Introduction
Osteosarcoma (OS) is the leading primary malignant
bone tumor in children and adolescence (1,2).
Pulmonary metastases, central presentation and local non-resectable
relapse are common in the majority of patients, contributing to a
high mortality rate (3). Although
advancements in surgical techniques, radiation and chemotherapy
strategies have increased the local control of OS, the overall
survival of OS patients has still remained relatively constant for
over two decades (4,5). Therefore, novel therapeutic agents are
desperately needed to further improve the prognosis of OS
patients.
Survivin, a member of the inhibitors of apoptosis
(IAP) protein family, encoded by the BIRC5 gene, is highly
expressed in various types of cancer cells while it is not
expressed or expressed at a substantially lower level in their
normal tissue counterparts (6).
Furthermore, the expression level of survivin has been found to
correlate with poor prognosis in gastric (7), colorectal (8), non-small cell lung (9), breast (10), pancreatic (11), ovarian (12), prostate cancers (13), and melanoma (14). Functionally, survivin has been shown
to counteract apoptosis induction upstream of effector caspases
(15) and to play a critical role
in the regulation of cell division by inducing exit from G1
checkpoint arrest and subsequent entry into the S phase (16). In addition, survivin also plays an
essential role in cell proliferation by regulating spindle assembly
and microtubule attachment to the kinetochore as a member of the
chromosomal passenger complex (17,18).
Growing evidence has demonstrated that inhibition of survivin
expression using antisense oligonucleotides, small-molecule
antagonists, or small interfering RNA (siRNA) suppresses tumor cell
proliferation and invasion, induces cell apoptosis, consequently
suppressing tumor growth (13–18).
Recently, YM155, a novel small-molecule inhibitor of
survivin, was identified by cell-based high throughput screening
using a survivin promoter luciferase assay (19). In vitro, YM155 exerts potent
antitumor activity in various types of cancer cells including
non-small cell lung cancer, non-Hodgkin’s lymphoma, melanoma and
hormone-refractory prostate cancer (20–24).
In vivo, YM155 has also shown anticancer efficacy in lung
and prostate cancer in xenograft models (21,25).
In addition, YM155 has been evaluated in phase II clinical trials
in patients with advanced refractory non-small cell lung carcinoma
and unresectable melanoma (26,27).
But little research has been carried out on the effects of YM155
against OS, and no tools are available to guide the use of YM155 in
the treatment of OS.
Therefore, in the present study, we evaluated the
anticancer effects of YM155 and the underlying molecular mechanism
in OS cell lines. We also investigated the anticancer effects of
YM155 on OS cell line MG63 xenografts.
Materials and methods
Reagents and antibodies
The small-molecule survivin inhibitor YM155
(Fig. 1A) was purchased from
Selleck Chemicals (Houston, TX, USA). For the in vitro
studies, YM155 was dissolved in dimethyl sulfoxide (DMSO) and
stored at −20°C.
Primary antibodies against the following proteins
were used for western blot analyses: survivin (monoclonal; Abcam,
Cambridge, MA, USA), GAPDH (Santa Cruz Biotechnology, Santa Cruz,
CA, USA), XIAP (monoclonal; BD Biosciences, San Jose, CA, USA),
phosphoinositide 3-kinase (PI3K), phospho (p)-PI3K (Tyr458), AKT,
p-AKT (Ser473), Bcl-2 and Mcl-1 (Cell Signaling Technology,
Danvers, MA, USA).
Cell culture
Two human OS cell lines, MG63 and Saos-2, were
purchased from the Cell Bank of the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). All cells were grown
in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) (both from Gibco-BRL, Grand Island, NY,
USA), 2 mM glutamine, 100 U/ml penicillin and 100 mg/ml
streptomycin at 37°C in a 5% CO2 atmosphere and at 95%
humidity.
Cell viability and colony formation
The MTT assay was used to determine the effect of
different concentrations of YM155 on the proliferation of cells.
Briefly, cells grown in monolayers were collected and dispensed in
96-well culture plates in 100 μl of DMEM at a concentration
of 5×103 cells/well. After 24 h, differential
concentrations of YM155 (0, 1, 10 and 100 nM) were added to the
cells. Seventy-two hours after treatment, cell proliferation was
measured as described previously (28). This assay was performed in
triplicate.
