Introduction
In 1937, Meigs and Cass reported the resolution of
hydrothorax and ascites after removal of a benign ovarian mass
which attracted widespread attention (1). In 1954, Meigs proposed to restrict
Meigs’ syndrome to a solid benign ovarian neoplasm such as fibroma
or thecoma accompanied by ascites and hydrothorax; the ascites and
hydrothorax must resolve fully after removal of the tumor (2). Pseudo-Meigs’ syndrome is often
characterized by pleural effusion and ascites caused by a pelvic
tumor other than an ovarian fibroma (such as struma ovarii tumors,
mucinous or serous cystadenomas, germ cell tumors) or uterine
leiomyoma (3). Uterine leiomyomas,
which are benign tumors of myometrial origin, are the most common
gynecological neoplasms, with an estimated prevalence of 25% in
women older than 30 years (4).
Leiomyoma variants include mitotically active, cellular and
atypical leiomyomas. Cellular leiomyomas (CLs) represent a subgroup
of leiomyoma variants and are defined by the World Health
Organization as typical leiomyomas that exhibit hypercellularity.
CLs are rare and do not account for >5% of leiomyomas (5). Patients with CLs have a risk of
distant metastasis after hysterectomy (6). CA125 is one of the most common tumor
markers for pelvic malignancy. Elevated serum CA125 is highly
suggestive of epithelial ovarian carcinoma. We here present a case
of pseudo-Meigs’ syndrome involving hemorrhagic necrosis and
mucinous degeneration of a pedunculated subserosal CL associated
with an elevated CA125 level.
Case report
A 37-year-old Chinese woman, gravida 3, para 1, was
referred to the gynecology department of our hospital for
complaining of right lower abdominal dull pain for 2 years and
abdominal distension for 6 days. The patient had cholecystitis for
6 months, but did not seek professional internal medical
assistance. Six days before admission, she presented with nausea
and abdominal swelling and pain after consuming greasy food. Her
condition did not improve after receiving antibiotics. Three days
before admission, she came to the emergency room to receive
anti-inflammatory, fluid replacement treatment. However, it was not
effective. The patient was not taking any medications. She had a
regular menstrual cycle and the last menstrual period was December
10. She had not been operated on previously and did not have a
family history of breast or gynecologic cancers.
