Introduction
RNA editing is a post-transcriptional process that
generates many different mRNAs from the same gene.
Adenosine-to-inosine (A-to-I) RNA editing is the most common type
of RNA editing in mammals, and is catalyzed by adenosine deaminases
acting on RNA (ADARs). ADAR2 is the main enzyme responsible for
A-to-I RNA editing. It has a catalytic domain and three
double-stranded RNA binding domains (1–4). The
potential impact of RNA editing on the etiology or progression of
human diseases is now recognized. Deficient or hyperactive A-to-I
RNA editing is associated with several types of human diseases,
including epilepsy, amyotrophic lateral sclerosis, immunological
disorders and cancers. Analysis of RNA editing in cancers is
expected to provide new diagnostic and prognostic markers (5–9).
Gliomas are the most common malignant tumors of the
central nervous system, and are almost always fatal. The molecular
basis for the malignant progression of glioma involves several
collaborative processes. Several studies have shown that a general
state of underediting exists in gliomas at several RNA editing
sites, including the glutamine (Q)/arginine (R) site of glutamate
receptor subunit B (GluR-B), the R/G site of GluR-B, and the
5-HT2C serotonin receptor site. Editing the Q/R site of
GluR-B is essential for the normal function of the neurotransmitter
receptor, and underediting at this site is associated with the
pathogenesis and invasiveness of glioma (5,10).
Significantly reduced editing at the Q/R site has been detected in
gliomas and correlates with the grade of the malignancy. A-to-I RNA
editing is catalyzed by ADAR2. The level of ADAR2 mRNA expression
is thought to play an important role in regulating the editing
activity of the enzyme. However, the levels of ADAR2 mRNA
expression in gliomas have been inconsistent in previous studies.
Furthermore, there is no consistent correlation between the editing
efficiency and ADAR2 mRNA expression in those studies. Therefore,
the regulation of the RNA editing activity of ADAR2 in vivo
remains largely unclear (10–13).
Human ADAR2 gene maps to chromosome 21 band
q22.3 and spans ~150 kb, and is now known to have 15 exons, as
summarized in Fig. 1. Two exons,
denoted exons -2 and -1, specify the 5′-untranslated region
(5′-UTR). Exons 0–9 represent the open reading frame that encodes
ADAR2. Various ADAR2 transcript isoforms result from multiple
alternative splicing events (3,4).
Nine splice sites have so far been detected in human
ADAR2 pre-mRNA, as summarized in Table
I. Among the possible alternative splicing events, five have
been demonstrated to affect the catalytic function of ADAR2
(14–20). In human ADAR2, the inclusion of exon
1a between exon -1 and 1 results in an N-terminal extension of 28
amino acids, which includes seven arginine (R)/lysine (K) residues.
An additional exon, designated exon 0, positioned between exon -1
and exon 1, has been characterized in human ADAR2. The inclusion of
exon 0, initiated at AUG49, results in the addition of 49 amino
acids at the N-terminus. Neither the relative protein abundance nor
the functional properties of the ADAR2 isoforms containing the
altered N-terminal residues have been determined.
 | Table IAlternative splicing sites in human
ADAR2 reported in previous studies. |
Table I
Alternative splicing sites in human
ADAR2 reported in previous studies.
| Authors (ref.) | Year | Alternative splice
site | Effect on ADAR2
transcript | Effect on ADAR2
protein | Effect on catalytic
activity |
|---|
| Gerber et al
(14) | 1997 | Between exons 5 and
6 | Inclusion of exon
5a | Adds an AluJ cassette
in the catalytic domain | Reduced |
| Lai et al
(15) | 1997 | Within exon 9 | Truncates the 3′ end
of the coding region | Replaces the 29
C-terminal residues with 2 amino acids | No catalytic
activity |
| Rueter et al
(16) | 1999 | Between exons 1 and
2 | Addition of 47
nucleotides to the 5′ end of exon 2 | Generates a 9-kDa
protein | Reduced |
| Slavov and Gardiner
(17) | 2002 | Between exons -1 and
1 | Inclusion of exon
1a | N-terminal extension
by 28 amino acids | Unknown |
| Kawahara et al
(18) | 2005 | Between exons 1 and
3 | Skipping of exon
2 | Produces a protein of
only 12 amino acids | No catalytic
activity |
| Kawahara et al
(18) | 2005 | Between exons 9 and
10 | Inclusion of intron
9 | Unknown | Unknown |
| Kawahara et al
(18) | 2005 | Within exon 9 | Splices exon 9 at a
position 83 nucleotides downstream from the stop codon | Unknown | Unknown |
| Mass and Gommans
(19) | 2009 | Between exons -1 and
1 | Inclusion of exon
0 | N-terminal extension
of 49 amino acids | Unknown |
| Agranat et al
(20) | 2010 | Between exons 7 and
8 | Inclusion of exon
7a | Leads to
nonsense-mediated mRNA decay | No catalytic
activity |
Another alternative splicing event in ADAR2 is
mediated by A-to-I auto-editing, which creates a new 3′ splice
acceptor site (changing the adenosine-adenosine to
adenosine-inosine) in the intron 1 sequence of the ADAR2 mRNA. This
editing-dependent splicing at this site results in the addition of
47 nt to the 5′ end of exon 2 in the ADAR2 mRNA, causing a
frameshift in the coding region. This is predicted to generate a
9-kDa ADAR2 protein because a premature translational termination
codon is introduced. Another ADAR2 isoform is produced by
alternative splicing in exon 5, which causes the inclusion of exon
5a. The alternatively spliced exon 5a encodes an in-frame AluJ
cassette in the catalytic domain. The inclusion of exon 5a results
in a protein with ~50% reduced activity. Another alternative
splicing event in the human ADAR2 mRNA causes the skipping of exon
2. The lack of exon 2 is predicted to result in a frameshift that
would introduce a stop codon in exon 3, producing a truncated
protein of only 12 amino acids if translated.