The effect of YM155 on cell colony formation was
assessed using a clonogenic assay. Briefly, 1.0×103
cells were plated in 6-well plates in growth medium, and after
overnight attachment, the cells were treated with different
concentrations of YM155 (0, 1, 10 and 100 nM) for 24 h. The cells
were then washed with PBS and allowed to grow for 14 days in
drug-free conditions, and the medium was replaced every 3 days. The
colonies were fixed with 4% paraformaldehyde for 20 min and stained
with 1% crystal violet for 10 min. Colonies containing >50 cells
were counted.
Cell apoptosis
The effect of YM155 on cell apoptosis was determined
by flow cytometry. Briefly, 5.0×105 cells were plated in
60-mm dishes and treated with different concentrations of YM155 (0,
1, 10 and 100 nM) for 24 h. After treatment, the cells were stained
with Annexin V (Molecular Probes) and propidium iodide
(Sigma-Aldrich, St. Louis, MO, USA) and analyzed using flow
cytometry (BD Biosciences, Mansfield, MA, USA). The apoptosis ratio
was analyzed by CellQuest software (BD Biosciences).
In addition, caspase-3, -8 and -9 activity was
detected as an additional indicator of apoptosis using Caspases
Colorimetric Protease Assay kits (Millipore Corporation, Billerica,
MA, USA) as previously described (28). The relative caspase-3, -8 and -9
activity of the control blank group was referred to as 100.
Cell migration and invasion assays
The migration assay was performed with Transwell
inserts with 8.0-mm pore size membrane (Corning, Tewksbury, MA,
USA). For the invasion assay, the previously mentioned inserts were
pre-coated with Matrigel matrix (BD Science, Sparks, MD, USA). The
cells (1×105) were resuspended in serum-free medium and
seeded into the upper chamber. DMEM with 10% FBS was added to the
lower chamber to serve as a chemoattractant. After incubation at
37°C for 48 h, the migrated and invaded cells present on the lower
side of the membrane were fixed with 70% ethanol for 30 min and
stained with 0.2% crystal violet for 10 min. The number of migrated
and invaded cells was counted in 5 randomly selected fields by an
inverted microscope (Olympus, Tokyo, Japan).
Western blot assays
The cells were lysed in lysis solution (Cell
Signaling Technology) supplemented with sodium fluoride (10 M;
Fisher) and phenylmethylsulfonyl fluoride (100 g/ml; Sigma-Aldrich)
for 30 min. Lysates were centrifuged at 14,000 × g for 10 min, and
the total protein concentration was determinated using the BCA
assay kit (Sigma-Aldrich). Each 20 μg of sample was
fractionated on either 8 or 12% SDS-polyacrylamide gel, and the
separated proteins were transferred to nitrocellulose membranes
(Bio-Rad, Munich, Germany). The membranes were blocked with 5%
non-fat dry milk for 2 h and incubated with the previously
mentioned primary antibody overnight at 4°C, followed by incubation
with horseradish peroxidase-conjugated goat anti-mouse IgG (Santa
Cruz Biotechnology) for 2 h at room temperature. Protein bands were
visualized with enhanced chemiluminescence reagent (ECL; Amersham,
GE Healthcare, Velizy-Villacoublay, France). Protein loading was
normalized by stripping the blots and then reprobing with the
anti-GAPDH antibody.
Xenograft model
Five-week-old male BALB/c nude mice (5–6 weeks old)
were purchased from the Experimental Animal Center of Changchun
Biological Institute (Changchun, China), and maintained under
specific pathogen-free (SPF) conditions. This study was approved by
the Animal Ethics Committee of Jilin University (Changchun,
China).
Tumors were established by injecting
2×106 MG63 cells subcutaneously into the right flank of
the mice. When subcutaneous tumors reached a size of 100
mm3, the xenografted animals were randomly divided into
vehicle and YM155 (10 mg/kg) groups. YM155 was subcutaneously
administered as a 3-day continuous infusion per week for 4 weeks
using an implanted micro-osmotic pump (Alzet Model 1003D; Durect,
Cupertino, CA, USA) as previously described (19). Tumors were measured every 5 days
with calipers, and the volume (mm3) was calculated
according to the following formula: [π/6 × length × width ×
height]. Thirty days after inoculation, the mice were sacrificed,
and tumors were stripped and weighed, and analyzed for survivin
expression by western blot analysis.