At physical examination she was cachectic, and a
massive abdominal mass reaching to the umbilicus was palpated. The
examination was difficult due to massive ascites. She had diffuse
and rebound tenderness, particularly in the right lower quadrant.
Gynecological examination revealed a mass in the right adnexal
region with a normal-sized mobile uterus. Laboratory studies showed
no abnormalities except for a serum CA125 level of 920.4 U/ml
(normal <35 U/ml). Abdominal and pelvic ultrasound confirmed the
presence of ascites and a 16×16 cm mixed mass at the upper right
anterior of the fundus uteri. The mass was heterogeneous in
echotexture showing flow signals. A computerized tomographic scan
revealed a heterogeneous mass within the pelvis and lower abdomen
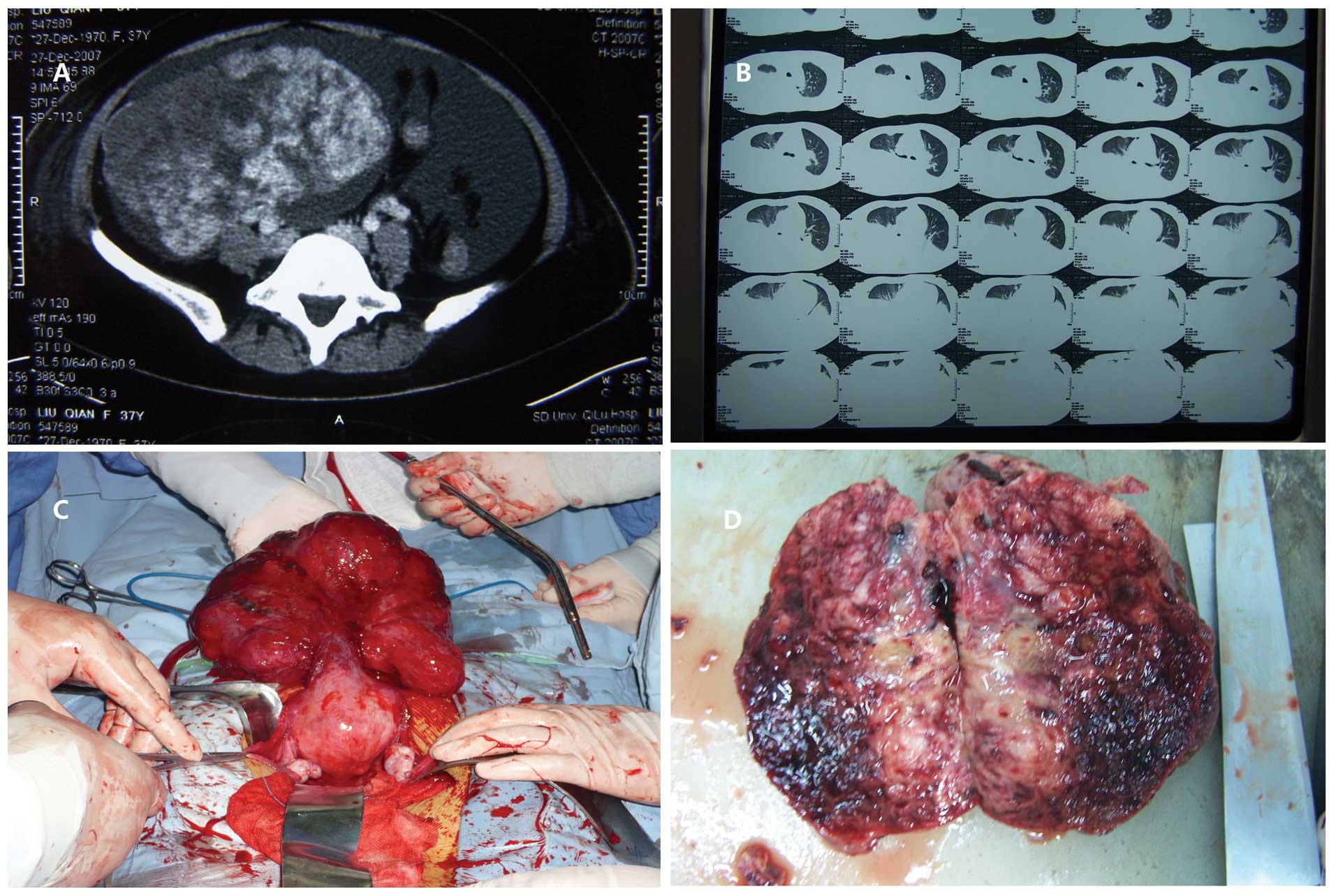
associated with ascites (Fig. 1A)
and massive bilateral hydrothorax resulting in nearly complete
collapse of the right middle, right lower and left lower lung lobes
(Fig. 1B).
In order to alleviate the symptoms and aid
diagnosis, paracentesis and thoracentesis were performed several
times each. The first paracentesis yielded a hematic exudate of
2,900 ml found to be negative for malignant cells and mycobacterium
tuberculosis. Five days later, a repeat paracentesis yielded 2,300
ml of straw-colored fluid containing reactive mesothelial cells
without evidence of malignancy. Four days later, a third
paracentesis yielded a hematic exudate of 2,900 ml. Thoracentesis
was conducted to yield hematic fluid of 1,000 ml consistent with an
exudative process. Two days after the thoracentesis, closed
thoracic drainage yielded 2,350 ml of straw-colored fluid.
An exploratory laparotomy was arranged for
diagnostic and therapeutic purposes. The patient was found to have
~1,000 ml of hematic ascites and a pedunculated mass protruding
from the uterine fundus. The mass was irregular, firm, hemorrhagic,
necrotic and dark red in appearance. The pedunculated mass measured
20×18×10 cm in size and the pedicle measured 5 cm in length and 5
cm in thickness (Fig. 1C and D).
The uterus was found to be enlarged similar to a two-month
pregnancy. There was no palpable pelvic or periaortic adenopathy,
and the liver, diaphragm, bowel and omentum were grossly free of
disease. A frozen section was suggestive of focal necrotic uterine
leiomyoma without significant cell atypia. The patient subsequently
underwent a total abdominal hysterectomy and omentum sampling.
