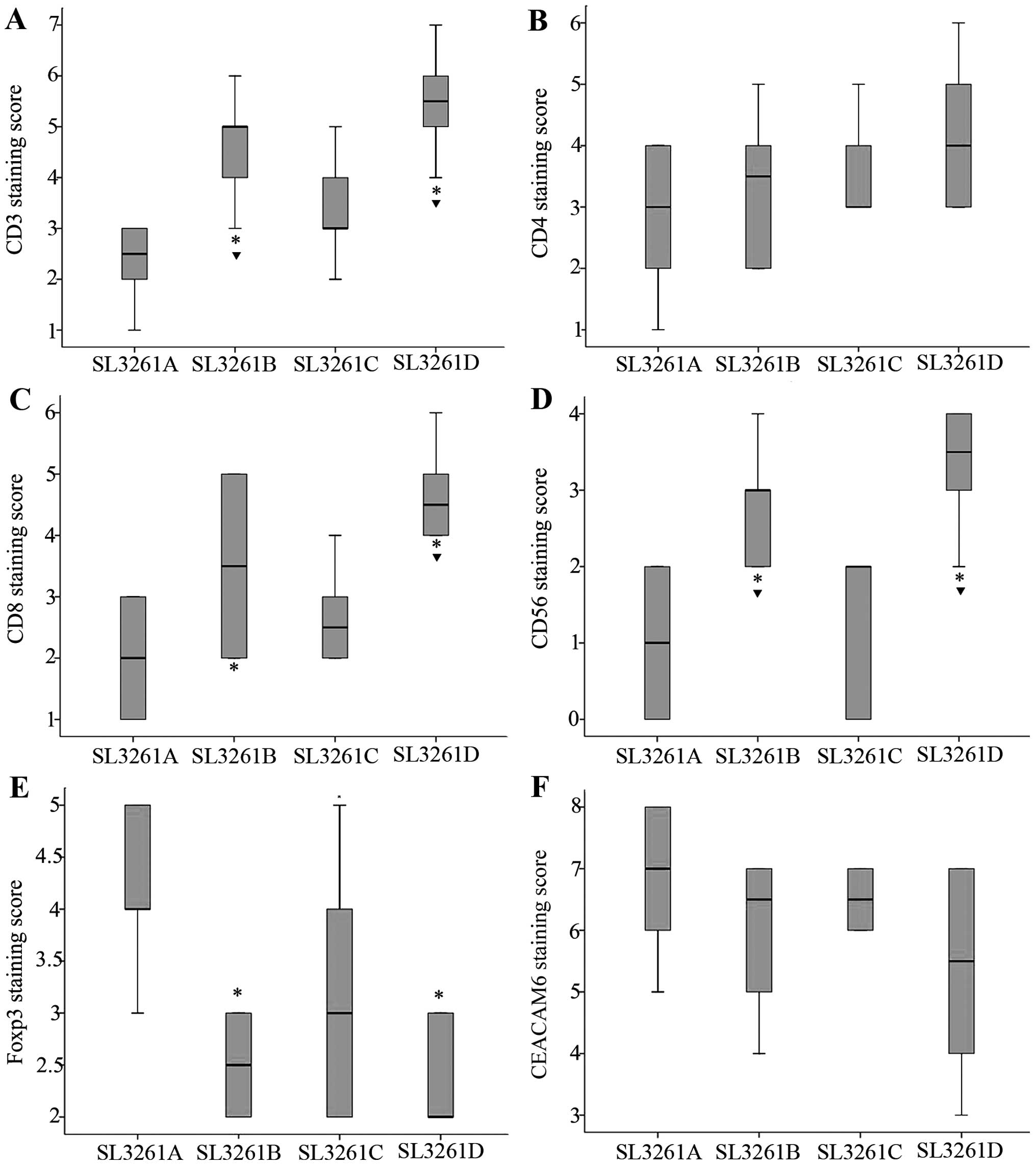
cells such as CD3, CD4, CD8, CD56, FOXP3 and CEACAM6 (Fig. 4). We found that CD3+ and
CD8+, but not the total number of CD4+ T
cells in the pIRES-4-1BBL/SL3261 and pIRES-CEACAM6-4-1BBL/SL3261
groups were significantly higher than that in the pIRES/SL3261
group (P<0.05) (Fig. 5A–C).