A newly discovered alternatively spliced isoform of
the human ADAR2 gene was reported by Agrant et al (20), in which a 93-nt sequence located in
intron 7 is included as exon 7a. The splicing event at this site
introduces a premature termination codon and may direct the
alternatively spliced transcripts to the nonsense-mediated mRNA
decay RNA surveillance pathway. Alternative splicing events between
exons 9 and 10 of the human ADAR2 mRNA produce multiple isoforms
with different C-terminal sequences or 3′-UTR regions. The use of
an alternative splice site within exon 9 of human ADAR2 leads to
the replacement of the 29 C-terminal residues with two amino acids
encoded by exon 10, which generates an inactive protein that cannot
edit the GluR-B pre-mRNA. Recent characterization of the human
brain ADAR2 transcripts in different developmental stages (fetal,
neonatal and adult) revealed four additional C-terminal variants
resulting from alternative splicing, which lead to the inclusion of
intron 9 and an alternative splicing site in exon 9 at a position
83 nt downstream from the stop codon. Whether variations within the
3′-UTR affect the stability, transport, or translational efficiency
of ADAR2 requires experimental evidence. The exceptional diversity
of the ADAR2 transcripts and consequent ADAR2 protein isoforms
implies a physiological mechanism that regulates the functional
activities of the ADAR2 enzyme. Alternative splicing is regulated
according to the cell type, and the developmental and disease
state. The molecular details of how the ADAR2 transcription
is regulated in specific tissues or at different developmental
stages are poorly understood.
Based on the results of previous studies, we
speculated that the level of total ADAR2 mRNA does not necessarily
reflect its editing activity in vivo, and that alternative
splicing events may regulate the editing activity of human ADAR2 in
glioma.
In the present study, we explored the mechanism
regulating A-to-I RNA editing in glioma. First, we determined the
A-to-I RNA editing level at the Q/R site in the GluR-B mRNA in the
human glioma cell lines U87, U251 and A172, and the NHAs HA1800. We
then analyzed the expression of ADAR2 mRNA in the glioma cell lines
and NHAs, and compared the alternative splicing patterns in these
cell lines. We attempt to explain the underediting at the GluR-B
Q/R site from the perspectives of the total ADAR2 mRNA expression
and the alternative splicing of the ADAR2 pre-mRNA.
Materials and methods
Cell culture
The human glioma cell lines U87, U251 and A172, and
NHAs HA1800 were purchased from Boster Biological Technology, Ltd.
(Wuhan, China), and were routinely cultured in Dulbecco’s modified
Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum
(FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml) in
a 5% CO2 atmosphere at 37°C. DMEM, FBS, and other tissue
culture reagents were purchased from Beijing DingGuo ChangSheng
Biotechnology Co., Ltd. (Beijing, China).
The study was approved by the Ethics Committee of
the China-Japan Union Hospital of Jilin University, Changchun,
Jilin, China.
RT-PCR, cloning and sequencing
Total RNA was isolated from the U87, U251, A172, and
HA1800 cells using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) and the RNA concentration was determined
according to the manufacturer’s instructions. Complementary DNA
(cDNA) was synthesized using a HiFi-MMLV cDNA kit (Beijing Kang
Century Biotechnology Co., Ltd., Beijing, China).
To analyze the alternative splicing of ADAR2
pre-mRNA, five specific primer pairs were designed to amplify
different regions that would collectively contain all the
alternative splicing sites. The first region is between exon -1 and
exon 1, in which alternative exon 1a is included. Exon 1a has an
alternative potential transcription initiation site. The second
region includes exons 1–4, and contains two alternative splice
sites: one allows the addition of a 47-nt cassette when
self-editing occurs in the upstream intron, the other allows exon 2
to be spliced out. The third region covers exons 4–6, and encodes
two alternative splice variants. Exon 5a encodes an in-frame Alu
sequence. The fourth region covers exons 6–8, and the inclusion of
exon 7a in this region can lead to nonsense-mediated mRNA decay.
The last region includes exons 8–10 and may contain three
alternative splice sites. The longest variant contains intron 9.
The second longest variant contains the whole of exon 9. In the
third longest variant, the latter half of exon 9 is spliced out at
a position 83 nt downstream from the stop codon in the long
C-terminus. The region encoding the long C-terminus is spliced out
from exon 9 in the short variant.
All the primers were designed using Primer 5
software (Premier Biosoft, Palo Alto, CA, USA) and synthesized by
GenScript Co., Ltd. (Nanjing, China). The sequences of the primers
for ADAR2 amplification are listed in Table II. Primers for the β-actin gene
were 5′-GGCACCACACCTTCTACAAT-3′ (forward) and
5′-GGCACCACACCTTCTACAAT-3′ (reverse). PCR was performed using
GoldStar Best DNA polymerase (Beijing Kang Century Biotechnology).