Microscopic examination showed uterine CL with significant
hemorrhage, necrosis and mucinous degeneration, adenomyosis,
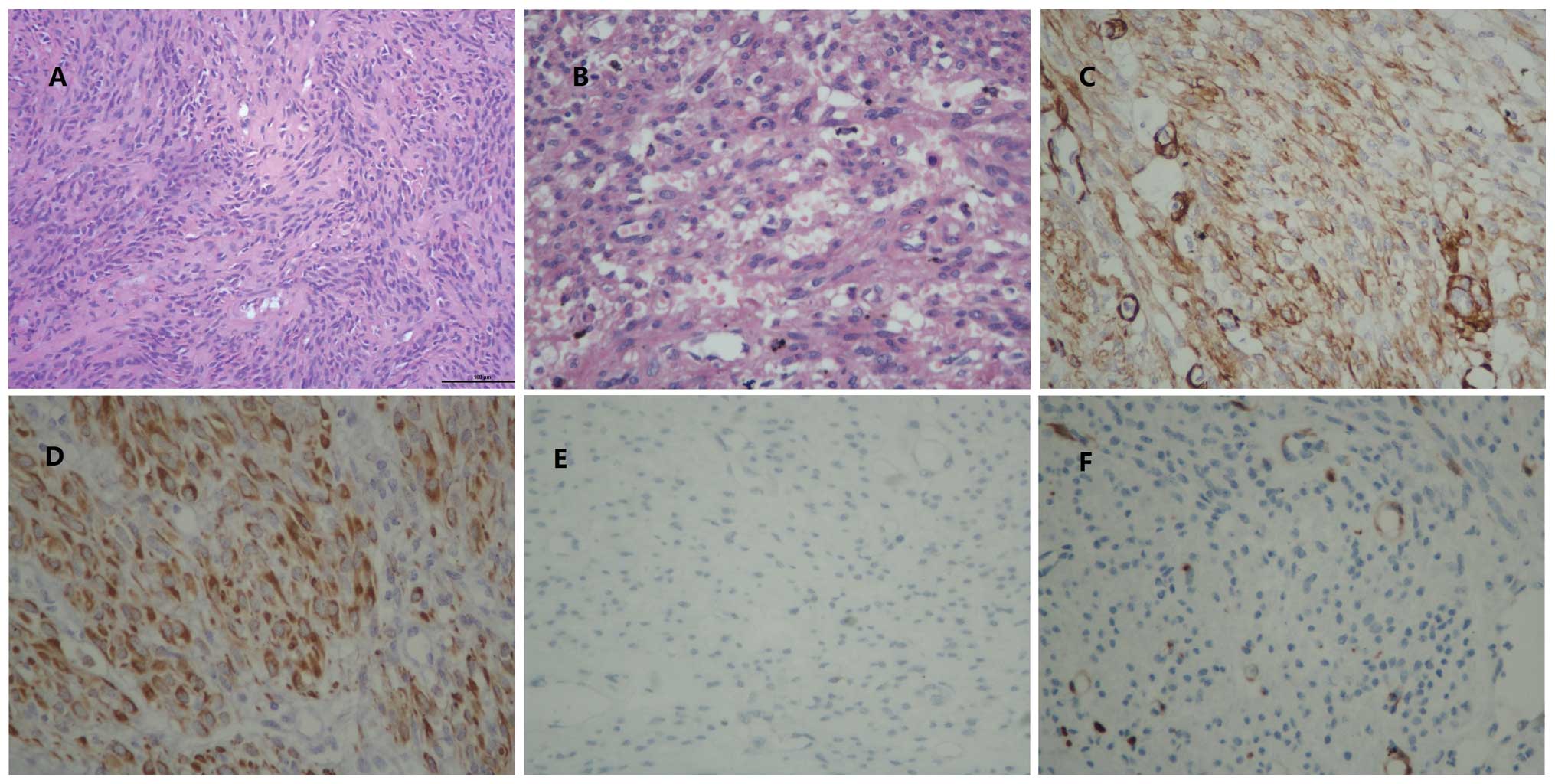
chronic cervicitis and normal omentum (Fig. 2A and B). Mitotic activity, cell
atypia, as well as coagulative tumor cell necrosis, were absent.
The patient recovered uneventfully and was discharged on day 13,
post-operatively. The patient recovered quickly with no evidence of
re-accumulation of the pleural effusions or ascites. At one month
follow-up, she was clinically well, with no evidence of disease on
physical examination and had a normal CA125 level. She remained
disease-free both clinically and on repeat CT scan after a
follow-up of >82 months.