Furthermore, the number of CD3+ TIL cells in
pIRES-4-1BBL/SL3261 and pIRES-CEACAM6-4-1BBL/SL3261 was higher than
that in the pIRES-CEACAM6/SL3261 group (P<0.05). The number of
CD8+ TIL cells in pIRES-CEACAM6-4-1BBL/SL3261 were
higher than that in the pIRES-CEACAM6/SL3261 group (P<0.05),
while CD56+ cells in the pIRES-4-1BBL/SL3261 and
pIRES-CEACAM6-4-1BBL/SL3261 groups were significantly higher than
that in the pIRES/SL3261 and pIRES-CEACAM6/SL3261 groups
(P<0.05). However, no significant difference was detected
between the pIRES-4-1BBL/SL3261 and pIRES-CEACAM6-4-1BBL/SL3261
groups (P>0.05) (Fig. 5D). The
number of Foxp3 cells in the pIRES-4-1BBL/SL3261,
pIRES-CEACAM6/SL3261 and pIRES-CEACAM6-4-1BBL/SL3261 groups was
significantly lower than that in the pIRES/SL3261 groups
(P<0.05), albeit there were no obvious difference among these
groups (P>0.05) (Fig. 5E).
Notably, the cell counts of CEACAM6 were the highest in
pIRES/SL3261, but the lowest in the pIRES-CEACAM6-4-1BBL/SL3261
groups, albeit there were no statistical significance among the
four groups.
Discussion
The 4-1BBL is a type II surface glycoprotein of the
tumor necrosis factor (TNF) superfamily, and is generally expressed
in APCs, such as dendritic cells, macrophages and activated B cells
(4). 4-1BBL binds to 4-1BB (also
known as CD137), a member of the TNFR superfamily and enhances
T-cell activation (4). Previous
studies have shown that the activation of 4-1BB by agonistic
monoclonal antibodies (Abs) or overexpression of 4-1BBL enhances
T-cell proliferation and cytokine production, upregulates
anti-apoptotic gene expression and prevents activation-induced cell
death (19–21). Our previous findings have shown that
recombinant attenuated Salmonella harboring the 4-1BBL gene
induces high levels of 4-1BBL expression in dendritic and other
immune cells (15). Vaccination
with this recombinant gene enhanced T-cell immunity and inhibited
the development of carcinogen-induced colorectal cancers in rats
(22).
CEACAM5 and CEACAM6 are members of the
carcinoembryonic antigen-related cell adhesion molecule (CEACAM)
family. CEACAM5 has been recognized as a clinically relevant target
for passive and active immunotherapy and has been the focus of
various preclinical and clinical human studies using vaccines for
the active stimulation of B- and T-cell immunity (23–25).
CEACAM6 is also an important protein associated with cell adhesion
and is an independent prognostic factor associated with a higher
risk of colorectal cancer relapse (26). Since in all tumors, CEACAM6
expression was greater than that of CEACAM5, CEACAM6 may be an
important therapeutic target to control malignancy and/or
metastasis. CEACAM6 is now considered a valid clinical biomarker
and promising therapeutic target in melanoma, lung, colorectal and
pancreatic cancers (27). Thus, we
constructed recombinant Salmonella-based CEACAM6 and 4-1BBL vaccine
to examine its effect on the development of DMH-induced colorectal
cancer in rats and the potential immune mechanisms involved.
A previous study indicated that abnormal morphology
characterized by the accumulation of undifferentiated cells with
pleiomorphic and conspicuous nucleoli in a small cluster of
neighboring crypts was found at 12 weeks after DMH admini stration.