Reaction conditions were as follows: 9°C for 10 min followed by 40
cycles of degradation at 94°C for 30 sec, annealing at 56°C for 30
sec and extension at 72°C for 60 sec, and then 72°C for 10 min. PCR
products were subjected to electrophoresis on a 1.0% agarose gel,
then scanned and analyzed with a gel imaging system. Different
bands were all extracted and purified with the Quick Gel Extraction
kit, and cloned the PCR products into the pUC-T vector (both from
Beijing Kang Century Biotechnology). Positive clones were sequenced
by Shanghai Sangon Biological Engineering Technology & Services
Co., Ltd. (Shanghai, China). Similarities between the nucleic acid
sequences were based on a two-sequence BLAST analysis.
 | Table IIPrimer pairs for RT-PCR. |
Table II
Primer pairs for RT-PCR.
| Regions | Forward | Reverse |
|---|
| Exon -1-Exon 1 |
TTCAGGCTGGCATGGAGAGCTT |
TGAGGGTTTCTTGACTGGCGGA |
| Exon 1-Exon 4 |
CCGCCAGTCAAGAAACCCTCAA |
ATGCAAGGCCACGATCACTCAT |
| Exon 4-Exon 6 |
TACATGAGTGATCGTGGCCTTGC |
GTCCGTAGCTGTCCTCTTGCTT |
| Exon 6-Exon 8 |
CAATGCGAGCATCCAAACGTGG |
ATGACCTCAATAGCGGAGTCGC |
| Exon 8-Exon 10 |
TCAGAGCTTTGAACAGGATACT |
GCTGCATGTAGTGGTTCTCC |
Real-time quantitative PCR (qPCR)
analysis
Primers were designed to amplify the total ADAR2
mRNA and the specific alternative splicing transcripts of ADAR2.
All the primers were designed using Primer 5 software and
synthesized by GenScript. The sequences of the primers used for
real-time PCR are listed in Table
III. Total RNA and cDNA were prepared as previously described.
RT-qPCR was performed with UltraSYBR mixture (Beijing Kang Century
Biotechnology). Reaction conditions were as follows: 95°C for 10
min followed by 40 cycles of degradation at 95°C for 15 sec,
annealing at 56°C for 30 sec and extension at 72°C for 30 sec. All
samples were run in triplicate and changes in the gene expression
were calculated using the ΔΔCt method.
 | Table IIIPrimer pairs for RT-qPCR. |
Table III
Primer pairs for RT-qPCR.
| Gene/Exons | Forward | Reverse |
|---|
| Total ADAR2 |
CAATGCGAGCATCCAAACGTGG |
ATGACCTCAATAGCGGAGTCGC |
| 1a (+) ADAR2 |
GGGCAACTGAAGGAGACACACT |
GGTGTGCTCATTGGCCATTTCA |
| 1a (−) ADAR2 |
CGCGGAGTTCTGCTTACTTCAG |
GCGGAGACTGTTTCTGTCTTGA |
| 2 (+) ADAR2 |
GCCTGGTTTGCAGTACACACTC |
CCTGGTCAGATGTGAAGTCCGT |
| 2 (−) ADAR2 |
GAAAACATGAGTTTTAGCTGACGC |
TAATGCAAGGCCACGATCACTC |
| 5a (+) ADAR2 |
TCCTGGAAGGGTCTCGCTCTTAC |
GTCCGTAGCTGTCCTCTTGCTT |
| 5a (−) ADAR2 |
TCCTGGAAGAACCAGCAGATAG |
CGAGAAGTAAATGGGCTCCACG |
| β-actin |
GGCACCACACCTTCTACAAT |
GGCACCACACCTTCTACAAT |
Quantification of RNA editing at the Q/R
site of GluR-B
The editing levels at the Q/R site of the GluR-B
mRNA were analyzed by RT-PCR and direct sequencing. Total RNA and
cDNA were prepared as previously described. Primers for the GluR-B
mRNA amplification were 5′-ACCCTTGTGAGAGAGAAGAGGTGAT-3′ (forward)
and 5′-TGGAGCCAGAGTCTAATGTTCCAT-3′ (reverse). An RT-PCR analysis of
the region surrounding the Q/R site was performed with GoldStar
Best DNA polymerase. Reaction conditions were as follows: 95°C for
10 min followed by 40 cycles of degradation at 94°C for 30 sec,
annealing at 57°C for 30 sec, and extension at 72°C for 60 sec and
then 72°C for 10 min. The PCR products were extracted with the
Quick Gel Extraction kit (Beijing Kang Century Biotechnology), and
the extracted DNA was sequenced by Shanghai Sangon Biological
Engineering Technology and Services. The editing level was
calculated from the ratio between the peak areas of the A and G
nucleotides occurring at identical positions in the DNA sequence
chromatogram. The ratio between the A and G peak heights in
individual chromatograms was calculated with FinchTV software
(Geospiza Inc., Seattle, WA, USA). The percentage editing was
calculated from the peak heights as: (G/(A + G)] ×100.
Statistical analysis
SPSS 16.0 statistical software (SPSS Inc., Chicago,
IL, USA) was used for the statistical analysis. Data are expressed
as means ± standard deviations (SD) of experiments performed in
triplicate. Statistical analyses were performed with one-way
analysis of variance (ANOVA) for multiple comparisons and the
Student’s t-test for the comparison of groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Q/R site of GluR-B mRNA is underedited in
the human glioma cell lines
We analyzed the editing status at the Q/R site of
GluR-B mRNA in the glioma-derived cell lines U87, U251 and A172,
and the normal control HA1800 cells. A GluR-B cDNA fragment
covering the Q/R site was PCR-amplified from reverse-transcribed
total RNA. We directly sequenced the GluR-B PCR amplicons. The
genomically encoded glutamine (Q) codon, CAG, was edited to an
arginine (R) codon (appearing as CGG in cDNA) in 100% of the
molecules in the normal HA1800 astrocytes. In contrast, all the
glioma cell lines showed a reduced level of editing at the Q/R
site, indicated by mixed A and G signals at the Q/R site. The
A-to-I RNA editing levels in the U87, U251 and A172 cells were 92,
89 and 83%, respectively (Fig.