Statistical analysis
All data are expressed as mean ± standard deviation
(SD). Statistical analysis between two samples was performed using
the Student’s t-test and for more than two groups, analysis was
performed using one-way ANOVA followed by a Tukey’s post hoc test
using GraphPad Prism 5.0 software (GraphPad Software, San Diego,
CA, USA). Significance was set at P<0.05.
Results
YM155 inhibits survivin expression in OS
cells
Recently, the small-molecule survivin inhibitor
YM155 (Fig. 1A) has been shown to
inhibit survivin expression in various types of cancers (13–18).
However, the effect of YM155 on survivin expression in OS cells has
been not reported to date. Next, we examined whether YM155 inhibits
survivin expression at the protein level in the OS cell lines by
western blot analysis 24 h after treatment with various doses of
YM155 (0, 1, 10 and 100 nM). The results of the western blot
analysis demonstrated that YM155 significantly inhibited survivin
expression in the MG63 and Saos-2 cells in a dose-dependent manner
(Fig. 1B).
YM155 inhibits cell proliferation, colony
formation, migration and invasion in OS cells
To evaluate the effect of YM155 on the cell
proliferation of OS cells, MG63 and Saos-2 cells were treated with
different concentrations of YM155 (0, 1, 10 and 100 nM) for 1–3
days, then an MTT assay was performed. The result showed that YM155
inhibited the cell proliferation of MG63 and Saos-2 cells in a
dose-dependent manner (Fig.
2A).
Next, the effects of YM155 on the cell colony
formation of OS cells were also determined. As shown Fig. 2B, YM155 obviously decreased colony
formation number of OS cells (MG63 and Saos-2) in a dose-dependent
manner (P<0.05).
We also investigate whether YM155 affects cell
migration and invasion of the OS cells by Transwell assay after
treatment with YM155. It was found that YM155 significantly
suppressed migration (Fig. 2C) and