Discussion
CL with necrosis and mucinous degeneration
presenting as pseudo-Meigs’ syndrome and highly elevated CA125 is a
rare condition. CLs are one of the variant forms of leiomyoma with
a favorable long-term prognosis. The most frequent symptoms of CLs
are menstrual abnormalities, a pelvic mass, abdominal pain and
pelvic pressure. Signs and symptoms of CLs are similar to those of
leiomyosarcomas (LMSs), but abdominal pain or distension is much
more common in women with LMS. Tumor growth or bleeding may
accompany CLs, particularly in postmenopausal women, and these can
be signs of a malignancy (7). The
recurrence and re-operation rates after myomectomy (28.6 and 14.3%,
respectively) in a CL group were found to be similar to those
reported for cohorts of patients with ordinary leiomyomas (8). Based on various reports CLs were
either the largest or the only uterine mass (9). CLs appear to have a distinct clinical
phenotype compared with typical leiomyomas and have various
characteristics that typically are associated with LMSs. Similarly
to LMSs, CLs were either the largest or the only uterine mass
(6).
The distinction of endometrial stromal sarcomas
(ESSs) from CLs of the uterus can sometimes be problematic for even
the experienced pathologist, since both can show a marked degree of
architectural and cytologic similarity, particularly as ESSs can
have smooth muscle-like differentiation and CLs, especially highly
cellular leiomyomas (HCLs) can exhibit dense cellularity, prominent
vascularity and irregular margins (5,10–12).
Yet, accurate classification is of considerable importance due to
the fact that CL, a benign neoplasm, and ESS, a low-grade malignant
neoplasm, are different in clinical behavior and require dissimilar
treatment (13).
Immunohistochemistry is currently an essential tool
for biomarker detection even in clinical practice.
Immunohistochemistry plays an important role for differentiating
between CLs and malignant disease. The immunohistochemical
detection of CD10, P16, SMA and the smooth muscle marker desmin can
be useful for differentiating CLs from ESSs (13).
Interstitial accumulation of mucus within a
leiomyoma is refered to as mucinous degeneration (Fig. 2A). Mucinous degeneration is often
associated with the most common form of degeneration, hyaline
change (14). CL is one of the
variant forms of leiomyoma. Compared with surrounding myometrium,
CL has an increased number of cells per unit area. The cells of CL
have scanty cytoplasm and are closed packed, so the section is dark
blue (Fig. 2B). As the
immunohistochemical analysis demonstrated positivity for SMA and
desmin and lack of CD10 and P16, CL was diagnosed (Fig. 2C–F). With mucinous degeneration,
hemorrhage, focal necrosis and abundance of cells, alteration of
the normal leiomyoma architecture undoubtedly increases the
difficulties in definitive diagnosis and differential
diagnosis.
The exact mechanisms of ascites and pleural
effusions remain unclear. Potential explanations include:
irritation of the peritoneum by the tumor, obstruction of the
lymphatics, toxins and release of inflammatory products,
hypoalbuminemia, and finally discrepancy between the arterial
supply and venous and lymphatic drainage (15,16).
Compression and subsequent congestion of the lymphatics and blood
vessels throughout the tumor by the tumor itself followed by
interstitial fluid secretion from the tumor surface have also been
suggested as causes for the development of ascites (17). As for the mechanism of pleural
effusions, dye tests have shown that these are likely to originate
from the peritoneal fluid via a mechanical transfer through the
diaphragmatic opening (15). The
connection between uterine leiomyoma and ascites or hydrothorax has
been confirmed by the rapid resolution of abdominal and pleural
fluid after removal of the tumor.
CA125 is a cell-surface antigen associated with a
high molecular weight glycoprotein. High levels of Ca125 are mostly
noted in pelvic malignancies, such as ovarian cancer with
dissemination. However, Ca125 does not have 100% specificity in the
diagnosis of epithelial ovarian cancers. Its elevation has also
been noted in other malignancies and benign, physiological states,
including pregnancy, endometriosis and menstruation (18). In pseudo-Meigs’ syndrome, Lin et
al (19) and Timmerman et
al (20) proposed that elevated
CA125 levels are caused by mesothelial expression of Ca125 rather
than by the fibroma. In addition, in pseudo-Meigs’ syndrome, it is
the peritoneal inflammation not the leiomyoma that may be the
primary cause of the elevated CA125 level.