2).
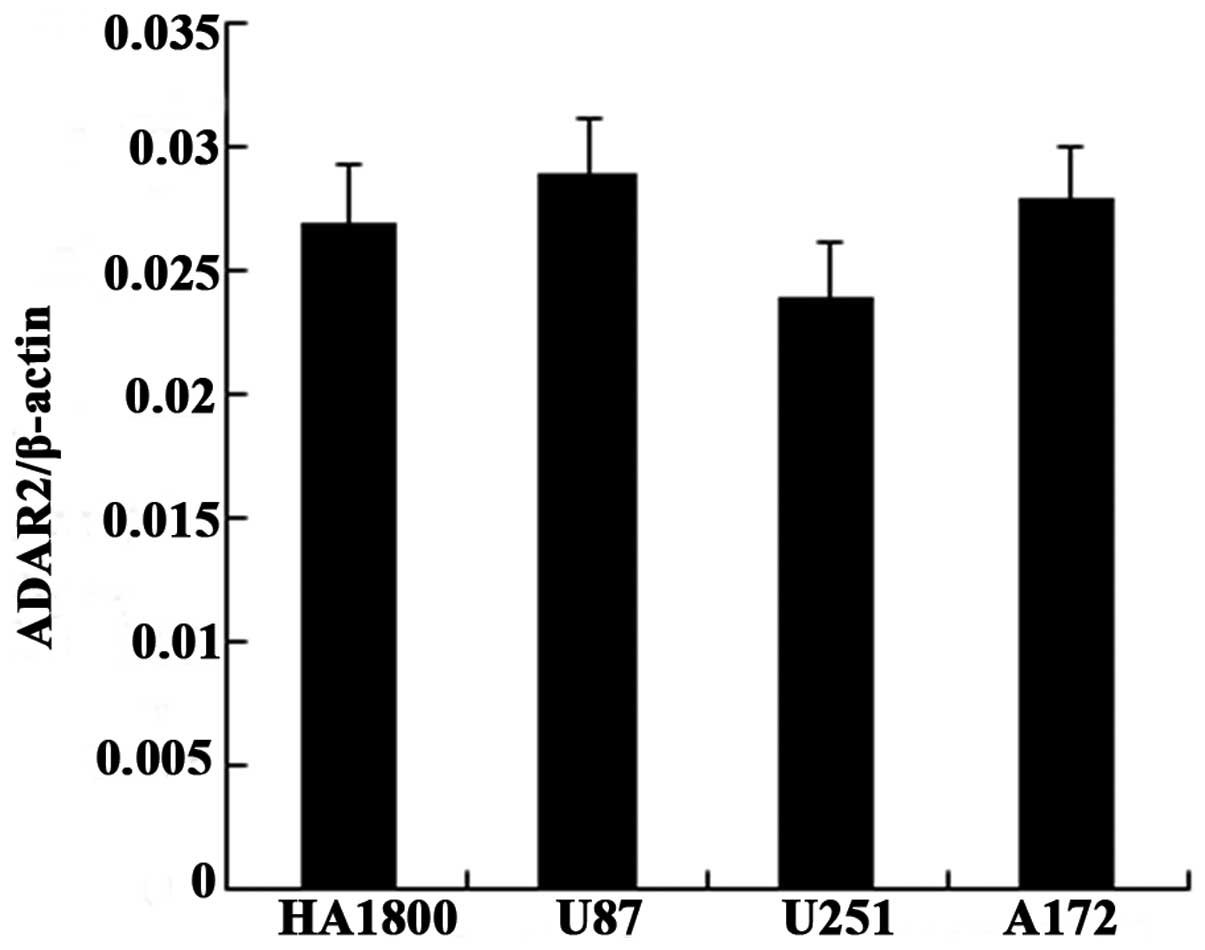
Expression of total ADAR2 mRNA is not
significantly altered in the human glioma cell lines
To investigate the mechanism underlying the
differences in the editing levels in the glioma cell lines and
NHAs, we assessed the expression of ADAR2 mRNA. Using RT-qPCR, we
found that the expression of ADAR2 mRNA was not significantly
altered in the glioma cell lines U87, U251 and A172 compared with
that in the NHAs HA1800 (Fig.
3).