invasion (Fig. 2D) in the MG63 and
Saos-2 cells in a dose-dependent manner (P<0.05).
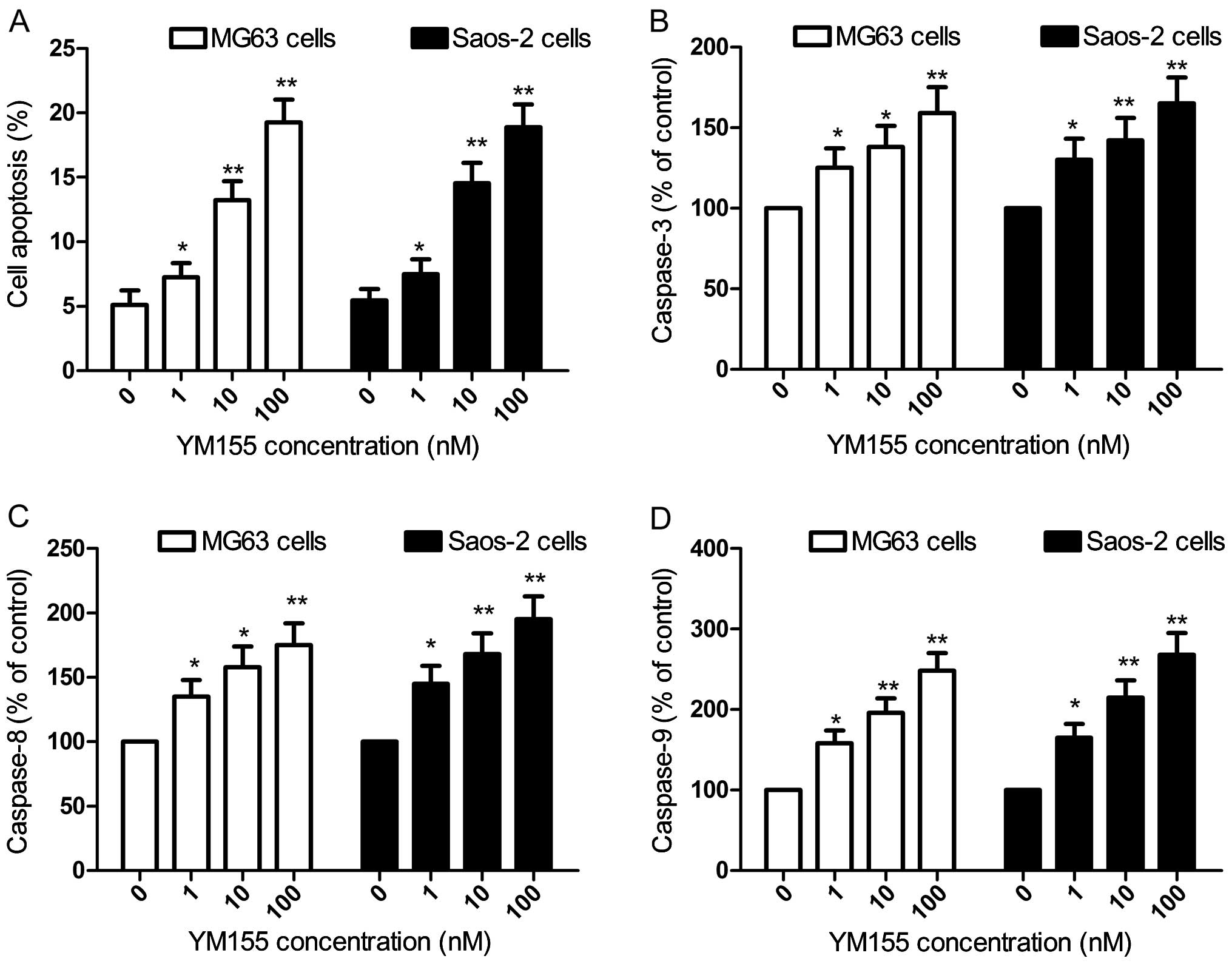
YM155 induces cell apoptosis and
increases caspase activity in OS cells
To examine the effect of YM155 on cell apoptosis, we
performed apoptosis assays using the Annexin V-FITC/PI staining
method. Our result showed that YM155 significantly induced
apoptosis in the MG63 and Saos-2 cells in a dose-dependent manner
(Fig. 3A, P<0.05).
In addition, we also evaluated the effect of YM155
on caspase activity in the OS cells as previously described
(28). YM155 significantly
increased caspase-3, -8 and -9 activity in the MG63 and Saos-2
cells in a dose-dependent manner (Fig.
3B-D, P<0.05).
YM155 affects the expression of
anti-apoptotic proteins in OS cells
To determine the potential mechanism of
YM155-induced cell apoptosis of OS cells, we investigated the
effects of YM155 on the expression of anti-apoptotic proteins, such
as XIAP, Bcl-2 and Mcl-1 in the OS cell lines (Fig. 4). Western blot assay showed that
YM155 significantly decreased Mcl-1 protein expression in the OS
cell lines in a dose-dependent manner. There was no change in Bcl-2
or XIAP levels in the OS cell lines after treatment with YM155.
YM155 affects the PI3K/AKT signaling
pathway in OS cells
It has been showed that the PI3K/AKT signaling
pathway plays a crucial role in cell proliferation, apoptosis,
migration and invasion (29).
Previous studies have shown that inhibition of PI3K and AKT
downregulates survivin protein expression (30,31).
Here, we aimed to ascertain whether YM155 affects the expression of
the PI3K/AKT signaling pathway in the OS cells. We examined PI3K,
AKT, p-PI3K and p-AKT expression by western blot analysis, and
found that YM155 reduced the expression of p-PI3K (Tyr458) and
p-AKT (Ser473) in the OS cells, whereas the total PI3K and AKT
remained unchanged (Fig. 5).