Benign leiomyomas presenting with pseudo-Meigs’
syndrome and elevated CA125 are rare. Few cases of benign leiomyoma
accompanied with pseudo-Meigs’ syndrome and elevated Ca125 have
been described. We performed a systematic review of related
literature in the PubMed database using a combination of free words
and MeSH. The search was limited to English language literature.
Eleven related case reports were found (Tables I and II). Here, we describe a twelfth case with
leiomyoma presenting with pseudo-Meigs’ syndrome and elevated CA125
level.
 | Table IGeneral characteristics of reported
uterine leiomyomas associated with pseudo-Meigs’ syndrome and
elevated CA125 level. |
Table I
General characteristics of reported
uterine leiomyomas associated with pseudo-Meigs’ syndrome and
elevated CA125 level.
| Authors (ref.) | Year | Age (years) | Tumor size (cm) | Ascites (ml) | Hydrothorax (ml) | CA125 U/ml) | Hydrothorax
disappeared after surgery | Uterine weight
(g) |
|---|
| Brown et al
(21) | 1998 | 31 | 17×11.5×8.5 | NR | NR | 83.0 | NR | – |
| Domingo et al
(22) | 1998 | 46 | 20 in diameter | NR | NR | 317.0 | NR | NR |
| Dunn et al
(23) | 1998 | 46 | 30×18×15 | 1,600 | 3,300 | 254.0 | 4 months | 3,094 |
| Migishima et
al (24) | 2000 | 51 | 12.3×24.3×12.5 | 19,600 | 3,060 | 820.0 | 4 months | 9,700 |
| Amant et al
(15) | 2001 | 39 | 30×30×15 | 12,000 | NR | 785.0 | 7 weeks | 7,840 |
| Kebapci et al
(25) | 2002 | 38 | 9×10×10.5 | NR | NR | 281.0 | 6 months | NR |
| Weise et al
(26) | 2002 | 27 | 7×8×6 | 22,500 | NR | 1854.0 | 3 months | – |
| Weinrach et al
(27) | 2004 | 40 | 19×11×10 | NR | NR | 734.0 | 6 months | 1,900 |
| Ricci et al
(28) | 2009 | 35 | 15×10×8.5 | 2,000 | – | 231.4 | – | NR |
| Yip et al
(29) | 2014 | 41 | 12×11×7.8 | 6,600 | – | 939.7 | – | NR |
| Present study | – | 37 | 20×18×10 | 9,100 | 3,900 | 920.4 | 1 month | NR |
 | Table IIClinical symptoms, characteristics and
the treatment of reported uterine leiomyomas associated with
pseudo-Meigs’ syndrome and elevated CA125 level. |
Table II
Clinical symptoms, characteristics and
the treatment of reported uterine leiomyomas associated with
pseudo-Meigs’ syndrome and elevated CA125 level.
| Authors (ref.) | Clinical
symptoms | Pathology | Treatments |
|---|
| Brown et al
(21) | Dyspnea, abdominal
swelling, an intermittent difficulty in passing urine, weight
loss | Broad ligament
leiomyoma | Myoectomy |
| Domingo et al
(22) | Respiratory
arrest | Multinodular
myoma | TAH, BSO |
| Dunn et al
(23) | Nausea, vomiting,
diarrhea, tachypnea | A bilobate,
pedunculated leiomyoma | TAH, BSO,
omentectomy, lymph node sampling |
| Migishima et
al (24) | Gradual abdominal
distension, progressive dyspnea | Leiomyoma with
myxoid degeneration and intercellular edema | TAH, BSO |
| Amant et al
(15) | Abdomen
swelling | A hydropic
leiomyoma | TAH |
| Kebapci et
al (25) | Low back pain,
abdominal distension, weakness, loss of appetite | Pedunculated
leiomyoma with parasitized blood supply from the omentum | Myoectomy,
omentectomy, appendectomy |
| Weise et al
(26) | Increasing
abdominal girth for 2 months | Pedunculated fundal
myoma attached to the bladder | Myomectomy |
| Weinrach et
al (27) | Abdominal
distension, shortness of breath | Uterine symplastic
leiomyoma | TAH, BSO |
| Ricci et al
(28) | Abdominal
distension 2 days after a vaginal delivery | Leiomyoma in
puerperium | Myoectomy |
| Yip et al
(29) | Abdominal fullness
and prolonged menstrual periods for 3 years | Pedunculated
leiomyoma with parasitized blood supply from the adjacent tissues
and organs | Myoectomy |
| Present | Right lower
abdominal dull pain and abdominal distension | Cellular leiomyoma
with necrosis and mucinous degeneration | TAH, omentum
sampling |
Most of the uterine leiomyomas can easily be
differentiated from other gynecologic or non-gynecologic pelvic
mass lesions. However, degeneration, hemorrhage and focal necrosis
may make the differential diagnosis difficult, and a degenerated
leiomyoma, HCL, or leiomyoma with edema may not have typical
imaging findings (30).