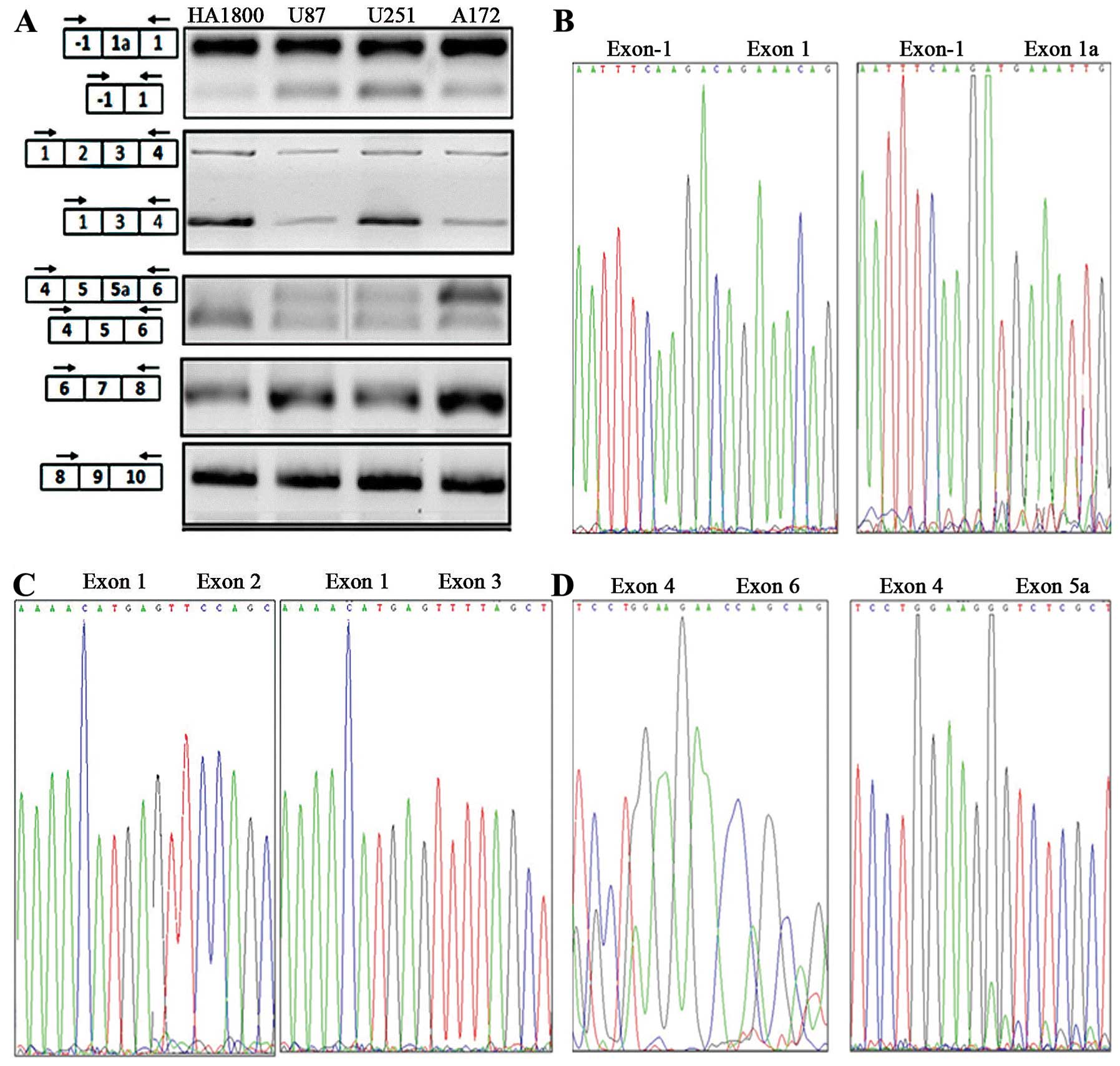
Three alternative splicing sites in ADAR2
pre-mRNA were detected in human glioma cell lines and NHAs
The whole ADAR2 cDNA was divided into five
overlapping fragments, collectively containing all the alternative
splicing sites. Five specific primer pairs were designed to amplify
the different regions. The PCR products were analyzed with agarose
gel electrophoresis, and then cloned and sequenced. When the
primers amplifying the region covering exon -1 and 1 were used, one
band joining exon -1 and 1, and a longer additional band were
amplified in all the cell lines. When the primers amplifying the
region covering exon 1 and 4 were used, one band joining exons 1,
2, 3, and 4, and a shorter additional band were amplified. When the
primers amplifying the region between exon 4 and 6 were used, one
band joining exons 4, 5 and 6, and a longer additional band were
amplified. When the primers amplifying the region covering exon 6
and 8 were used, only one band was amplified, and when the primers
amplifying the region covering exon 8 and 10 were used, only one
band was amplified (Fig. 4A).
Sequencing showed that all the bands corresponded to some specific
fragment of the ADAR2 mRNA. Among these bands, the longer product
between exon -1 and exon 1 contained an additional 47-nt exon,
which was identical to exon 1a (Fig.
4B). The shorter product between exon 1 and 4 lacked a 935-nt
exon corresponding to exon 2 (Fig.
4C), and the longer fragment between exon 4 and 6 contained an
additional 120-nt exon identical to exon 5a (Fig. 4D).
RT-PCR and sequencing showed that three alternative
splice sites in the ADAR2 mRNA were utilized in both the human
glioma cell lines and the NHAs. We designated these alternative
splice sites ASS1, ASS2 and ASS3. ASS1 is located between exon -1
and 1. Alternative splicing at this site creates exon 1a, the
inclusion of which results in an alternative potential
transcription initiation site and an N-terminal extension of 28
amino acids. ASS2 is located between exon 1 and 3. Alternative
splicing at ASS2 removes the whole of exon 2, resulting in a
frameshift that introduces a downstream stop codon. ASS3 is located
between exon 4 and 6. Alternative splicing at this site involves
the inclusion of alternative exon 5a, which introduces an in-frame
120-nt Alu-repeat-encoding sequence. No other previously reported
alternative splicing sites in ADAR2 were utilized in the
human glioma cell lines or NHAs.