Antitumor effect of YM155 in a xenograft
model
To confirm the antitumor effects of YM155 in
vivo, we used a BALB/c mouse xenograft model. Animals bearing
MG63 tumors were treated with YM155. Control mice were treated with
saline (carrier) equivalently delivered. YM155 was well-tolerated,
and it did not cause any animal mortality or induce significant
decrease in body weight compared to the control group. Mice were
sacrificed 30 days after treatment initiation, and tumor tissue was
excised. YM155 treatment markedly abrogated tumor growth (Fig. 6A); the average size of the tumors
from the control group at the study termination was 1,423±113 vs.
624±67 mm3 in the YM155-treated group (P<0.05,
Fig. 6B). Moreover, treatment with
YM155 significantly reduced tumor weight compared with the control
(P<0.05). Average tumor weights at study termination were
1.42±0.13 and 0.62±0.10 g in the control and YM155 group,
respectively (Fig. 6C). In
addition, survivin expression in the xenograft tumors was
determined by western blot analysis. The results revealed that
survivin expression was downregulated in the YM155 treatment group
compared to the other groups (Fig.
6D). These results suggest that YM155 markedly suppressed OS
tumorigenicity in nude mice through inhibition of survivin
expression.
Discussion
Survivin, a member of the inhibitor of apoptosis
protein family, is involved in cell survival and regulation of
mitosis in various types of cancer (6–10).
Growing evidence has demonstrated that survivin suppression induces
tumor cell apoptosis and enhances sensitivity to apoptosis induced
by existing anticancer drugs and other apoptotic stimuli (13–18),
suggesting that survivin may be a new avenue for anticancer
treatment. Overexpression of survivin has been reported to be
important in the development and progression of OS cells (32,33).
Shoeneman et al found that inhibition of survivin expression
in canine OS cells by siRNA inhibited cell-cycle progression,
increased apoptosis, mitotic arrest, and chemosensitivity, and
cooperated with chemotherapy to obviously improve in vivo
tumor control (34). Zou et
al found that that in OS cells, downregulation of survivin with
the use of the pSUPER-sh vector induced cell apoptosis and promoted
induction of cell death by chemotherapy (35). In agreement with these results, in
the present study, our results showed that inhibition of survivin
expression by YM155, a small-molecule inhibitor of survivin,
significantly suppressed OS growth in vitro and in
vivo, suggesting that survivin is a novel target for anticancer
therapy for OS.
Several therapeutic approaches for inhibiting
survivin expression, such as immunotherapy or small-molecule
antagonists, siRNA, either as single agents or in combination with
conventional chemotherapeutic agents, are currently in clinical
trials (36). YM155, identified as
a novel small-molecule survivin suppressant, has been showed to
have potent anticancer activity against a broad spectrum of human
cancer cell lines, such as lung, breast, prostate, ovarian and
bladder cancer, and various human-derived tumor xenograft mouse
models (21). However, there are
few studies describing the inhibitory effect of YM155 on human OS
which highly expresses survivin. Here, we tested the effects of
YM155 on OS cells. The present study showed that YM155 inhibited
MG63 and Saos-2 cell proliferation and migration and invasion, as
well as induced cell apoptosis and increased caspase-3, -8 and -9
activity, suggesting that YM155 has effective anticancer activity
against OS.
Although YM155 has been shown to have antitumor
activities in vitro, in vivo and in clinical trials,
the mechanism underlying human cancer susceptibility to YM155
remains to be fully elucidated. Recently, it was reported that
YM155 exerts an anti-proliferative effect, inducing cell apoptosis
through Mcl-1 in various cancer cell types (37). Feng et al (38) also demonstrated that YM155 exhibited
robust cytotoxic activity through downregulation of survivin and
Mcl-1 in human leukemia cells. Mcl-1 is a widely expressed
pro-survival Bcl-2 family member. It protects cells from
stimulus-triggered apoptosis, while inhibition of Mcl-1 usually
correlates with induction of cellular apoptosis (39). Consistent with these results, the
present study showed that YM155 significantly inhibited survivin
and Mcl-1 protein expression in OS cell lines in a dose-dependent
manner, without altering the Bcl-2 or XIAP levels in the OS cell
lines, suggesting that YM155 suppresses OS growth through
inhibition of survivin and Mcl-1 expression.
An increasing body of evidence has shown that the
PI3K/AKT pathway is frequently hyperactivated in OS and contributes
to disease initiation and development, including tumorigenesis,
proliferation, invasion, cell cycle progression, inhibition of
apoptosis, angiogenesis, metastasis and chemoresistance (29,40).