Microscopic carcinomatous foci were observed at 15 weeks after DMH
treatment (28). Therefore, in the
present study, the vaccine administration was initiated 8 weeks
after DMH treatment. We found that in the pIRES-4-1BBL/SL3261,
pIRES-CEACAM6/SL3261 and pIRES-CEACAM6-4-1BBL/SL3261 groups tumor
growth was suppressed as compared with the pIRES/SL3261 group
(P<0.05). However, no significant difference was found in the
pIRES-4-1BBL/SL3261, pIRES-CEACAM6/SL3261 and
pIRES-CEACAM6-4-1BBL/SL3261 groups (P>0.05), although a slightly
decreased number of colorectal tumors was observed in the
pIRES-CEACAM6-4-1BBL/SL3261 group. We induced colon cancer and
found that most of the tumors in the pIRES/SL3261 groups were in
late stage, while early and middle stages were found in the
pIRES-4-1BBL/SL3261 and pIRES-CEACAM6/SL3261 groups, whereas tumors
in the pIRES-CEACAM6-4-1BBL/SL3261 group were in early stage only.
Thus, the results suggest that recombinant bacteria-based 4-1BBL
and CEACAM6 vaccines effectively inhibited the development of
colorectal cancer.
Tumor-infiltrating lymphocytes (TILs) have been
considered to reflect host immune response against malignant tumors
(29). Tumor infiltration by T
lymphocytes has been shown to be associated with favorable
prognosis, particularly in colorectal cancers (30). On the other hand, tumor infiltration
by T-lymphocyte subsets endowed with immunoregulatory or
suppressive potential, e.g., CD4+ T cells expressing
FoxP3 transcription factor, has been suggested to be associated
with tumor progression and unfavorable prognosis (31). Thus, TIL cells in the present study
indicate the immune mechanisms of recombinant vaccine-suppressing
tumors.
CD3+CD8+ cells are involved in
T-cell immunity against tumors (32). To induce antigen-specific
CD8+ cytotoxic T lymphocytes (CTLs), peptides derived
from tumor associated antigens (TAAs) should be presented on the
surface of antigen-presenting cells (APCs) in the context of MHC
class I molecules. Previous findings have shown that vaccination
with antigen peptide connected with 4-1BBL enhances dendritic cell
activation and antigen uptake and promotes CD8+ T-cell
effector/memory responses (33,34).
As a TAA, CEACAM6 can present on the surface of APCs and induce
antigen-specific CTLs. CTL is also the main component of TIL cells.
In the present study, higher counts of CD8+ TIL cells
were evident in the pIRES-CEACAM6-4-1BBL/SL3261 group, suggesting
that the recombinant vaccine harboring the 4-1BBL and
CEACAM6 genes may induce specific immune and enhance T-cell
immunity and inhibit the development of colorectal cancer in rats.
As the first signal, the protein encoded by the transfected
CEACAM6 gene presented on the surface of APC, whereas as the
costimulatory molecule, the overexpressed 4-1BBL strengthened the
second signal, both of which induced the activation of specific
anti-CEACAM6 CTL.
Natural killer (NK) cells are part of the innate
immune system and have diverse biological functions including the
ability to recognize and kill a variety of tumor cells (35). Cytolysis is performed via the
release of cytotoxic granula-containing perforin and granzyme B
(GrmB) or by the induction of death receptor-mediated apoptosis. NK
cells are also able to produce a broad spectrum of chemokines and
cytokines. Previous findings (36)
have shown that NK cells are generally absent in CRC of all stages.
NK cell migration into CRC tumor tissue is obviously impaired early
during tumor development by mechanisms that do not affect T-cell
infiltration. In the present study, we found that the
CD56+ TIL cell densities were lower in the pIRES/SL3261
group, but higher in the pIRES-4-1BBL/SL3261 and
pIRES-CEACAM6-4-1BBL/SL3261 groups. Zhang et al (37) found that CD137 signaling can
increase the ability of CD3-CD56+NK cells to kill cancer
cells by upregulating the expression of NKG2D and LFA-1. Thus,
recombinant attenuated Salmonella harboring the 4-1BBL gene
can upregulate the expression of 4-1BBL and kill cancer cells by
the above mechanism.