Our patient demonstrated several unusual features.
Firstly, she presented with massive hematic ascites and pleural
effusions. Secondly, microscopic examination showed CL with
significant hemorrhage, necrosis and mucinous degeneration.
Thirdly, we followed up the patient for >82 months and she
remained disease-free both clinically and on repeat CT scan. A CL
should be considered in differential diagnosis when a
hypervascular, heterogeneous solid pelvic mass that shows no
relation to the uterus is encountered in association with massive
ascites, pleural effusion and elevated levels of serum CA125.
References
|
1
|
Meigs JV and Cass J: Fibroma of the ovary
with ascites and hydrothorax: with a report of seven cases. Am J
Obstet Gynecol. 33:249–267. 1937.
|
|
2
|
Meigs JV: Fibroma of the ovary with
ascites and hydrothorax; Meigs’ syndrome. Am J Obstet Gynecol.
67:962–985. 1954.PubMed/NCBI
|
|
3
|
Meigs JV: Pelvic tumors other than
fibromas of the ovary with ascites and hydrothorax. Obstet Gynecol.
3:471–486. 1954.PubMed/NCBI
|
|
4
|
Solomon LA, Schimp VL, Ali-Fehmi R,
Diamond MP and Munkarah AR: Clinical update of smooth muscle tumors
of the uterus. J Minim Invasive Gynecol. 12:401–408. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilkinson N and Rollason TP: Recent
advances in the pathology of smooth muscle tumours of the uterus.
Histopathology. 39:331–341. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taran FA, Weaver AL, Gostout BS and
Stewart EA: Understanding cellular leiomyomas: a case-control
study. Am J Obstet Gynecol. 203:109.e1–109.e6. 2010. View Article : Google Scholar
|
|
7
|
Guan R, Zheng W and Xu M: A retrospective
analysis of the clinicopathologic characteristics of uterine
cellular leiomyomas in China. Int J Gynaecol Obstet. 118:52–55.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reed SD, Newton KM, Thompson LB, McCrummen
BA and Warolin AK: The incidence of repeat uterine surgery
following myomectomy. J Womens Health (Larchmt). 15:1046–1052.
2006. View Article : Google Scholar
|
|
9
|
Schwartz LB, Diamond MP and Schwartz PE:
Leiomyosarcomas: clinical presentation. Am J Obstet Gynecol.
168:180–183. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Agoff SN, Grieco VS, Garcia R and Gown AM:
Immunohistochemical distinction of endometrial stromal sarcoma and
cellular leiomyoma. Appl Immunohistochem Mol Morphol. 9:164–169.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nucci MR, O’Connell JT, Huettner PC, Cviko
A, Sun D and Quade BJ: h-Caldesmon expression effectively
distinguishes endometrial stromal tumors from uterine smooth muscle
tumors. Am J Surg Pathol. 25:455–463. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oliva E, Young RH, Amin MB and Clement PB:
An immunohistochemical analysis of endometrial stromal and smooth
muscle tumors of the uterus: a study of 54 cases emphasizing the
importance of using a panel because of overlap in immunoreactivity
for individual antibodies. Am J Surg Pathol. 26:403–412. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu XQ, Shi YF, Cheng XD, Zhao CL and Wu
YZ: Immunohistochemical markers in differential diagnosis of
endometrial stromal sarcoma and cellular leiomyoma. Gynecol Oncol.