Inhibition of this pathway using small-molecule compounds
represents an attractive potential therapeutic approach for OS
(40). Previous studies have shown
that inhibition of PI3K and AKT through a PI3K or an AKT-specific
inhibitor decreased the survivin protein expression in cancer cells
(30,31). Of note, recently a study showed that
YM155 downregulates PI3K expression in human pancreatic cancer
cells (41). Here, we found that
YM155 reduced the expression of p-PI3K (Tyr458) and p-AKT (Ser473)
expression, whereas the total PI3K and AKT remained unchanged
(Fig. 5), suggesting that YM155
inhibits OS cell growth in vitro and in vivo, at
least in part, via inhibiting the activation of the PI3K/AKT
signaling pathway.
In summary, we present evidence that YM155
significantly inhibits OS cell proliferation, colony formation,
migration and invasion, and induces cell apoptosis and increases
caspase-3, -8 and -9 activities in vitro, as well as
suppresses tumor growth in vivo through inhibition of
survivin and Mcl-1 expression. In addition, YM155 inhibits the
activation of the PI3K/AKT signaling pathway, which contributes to
inhibition of OS cell survival. These findings suggest that YM155
may be a promising drug candidate for the treatment of OS.
References
|
1
|
Ottaviani G, Robert RS, Huh WW, Palla S
and Jaffe N: Sociooccupational and physical outcomes more than 20
years after the diagnosis of osteosarcoma in children and
adolescents: Limb salvage versus amputation. Cancer. 119:3727–3736.
2013.PubMed/NCBI
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
3
|
Ozaki T, Flege S, Kevric M, Lindner N,
Maas R, Delling G, Schwarz R, von Hochstetter AR, Salzer-Kuntschik
M, Berdel WE, et al: Osteosarcoma of the pelvis: Experience of the
Cooperative Osteosarcoma Study Group. J Clin Oncol. 21:334–341.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chou AJ, Kleinerman ES, Krailo MD, Chen Z,
Betcher DL, Healey JH, Conrad EU III, Nieder ML, Weiner MA, Wells
RJ, et al Children’s Oncology Group: Addition of muramyl tripeptide
to chemotherapy for patients with newly diagnosed metastatic
osteosarcoma: A report from the Children’s Oncology Group. Cancer.
115:5339–5348. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grignani G, Palmerini E, Dileo P, Asaftei
SD, D’Ambrosio L, Pignochino Y, Mercuri M, Picci P, Fagioli F,
Casali PG, et al: A phase II trial of sorafenib in relapsed and
unresectable high-grade osteosarcoma after failure of standard
multimodal therapy: An Italian Sarcoma Group study. Ann Oncol.
23:508–516. 2012. View Article : Google Scholar
|
|
6
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar
|
|
7
|
Lu CD, Altieri DC and Tanigawa N:
Expression of a novel anti-apoptosis gene, survivin, correlated
with tumor cell apoptosis and p53 accumulation in gastric
carcinomas. Cancer Res. 58:1808–1812. 1998.PubMed/NCBI
|
|
8
|
Kawasaki H, Altieri DC, Lu CD, Toyoda M,
Tenjo T and Tanigawa N: Inhibition of apoptosis by survivin
predicts shorter survival rates in colorectal cancer. Cancer Res.
58:5071–5074. 1998.PubMed/NCBI
|
|
9
|
Monzó M, Rosell R, Felip E, Astudillo J,
Sánchez JJ, Maestre J, Martín C, Font A, Barnadas A and Abad A: A
novel anti-apoptosis gene: Re-expression of survivin messenger RNA
as a prognosis marker in non-small-cell lung cancers. J Clin Oncol.
17:2100–2104. 1999.PubMed/NCBI
|
|
10
|
Tanaka K, Iwamoto S, Gon G, Nohara T,
Iwamoto M and Tanigawa N: Expression of survivin and its
relationship to loss of apoptosis in breast carcinomas. Clin Cancer
Res. 6:127–134. 2000.PubMed/NCBI
|
|
11
|
Liggins C, Orlicky DJ, Bloomquist LA and
Gianani R: Developmentally regulated expression of Survivin in
human pancreatic islets. Pediatr Dev Pathol. 6:392–397. 2003.