Tregs are a small subset of
CD4+CD25+ T cells and are identified by their
expression of FOXP3, a transcription factor critical to their
differentiation and suppressive function (38). FOXP3+ cell counts have
been associated with a higher likelihood of disease progression in
several malignancies (39,40). Consequently, we analyzed
FOXP3+ TIL cells and found they were lower in the
pIRES-CEACAM6-4-1BBL/SL3261 group, but higher in the pIRES/SL3261
group. These findings suggest that a high number of Treg leads to a
more suppressive tumor microenvironment. However, the recombinant
Salmonella-based CEACAM6 and 4-1BBL vaccine can break this
microenvironment, decrease FOXP3 and inhibit colorectal cancer in
rats, although the underlying mechanism remains to be
determined.
We detected the expression of CEACAM6 in each group,
and found that there were no more counts of intratumoral CEACAM6 in
the group in which the vaccine harbored the CEACAM6 gene. By
contrast, more counts of intratumoral CEACAM6 were identified in
the pIRES/SL3261 group. Given that CEACAM6 is a TAA, more tumors
were identified in the pIRES/SL3261 group. Consequently, the
expression of TAA-CEACAM6 was higher, but due to inhibition of the
tumor microenvironment, the TAA could not be recognized by the
immune system and killed. The vaccine containing the CEACAM6
gene bypassed the tumor microenvironment, while as an endogenous
antigen, CEACAM6 could present on the surface of APCs more
efficiently and induce the body to produce a specific antitumor
immune response to inhibit tumor growth, while
pIRES-CEACAM6-4-1BBL/SL3261 group had the least number of tumors
and the earlier stage, presumably because of the existence of
costimulatory molecule 4-1 BBL to amplify the antitumor effect.
In summary, the results indicate that recombinant
CEACAM6 and 4-1BBL genes effectively delivered
CEACAM6 and 4-1BBL antigen to attenuated Salmonella and the
Salmonella-based vaccine efficiently inhibited the development of
DMH-induced colorectal tumors in rats, accompanied by increasing of
the number of CD3+CD8+ and NK TIL cells and
decreasing the number of FOXP3 cells by inducing specific and
non-specific immunity and breaking the suppressive tumor
microenvironment. Moreover, it was technically much simpler to
administer. Our findings therefore may provide a new basis for the
design of immunotherapies for the intervention of colorectal
cancer.
Acknowledgments
The present study was supported by the grants from
the National Natural Science Foundation of China (grant no.
81172166), the Natural Science Foundation of Jiangsu Province of
China (grant no. BK2008171), and the Natural Science Foundation of
the Jiangsu Higher Education Institutions of China (grant no.
11KJB320016).
Abbreviations:
|
CEACAM6
|
non-specific cross-reacting
antigen
|
|
4-1BBL
|
4-1BB ligand
|
|
CTL
|
cytotoxic T lymphocyte
|
|
DMH
|
1, 2-dimethylhydrazine
|
|
FOXP3
|
forkhead/winged-helix transcription
factor box P3
|
|
TIL
|
tumor-infiltrating lymphocyte
|
References
|
1
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vinay DS and Kwon BS: Immunotherapy of
cancer with 4-1BB. Mol Cancer Ther. 11:1062–1070. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hodge JW, Greiner JW, Tsang KY, Sabzevari
H, Kudo-Saito C, Grosenbach DW, Gulley JL, Arlen PM, Marshall JL,
Panicali D, et al: Costimulatory molecules as adjuvants for
immunotherapy. Front Biosci. 11:788–803. 2006. View Article : Google Scholar
|
|
4
|
Cheuk AT, Mufti GJ and Guinn BA: Role of
4-1BB: 4-1BB ligand in cancer immunotherapy. Cancer Gene Ther.
11:215–226. 2004. View Article : Google Scholar
|
|
5
|
Laderach D, Movassagh M, Johnson A,
Mittler RS and Galy A: 4-1BB co-stimulation enhances human
CD8+ T cell priming by augmenting the proliferation and
survival of effector CD8+ T cells. Int Immunol.