Unbalanced expression among alternatively
spliced ADAR2 transcripts in human glioma cell lines
Since three alternative splice sites in the ADAR2
pre-mRNA are spliced in the human glioma cell lines and NHAs, the
downregulated catalytic activity of ADAR2 in gliomas cannot be
simply attributed to differences in the alternative splice sites
utilized. Therefore, we investigated whether the expression ratios
of the different ADAR2 transcripts produced by alternative splicing
are disturbed in the human glioma cell lines.
Alternative splicing yields two types of transcripts
at each of the three alternative splice sites. Alternative splicing
at ASS1 produces ADAR2 transcripts with and without exon 1a,
designated exon 1a (+) and 1a (−), respectively. Similarly,
alternative splicing at ASS2 produces two transcripts designated
exon 2 (+) and 2 (−), and alternative splicing at ASS3 produces two
transcripts, designated exon 5a (+) and 5a (−).
To investigate the expression ratios of the
different ADAR2 transcripts produced by specific alternative
splicing events, we analyzed the products of each of the three
alternative splice sites independently. A quantitative analysis was
performed with RT-qPCR. Two primer pairs were designed
complementary to each alternative splice site. To amplify the exon
1a (+) transcripts, the sense primer was located in exon -1 and the
antisense primer in exon 1a. For the exon 1a (−) transcripts, the
sense primer was located in exon -1, and the antisense primer
flanked the exon -1/exon 1 splice junction. RT-qPCR revealed that
the relative expression levels of exon 1a (−)/exon 1a (+) did not
differ between the human glioma cell lines and NHAs (Fig. 5A). Similarly, the sense primer for
exon 2 (+) was located in exon 2 and the antisense primer was
located in exon 4. The sense primer for exon 2 (−) flanked the exon
2/exon 3 splice junction and the antisense primer was located in
exon 4. There was no significant difference in the exon 2 (−)/exon
2 (+) expression levels in the glioma cell lines and NHAs (Fig. 5B).
At the third alternative splice site, ASS3, the
sense primer for exon 5a (+) was located at the exon 5a/exon 6
splice junction and the antisense primer flanked exon 8. The sense
primer for exon 5a (−) flanked the exon 5/exon 6 splice junction
and the antisense primer was located in exon 8. With RT-qPCR, we
demonstrated that the exon 5a (−)/exon 5a (+) ratio in the human
glioma cell lines was lower than that in the NHAs. Therefore, in
the glioma cell lines, the exon 5a (+) transcripts were
predominantly expressed, whereas the exon 5a (−) transcripts were
predominantly expressed in the NHAs (Fig. 5C).
Discussion
Gliomas are the most aggressive and lethal tumors of
the central nervous system. In contrast to patients with other
types of cancer, the outcomes of patients with glioma have only
marginally improved in the past decades mainly due to the lack of a
comprehensive understanding of the molecular mechanisms underlying
glioma. A-to-I RNA editing is an essential post-transcriptional
modification in the brain and varies little among healthy
individuals. However, deregulated RNA editing has been found in
many different human cancers, including glioma. Many studies have
shown that the Q/R site of the GluR-B mRNA is underedited in
gliomas compared with its editing in normal tissue. The A-to-I
editing catalyzed by ADAR2 at this site is essential for reducing
the invasiveness of glioma, and its dysregulation may be crucial in
glioma pathogenesis. Galeano et al (21) demonstrated that the editing activity
of ADAR2 inhibits glioblastoma growth by modulating the
CDC14B/SKP2/p21/p27 axis. In this study, we analyzed the editing
status of the GluR-B Q/R site in the glioma cell lines U87, U251
and A172, and in NHAs. The editing levels at the Q/R site were
reduced in all three glioma cell lines compared with that in NHAs,
confirming that ADAR2-mediated A-to-I editing is significantly
reduced in gliomas.
ADAR2 is the enzyme responsible for A-to-I editing
at the Q/R site of the GluR-B mRNA. Two factors may account for the
reduced A-to-I editing in gliomas. First, the levels of ADAR2 mRNA
expressed may be reduced in glioma. However, recent findings
regarding ADAR2 mRNA expression in gliomas have been inconsistent.
The first study by Maas et al (10) showed that ADAR2 mRNA expression was
not significantly altered in one oligodendroglioma and seven
glioblastoma multiforme samples, whereas Paz et al (11) observed a pronounced reduction in
ADAR2 mRNA in gliomas of different grades in an analysis of 18
brain tumors. Cenci et al (12) analyzed 14 astrocytoma tissue samples
from 10 children, and found no significant difference in the ADAR2
mRNA levels in the tumor tissues and normal white matter. The other
possible explanation is that the catalytic activity of ADAR2 is
abnormal. ADAR2 pre-mRNA undergoes a number of well-known
alternative splicing events, some affecting its function. A
combination of genetic and epigenetic factors influences the
precise cell-type- and developmental-stage-specific selection of
alternative splice sites. The mechanisms underlying the regulation
of both the expression and the enzymatic activity of human ADAR2
remain unknown. There is little knowledge of the mechanisms that
regulate RNA editing in vivo. Therefore, the
characterization of ADAR expression and the identification of
alternative ADAR2 variants is an important prerequisite for
understanding the mechanisms that regulate RNA editing and
deregulate it in diseases.
To identify the factors that are responsible for the
reduced RNA-editing activity of ADAR2, we first examined the
expression of ADAR2 mRNA in the human glioma cell lines and NHAs
using RT-qPCR. We found no differences in the ADAR2 mRNA expression
in these cell types. Our results are consistent with most studies
that have analyzed ADAR2 mRNA expression. Based on the results of
our study and previous reviews, we consider that the expression of
ADAR2 mRNA is not significantly altered in glioma and that the
underediting at the Q/R site of GluR-B in glioma is not
attributable to altered ADAR2 expression. Therefore, we speculated
that the unde-rediting at the Q/R site of GluR-B mRNA is
attributable to the abnormal catalytic activity of ADAR2 caused by
alternative splicing.