View Article : Google Scholar
|
|
12
|
Xing J, Jia CR, Wang Y, Guo J and Cai Y:
Effect of shRNA targeting survivin on ovarian cancer. J Cancer Res
Clin Oncol. 138:1221–1229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krajewska M, Krajewski S, Banares S, Huang
X, Turner B, Bubendorf L, Kallioniemi OP, Shabaik A, Vitiello A,
Peehl D, et al: Elevated expression of inhibitor of apoptosis
proteins in prostate cancer. Clin Cancer Res. 9:4914–4925.
2003.PubMed/NCBI
|
|
14
|
McKenzie JA and Grossman D: Role of the
apoptotic and mitotic regulator survivin in melanoma. Anticancer
Res. 32:397–404. 2012.PubMed/NCBI
|
|
15
|
Dohi T, Okada K, Xia F, Wilford CE, Samuel
T, Welsh K, Marusawa H, Zou H, Armstrong R, Matsuzawa S, et al: An
IAP-IAP complex inhibits apoptosis. J Biol Chem. 279:34087–34090.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wheatley SP and McNeish IA: Survivin: A
protein with dual roles in mitosis and apoptosis. Int Rev Cytol.
247:35–88. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tulu US, Fagerstrom C, Ferenz NP and
Wadsworth P: Molecular requirements for kinetochore-associated
microtubule formation in mammalian cells. Curr Biol. 16:536–541.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sampath SC, Ohi R, Leismann O, Salic A,
Pozniakovski A and Funabiki H: The chromosomal passenger complex is
required for chromatin-induced microtubule stabilization and
spindle assembly. Cell. 118:187–202. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakahara T, Kita A, Yamanaka K, Mori M,
Amino N, Takeuchi M, Tominaga F, Hatakeyama S, Kinoyama I,
Matsuhisa A, et al: YM155, a novel small-molecule survivin
suppressant, induces regression of established human
hormone-refractory prostate tumor xenografts. Cancer Res.
67:8014–8021. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawano H, Shakushiro K, Nakata M, Kita A,
Maeda A, Watanabe S, Sako K and Oku N: Antitumor efficacy and
biodistribution of liposomal sepantronium bromide (YM155), a novel
small-molecule survivin suppressant. Eur J Pharm Biopharm.
88:283–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakahara T, Kita A, Yamanaka K, Mori M,
Amino N, Takeuchi M, Tominaga F, Kinoyama I, Matsuhisa A, Kudou M,
et al: Broad spectrum and potent antitumor activities of YM155, a
novel small-molecule survivin suppressant, in a wide variety of
human cancer cell lines and xenograft models. Cancer Sci.
102:614–621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Minematsu T, Iwai M, Sugimoto K, Shirai N,
Nakahara T, Usui T and Kamimura H: Carrier-mediated uptake of
1-(2-methoxyethyl)-2-methyl-4,9-dioxo-3-(pyrazin-2-ylmethyl)-4,9-dihydro-1H-naphtho[2,3-d]imidazolium
bromide (YM155 monobromide), a novel small-molecule survivin
suppressant, into human solid tumor and lymphoma cells. Drug Metab
Dispos. 37:619–628. 2009. View Article : Google Scholar
|
|
23
|
Kita A, Nakahara T, Yamanaka K, Nakano K,
Nakata M, Mori M, Kaneko N, Koutoku H, Izumisawa N and Sasamata M:
Antitumor effects of YM155, a novel survivin suppressant, against
human aggressive non-Hodgkin lymphoma. Leuk Res. 35:787–792. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tolcher AW, Quinn DI, Ferrari A, Ahmann F,
Giaccone G, Drake T, Keating A and de Bono JS: A phase II study of
YM155, a novel small-molecule suppressor of survivin, in
castration-resistant taxane-pretreated prostate cancer. Ann Oncol.
4:968–973. 2012. View Article : Google Scholar
|
|
25
|
Zhang K, Li Y, Liu W, Gao X and Zhang K:
Silencing survivin expression inhibits the tumor growth of
non-small-cell lung cancer cells in vitro and in vivo. Mol Med Rep.