14:1155–1167. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shuford WW, Klussman K, Tritchler DD, Loo
DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen
CP, et al: 4-1BB costimulatory signals preferentially induce
CD8+ T cell proliferation and lead to the amplification
in vivo of cytotoxic T cell responses. J Exp Med. 186:47–55. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cannons JL, Lau P, Ghumman B, DeBenedette
MA, Yagita H, Okumura K and Watts TH: 4-1BB ligand induces cell
division, sustains survival, and enhances effector function of CD4
and CD8 T cells with similar efficacy. J Immunol. 167:1313–1324.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cooper D, Bansal-Pakala P and Croft M:
4-1BB (CD137) controls the clonal expansion and survival of CD8 T
cells in vivo but does not contribute to the development of
cytotoxicity. Eur J Immunol. 32:521–529. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee HW, Park SJ, Choi BK, Kim HH, Nam KO
and Kwon BS: 4-1BB promotes the survival of CD8+ T
lymphocytes by increasing expression of Bcl-xL and Bfl-1. J
Immunol. 169:4882–4888. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuroki M, Matsuo Y, Kinugasa T and
Matsuoka Y: Three different NCA species, CGM6/CD67, NCA-95, and
NCA-90 are comprised in the major 90 to 100 kDa band of granulocyte
NCA detectable upon SDS-polyacrylamide gel electrophoresis. Biochem
Biophys Res Comm. 182:501–506. 1992. View Article : Google Scholar
|
|
11
|
Scholzel S, Zimmermann W, Schwarzkopf G,
Grunert F, Rogaczewski B and Thompson J: Carcinoembryonic antigen
family members CEACAM6 and CEACAM7 are differentially expressed in
normal tissues and oppositely deregulated in hyperplastic
colorectal polyps and early adenomas. Am J Pathol. 157:1051–1052.
2000.
|
|
12
|
Kuroki M, Matsushita H, Matsumoto H,
Hirose Y, Senba T and Yamamoto T: Nonspecific cross-reacting
antigen 50/90 (NCA-50/90) as a new tumor marker. Anticancer Res.
19:5599–5606. 1999.
|
|
13
|
Hinoda Y, Saito T, Takahashi H, Itoh F,
Adachi M and Imai K: Induction of nonspecific cross-reacting
antigen mRNA by interferongamma and anti-fibronectin receptor
antibody in colon cancer cells. J Gastroenterol. 32:200–205. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blumenthal RD, Leon E, Hansen HJ and
Goldenberg DM: Expression patterns of CEACAM5 and CEACAM6 in
primary and metastatic cancers. BMC Cancer. 7:22007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye JX, Zhang YT, Zhang XG, Ren DM and Chen
WC: Recombinant attenuated Salmonella harboring 4-1BB ligand gene
enhances cellular immunity. Vaccine. 27:1717–1723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan JX, Chen WC, Zhang XG and Zhang GB:
The change of immunoactivity of dendritic cells induced by
recombinant rat 4-1BBL. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
24:764–767. 2008.In Chinese. PubMed/NCBI
|
|
17
|
Kyriakos M: The President’s cancer, the
Dukes classification, and confusion. Arch Pathol Lab Med.
109:1063–1066. 1985.PubMed/NCBI
|
|
18
|
Kawai H, Ishii A, Washiya K, Konno T, Kon
H, Yamaya C, Ono I, Minamiya Y and Ogawa J: Estrogen receptor alpha
and beta are prognostic factors in non-small cell lung cancer. Clin
Cancer Res. 11:5084–5089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watts TH: TNF/TNFR family members in
costimulation of T cell responses. Annu Rev Immunol. 23:23–68.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Croft M: Co-stimulatory members of the
TNFR family: keys to effective T-cell immunity? Nat Rev Immunol.
3:609–620. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lim HY, Kim KK, Zhou FC, Yoon JW, Hill JM
and Kwon BS: 4-1BB-like molecule is expressed in islet-infiltrating
mono-nuclear cells and in the gray matter of the brain. Cell Biol
Int. 26:271–278. 2002. View Article : Google Scholar
|
|
22
|
Ye J, Li L, Zhang Y, Zhang X, Ren D and
Chen W: Recombinant salmonella-based 4-1BBL vaccine enhances T cell
immunity and inhibits the development of colorectal cancer in rats:
in vivo effects of vaccine containing 4-1BBL. J Biomed Sci.
20:82013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hou Y, Kavanagh B and Fong L: Distinct
CD8+ T cell repertoires primed with agonist and native
peptides derived from a tumor-associated antigen. J Immunol.