92:71–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Robboy SJ, Bentley RC, Butnor K and
Anderson MC: Pathology and pathophysiology of uterine smooth-muscle
tumors. Environ Health Perspect. 108(Suppl 5): 779–784. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amant F, Gabriel C, Timmerman D and
Vergote I: Pseudo-Meigs’ syndrome caused by a hydropic degenerating
uterine leiomyoma with elevated CA 125. Gynecol Oncol. 83:153–157.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zannoni GF, Gallotta V, Legge F, Tarquini
E, Scambia G and Ferrandina G: Pseudo-Meigs’ syndrome associated
with malignant struma ovarii: a case report. Gynecol Oncol.
94:226–228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Terada S, Suzuki N, Uchide K and Akasofu
K: Uterine leiomyoma associated with ascites and hydrothorax.
Gynecol Obstet Invest. 33:54–58. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jacobs I and Bast RC JR: The Ca 125
tumour-associated antigen: a review of the literature. Hum Reprod.
4:1–12. 1989.PubMed/NCBI
|
|
19
|
Lin JY, Angel C and Sickel JZ: Meigs
syndrome with elevated serum CA 125. Obstet Gynecol. 80:563–566.
1992.PubMed/NCBI
|
|
20
|
Timmerman D, Moerman P and Vergote I:
Meigs’ syndrome with elevated serum CA 125 levels: two case reports
and review of the literature. Gynecol Oncol. 59:405–408. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brown RSD, Marley JL and Cassoni AM:
Pseudo-Meigs’ syndrome due to broad ligament leiomyoma: a mimic of
metastatic ovarian carcinoma. Clin Oncol (R Coll Radiol).
10:198–201. 1998. View Article : Google Scholar
|
|
22
|
Domingo P, Montiel JA, Monill JM and Prat
J: Pseudo-Meigs syndrome with elevated CA 125 levels. Arch Intern
Med. 158:1378–1379. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dunn JS JR, Anderson CD, Method MW and
Brost BC: Hydropic degenerating leiomyoma presenting as
pseudo-Meigs syndrome with elevated CA 125. Obstet Gynecol.
92:648–649. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Migishima F, Jobo T, Hata H, Sato R, Ikeda
Y, Arai M and Kuramoto H: Uterine leiomyoma causing massive ascites
and left pleural effusion with elevated CA 125: a case report. J
Obstet Gynaecol Res. 26:283–287. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kebapci M, Aslan O, Kaya T, Yalcin OT and
Ozalp S: Pedunculated uterine leiomyoma associated with
pseudo-Meigs’ syndrome and elevated CA-125 level: CT features. Eur
Radiol. 12(Suppl 3): S127–S129. 2002.
|
|
26
|
Weise M, Westphalen S, Fayyazi A, Emons G
and Krauss T: Pseudo-Meigs syndrome: uterine leiomyoma with bladder
attachment associated with ascites and hydrothorax - a rare case of
a rare syndrome. Onkologie. 25:443–446. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weinrach DM, Wang KL, Keh P and Sambasiva
Rao M: Pathologic quiz case: a 40-year-old woman with a large
pelvic mass, ascites, massive right hydrothorax, and elevated CA
125. Uterine symplastic leiomyoma associated with pseudo-Meigs
syndrome and elevated CA 125. Arch Pathol Lab Med. 128:933–934.
2004.PubMed/NCBI
|
|
28
|
Ricci G, Inglese S, Candiotto A, Maso G,
Piccoli M, Alberico S and Guaschino S: Ascites in puerperium: a
rare case of atypical pseudo-Meigs’ syndrome complicating the
puerperium. Arch Gynecol Obstet. 280:1033–1037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yip HK, Huang LW, Lin YH and Hwang JL:
Massive ascites caused by a large pedunculated subserosal uterine
leiomyoma that has feeding arteries from peripheral tissues and
exhibits elevated Ca125: a case report of atypical pseudo-Meigs’
syndrome. J Obstet Gynaecol. 34:1072014. View Article : Google Scholar
|
|
30
|
Ueda H, Togashi K, Konishi I, Kataoka ML,
Koyama T, Fujiwara T, Kobayashi H, Fujii S and Konishi J: Unusual
appearances of uterine leiomyomas: MR imaging findings and their
histopathologic backgrounds. Radiographics. 19:S131–S145. 1999.
View Article : Google Scholar : PubMed/NCBI
|