11:639–644. 2015.
|
|
26
|
Giaccone G, Zatloukal P, Roubec J, Floor
K, Musil J, Kuta M, van Klaveren RJ, Chaudhary S, Gunther A and
Shamsili S: Multicenter phase II trial of YM155, a small-molecule
suppressor of survivin, in patients with advanced, refractory,
non-small-cell lung cancer. J Clin Oncol. 27:4481–4486. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lewis KD, Samlowski W, Ward J, Catlett J,
Cranmer L, Kirkwood J, Lawson D, Whitman E and Gonzalez R: A
multi-center phase II evaluation of the small molecule survivin
suppressor YM155 in patients with unresectable stage III or IV
melanoma. Invest New Drugs. 29:161–166. 2011. View Article : Google Scholar
|
|
28
|
Yang Q, Zhang S, Kang M, Dong R and Zhao
J: Synergistic growth inhibition by sorafenib and cisplatin in
human osteosarcoma cells. Oncol Rep. 33:2537–2544. 2015.PubMed/NCBI
|
|
29
|
Follo MY, Manzoli L, Poli A, McCubrey JA
and Cocco L: PLC and PI3K/Akt/mTOR signalling in disease and
cancer. Adv Biol Regul. 57:10–16. 2015. View Article : Google Scholar
|
|
30
|
Hideshima T, Catley L, Raje N, Chauhan D,
Podar K, Mitsiades C, Tai YT, Vallet S, Kiziltepe T, Ocio E, et al:
Inhibition of Akt induces significant downregulation of survivin
and cytotoxicity in human multiple myeloma cells. Br J Haematol.
138:783–791. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siddiqa A, Long LM, Li L, Marciniak RA and
Kazhdan I: Expression of HER-2 in MCF-7 breast cancer cells
modulates anti-apoptotic proteins Survivin and Bcl-2 via the
extracellular signal-related kinase (ERK) and phosphoinositide-3
kinase (PI3K) signalling pathways. BMC Cancer. 8:1292008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Osaka E, Suzuki T, Osaka S, Yoshida Y,
Sugita H, Asami S, Tabata K, Hemmi A, Sugitani M, Nemoto N, et al:
Survivin as a prognostic factor for osteosarcoma patients. Acta
Histochem Cytochem. 39:95–100. 2006. View Article : Google Scholar
|
|
33
|
Trieb K, Lehner R, Stulnig T, Sulzbacher I
and Shroyer KR: Survivin expression in human osteosarcoma is a
marker for survival. Eur J Surg Oncol. 29:379–382. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shoeneman JK, Ehrhart EJ III, Eickhoff JC,
Charles JB, Powers BE and Thamm DH: Expression and function of
survivin in canine osteosarcoma. Cancer Res. 72:249–259. 2012.
View Article : Google Scholar
|
|
35
|
Zou J, Gan M, Mao N, Zhu X, Shi Q and Yang
H: Sensitization of osteosarcoma cell line SaOS-2 to chemotherapy
by downregulating survivin. Arch Med Res. 41:162–169. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Church DN and Talbot DC: Survivin in solid
tumors: Rationale for development of inhibitors. Curr Oncol Rep.
14:120–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang H, Shao H, Yu C and Hou J: Mcl-1
downregulation by YM155 contributes to its synergistic anti-tumor
activities with ABT-263. Biochem Pharmacol. 82:1066–1072. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feng W, Yoshida A and Ueda T: YM155
induces caspase-8 dependent apoptosis through downregulation of
survivin and Mcl-1 in human leukemia cells. Biochem Biophys Res
Commun. 435:52–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thomas LW, Lam C and Edwards SW: Mcl-1;
the molecular regulation of protein function. FEBS Lett.
584:2981–2989. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Na YS, Yang SJ, Kim SM, Jung KA, Moon JH,
Shin JS, Yoon DH, Hong YS, Ryu MH, Lee JL, et al: YM155 induces
EGFR suppression in pancreatic cancer cells. PLoS One.
7:e386252012. View Article : Google Scholar : PubMed/NCBI
|