180:1526–1534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Loisel-Meyer S, Felizardo T, Mariotti J,
Mossoba ME, Foley JE, Kammerer R, Mizue N, Keefe R, McCart JA and
Zimmermann W: Potent induction of B- and T-cell immunity against
human carcinoembryonic antigen-expressing tumors in human
carcinoembryonic antigen transgenic mice mediated by direct
lentivector injection. Mol Cancer Ther. 8:692–702. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hörig H, Medina FA, Conkright WA and
Kaufman HL: Strategies for cancer therapy using carcinoembryonic
antigen vaccines. Expert Rev Mol Med. 2:1–24. 2000.
|
|
26
|
Jantscheff P, Terracciano L, Lowy A,
Glatz-Krieger K, Grunert F, Micheel B, Brümmer J, Laffer U, Metzger
U, Herrmann R, Rochlitz C, et al: Expression of CEACAM6 in
resectable colorectal cancer: a factor of independent prognostic
significance. J Clin Oncol. 21:3638–3846. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beauchemin N and Arabzadeh A:
Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs)
in cancer progression and metastasis. Cancer Metastasis Rev.
32:643–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maskens AP: Histogenesis and growth
pattern of 1, 2-dimethylhydrazine-induced rat colon adenocarcinoma.
Cancer Res. 36:1585–1592. 1976.PubMed/NCBI
|
|
29
|
Takagi S, Chen K, Schwarz R, Iwatsuki S,
Herberman RB and Whiteside TL: Functional and phenotypic analysis
of tumor-infiltrating lymphocytes isolated from human primary and
metastatic liver tumors and cultured in recombinant interleukin-2.
Cancer. 63:102–111. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B and Lagorce-Pages C: Type, density, and
location of immune cells within human colorectal tumors predict
clinical outcome. Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Curiel TJ: Regulatory T cells and
treatment of cancer. Curr Opin Immunol. 20:241–246. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schiavon V, Roth P, Bolton WE, Farcet JP,
Bensussan A and Boumsell L: Lymphocytes subsets in normal
individuals: analysis by four color immunofluorescence and flow
cytometry on whole blood. Tissue Antigens. 48:312–318. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sharma RK, Elpek KG, Yolcu ES, Schabowsky
RH, Zhao H, Bandura-Morgan L and Shirwan H: Costimulation as a
platform for the development of vaccines: a peptide-based vaccine
containing a novel form of 4-1BB ligand eradicates established
tumors. Cancer Res. 69:4319–4326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharma RK, Schabowsky RH, Srivastava AK,
Elpek KG, Madireddi S, Zhao H, Zhong Z, Miller RW, Macleod KJ,
Yolcu ES, et al: 4-1BB ligand as an effective multifunctional
immunomodulator and antigen delivery vehicle for the development of
therapeutic cancer vaccines. Cancer Res. 70:3945–3954. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Walzer T, Jaeger S, Chaix J and Vivier E:
Natural killer cells: from CD3−NKp46+ to
post-genomics meta-analyses. Curr Opin Immunol. 19:365–372. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Halama N, Braun M, Kahlert C, Spille A,
Quack C, Rahbari N, Koch M, Weitz J, Kloor M, Zoernig I,
Schirmacher P, et al: Natural killer cells are scarce in colorectal
carcinoma tissue despite high levels of chemokines and cytokines.
Clin Cancer Res. 17:678–689. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Ju S and Shu Y: Research on the
effects of CD137 signaling on the function of
CD3-CD56+NK cells. J Nanjing Medical University.
23:10–14. 2009. View Article : Google Scholar
|
|
38
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hiraoka N, Onozato K, Kosuge T and
Hirohashi S: Prevalence of FOXP3+ regulatory T cells
increases during the progression of pancreatic ductal
adenocarcinoma and its premalignant lesions. Clin Cancer Res.
12:5423–5434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kobayashi N, Hiraoka N, Yamagami W, Ojima
H, Kanai Y and Kosuge T: FOXP3+ regulatory T cells
affect the development and progression of hepatocarcinogenesis.
Clin Cancer Res. 13:902–911. 2007. View Article : Google Scholar : PubMed/NCBI
|