Alternative splicing is a key molecular event that
increases protein diversity. Through this process, the coding
capacity of a single gene is increased and several related proteins
with diverse and even antagonistic functions are expressed.
Aberrant splicing is associated with various diseases, including
cancer. Increasing evidence indicates that cancer-associated
splicing variants play an important role in tumor initiation and
progression. Changes in the normal process of alternative splicing
in cancer cells cause the production of novel mRNAs or the
modification of the tissue-specific ratios between normal mRNA
isoforms. Although most aberrant mRNAs are degraded by the
nonsense-mediated mRNA decay pathway, some new mRNA species can
reduce the levels of normal protein or give rise to different
protein isoforms with potentially tumorigenic properties. Several
reviews have provided lists of many genes that display
cancer-related splicing changes. The alternative splicing of
cancer-related genes could affect cell-cycle control, signal
transduction pathways, apoptosis, angiogenesis, invasion and
metastasis (22–27).
To investigate whether alternative splicing plays a
role in regulating the catalytic activity of ADAR2 and affects
A-to-I editing at the Q/R site of GluR-B mRNA in gliomas, we
analyzed the alternative splicing patterns of ADAR2 in the glioma
cell lines and NHAs.
As the first step, we analyzed the alternative
splicing sites in the ADAR2 pre-mRNA in human glioma cells and
NHAs. Five specific primer pairs were designed to amplify different
regions of the ADAR2 cDNA, which collectively contained all
the alternative splicing sites. The PCR products were analyzed with
agarose gel electrophoresis and then cloned and sequenced. The
RT-PCR and sequencing results showed that three alternative
splicing sites were utilized in both the human glioma cell lines
and NHAs. ASS1 is located between exon -1 and exon 1. Alternative
splicing at this site creates exon 1a, the inclusion of which
results in an alternative potential transcription initiation site
and an N-terminal extension of 28 amino acids. ASS2 is located
between exon 1 and 3. Alternative splicing at ASS2 removes the
whole exon 2 and produces a frameshift that introduces a downstream
stop codon. ASS3 is located between exon 4 and 6. Alternative
splicing at this site involves the inclusion of alternative exon
5a, which introduces an in-frame 120-nt Alu-repeat encoding
sequence. No utilization of the other previously reported
alternative splice sites in ADAR2 was detected in the human
glioma cell lines or NHAs. The alternative splice sites in
ADAR2 utilized in the glioma cell lines were identical to
those utilized in NHAs.
We also investigated whether the expression ratios
of different ADAR2 transcripts differed between the glioma cell
lines and NHAs. The expression ratios of the different transcripts
of ADAR2 generated by alternative splicing at each of the three
splicing sites were measured with RT-qPCR. The relative expression
levels of exon 1a (−)/exon 1a (+) did not differ between the human
glioma cell lines and NHAs. Similarly, the relative expression of
exon 2 (−)/exon 2 (+) did not differ significantly between the
glioma cell lines and NHAs. However, the exon 5a (−)/exon 5a (+)
ratio was lower in the human glioma cell lines than that in the
NHAs. Thus, in the glioma cell lines, the exon 5a (+) transcripts
predominated, whereas the exon 5a (−) transcripts predominated in
the NHAs.
Changes in the normal alternative splicing processes
in cancer cells result in the production of novel mRNAs or in the
modification of the tissue-specific ratios between the normal mRNA
isoforms. In this study, we found that the alternatively splice
sites utilized in the glioma cell lines and NHAs were identical.
However, the expression ratios between the normal mRNA isoforms
generated by splicing at ASS3 differed in the two types of cells.
However, whether the altered forms caused the cancer or are merely
innocent bystanders generated by the changes in the mRNA processing
machinery remains unknown.
We investigated, for the first time, the
underediting of GluR-B Q/R by ADAR2 in glioma from the perspectives
of the total ADAR2 mRNA expression and the alternative splicing of
ADAR2 pre-mRNA. We found that the total ADAR2 mRNA expression did
not differ between the glioma cell lines and NHAs and that the same
three alternative splicing sites were used in both the glioma cell
lines and NHAs. However, the relative expression of the different
transcripts differed in the glioma and normal cells. In the glioma
cell lines, exon 5a (+) transcripts, which generate an ADAR2
isoform with ~50% reduced activity, predominated. Thus, we conclude
that an aberrant alternative splicing pattern in the ADAR2 pre-mRNA
contributes to the downregulation of A-to-I editing in glioma.
Acknowledgments
The authors thank Professor Chunlei Fan for
technical assistance. The present study was supported by grants
from the National Science Foundation of China (no. 30672159) and
New Century Excellent Talents in Chinese Universities
(NCET-06–0306).
Abbreviations:
|
A-to-I
|
adenosine-to-inosine
|
|
ADAR
|
adenosine deaminase acting on RNA
|
|
NHAs
|
normal human astrocytes
|
|
GluR-B
|
glutamate receptor subunit B
|
|
PCR
|
polymerase chain reaction
|
|
RT-PCR
|
reverse transcription-PCR
|
References
|
1
|
Tang W, Fei Y and Page M: Biological
significance of RNA editing in cells. Mol Biotechnol. 52:91–100.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jacobs MM, Fogg RL, Emeson RB and Stanwood
GD: ADAR1 and ADAR2 expression and editing activity during
forebrain development. Dev Neurosci. 31:223–237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goodman RA, Macbeth MR and Beal PA: ADAR
proteins: structure and catalytic mechanism. Curr Top Microbiol
Immunol. 353:1–33. 2012.
|
|
4
|
George CX, Gan Z, Liu Y and Samuel CE:
Adenosine deaminases acting on RNA, RNA editing, and interferon
action. J Interferon Cytokine Res. 31:99–117. 2011. View Article : Google Scholar :
|
|
5
|
Kubota-Sakashita M, Iwamoto K, Bundo M and
Kato T: A role of ADAR2 and RNA editing of glutamate receptors in
mood disorders and schizophrenia. Mol Brain. 7:52014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slotkin W and Nishikura K:
Adenosine-to-inosine RNA editing and human disease. Genome Med.
5:105–112. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Avesson L and Barry G: The emerging role
of RNA and DNA editing in cancer. Biochim Biophys Acta.
1845:308–316. 2014.PubMed/NCBI
|
|
8
|
Galeano F, Tomaselli S, Locatelli F and
Gallo A: A-to-I RNA editing: The ‘ADAR’ side of human cancer. Semin
Cell Dev Biol. 23:244–250. 2012. View Article : Google Scholar
|
|
9
|
Dominissini D, Moshitch-Moshkovitz S,
Amariglio N and Rechavi G: Adenosine-to-inosine RNA editing meets
cancer. Carcinogenesis. 32:1569–1577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maas S, Patt S, Schrey M and Rich A:
Underediting of glutamate receptor GluR-B mRNA in malignant
gliomas. Proc Natl Acad Sci USA. 98:14687–14692. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paz N, Levanon EY, Amariglio N, Heimberger
AB, Ram Z, Constantini S, Barbash ZS, Adamsky K, Safran M,
Hirschberg A, et al: Altered adenosine-to-inosine RNA editing in
human cancer. Genome Res. 17:1586–1595. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cenci C, Barzotti R, Galeano F, Corbelli
S, Rota R, Massimi L, Di Rocco C, O’Connell MA and Gallo A:
Down-regulation of RNA editing in pediatric astrocytomas: ADAR2
editing activity inhibits cell migration and proliferation. J Biol
Chem. 283:7251–7260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tomaselli S, Galeano F, Massimi L, Di
Rocco C, Lauriola L, Mastronuzzi A, Locatelli F and Gallo A: ADAR2
editing activity in newly diagnosed versus relapsed pediatric
high-grade astrocytomas. BMC Cancer. 13:255–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gerber A, O’Connell MA and Keller W: Two
forms of human double-stranded RNA-specific editase 1 (hRED1)
generated by the insertion of an Alu cassette. RNA. 3:453–463.
1997.PubMed/NCBI
|
|
15
|
Lai F, Chen CX, Carter KC and Nishikura K:
Editing of glutamate receptor B subunit ion channel RNAs by four
alternatively spliced DRADA2 double-stranded RNA adenosine
deaminases. Mol Cell Biol. 17:2413–2424. 1997.PubMed/NCBI
|
|
16
|
Rueter SM, Dawson TR and Emeson RB:
Regulation of alternative splicing by RNA editing. Nature.
399:75–80. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Slavov D and Gardiner K: Phylogenetic
comparison of the pre-mRNA adenosine deaminase ADAR2 genes and
transcripts: conservation and diversity in editing site sequence
and alternative splicing patterns. Gene. 299:83–94. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawahara Y, Ito K, Ito M, Tsuji S and Kwak
S: Novel splice variants of human ADAR2 mRNA: skipping of the exon
encoding the dsRNA-binding domains, and multiple C-terminal splice
sites. Gene. 363:193–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maas S and Gommans WM: Novel exon of
mammalian ADAR2 extends open reading frame. PLoS One. 4:e42252009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Agranat L, Sperling J and Sperling R: A
novel tissue-specific alternatively spliced form of the A-to-I RNA
editing enzyme ADAR2. RNA Biol. 7:253–262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galeano F, Rossetti C, Tomaselli S,
Cifaldi L, Lezzerini M, Pezzullo M, Boldrini R, Massimi L, Di Rocco
CM, Locatelli F, et al: ADAR2-editing activity inhibits
glioblastoma growth through the modulation of the
CDC14B/Skp2/p21/p27 axis. Oncogene. 32:998–1009. 2013. View Article : Google Scholar :
|
|
22
|
Kim YJ and Kim HS: Alternative splicing
and its impact as a cancer diagnostic marker. Genomics Inform.
10:74–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J and Manley JL: Misregulation of
pre-mRNA alternative splicing in cancer. Cancer Discov.
3:1228–1237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oltean S and Bates DO: Hallmarks of
alternative splicing in cancer. Oncogene. 33:5311–5318. 2014.
View Article : Google Scholar
|
|
25
|
Sette C, Ladomery M and Ghigna C:
Alternative splicing: role in cancer development and progression.
Int J Cell Biol. 2013:4216062013.PubMed/NCBI
|
|
26
|
Bonomi S, Gallo S, Catillo M, Pignataro D,
Biamonti G and Ghigna C: Oncogenic alternative splicing switches:
role in cancer progression and prospects for therapy. Int J Cell
Biol. 2013:9620382013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu S and Cheng C: Alternative RNA
splicing and cancer. Wiley Interdiscip Rev RNA. 4:547–566. 2013.
View Article : Google Scholar : PubMed/NCBI
|