Introduction
The optimal use of chemotherapeutics, which allows
patients to achieve a completely hematologic remission, is an
effective and crucial strategy for leukemia patients (1). However, only 40% of patients achieve a
5-year survival (2), since most
patients relapse quickly after withdrawal of anticancer drugs. What
is worse, advanced stage patients frequently develop resistance to
drugs while receiving treatment. Intrinsic and acquired multidrug
resistance (MDR) is a phenomenon whereby tumor cells are resistant
to structurally and mechanistically distinct classes of compounds.
MDR remains a major impediment to the decrease in relapse rates in
leukemia patients (3). At present,
one of the most recognized mechanisms of MDR is overexpression of
ATP-binding cassette (ABC) transporters which reduce intracellular
drug concentrations leading to cancer cell resistance. Among the
ABC family proteins, ABC subfamily B member 1 (ABCB1) is closely
associated with recurrence and a poor therapeutic response in
leukemia patients (4).
ABCB1 (also named P-glycoprotein or MDR1), which is
a 170-kDa transporter, consists of two nucleotide binding domains
(NBDs) and two transmembrane domains (TMDs) (5). Leukemia cells often express a high
level of ABCB1 and more than 50% of conventional chemotherapeutics
undergo ABCB1-mediated efflux (6).
Accordingly, ABCB1 is regarded as an indicator of malignancy.
Interfering with the activity of ABCB1 may effectively circumvent
ABCB1-mediated drug resistance and improve the clinical efficacy of
leukemia chemotherapy (7).
Currently, numerous studies have sought to identify and develop
effective and safe inhibitors of the ABCB1 transporter.
Unfortunately, most studies have not confirmed sufficient efficacy
or have been terminated due to the nonspecific toxicity associated
with conventional chemotherapy drugs (8). The preliminary findings have aroused
intensive interest to tackle these hurdles. Exploring natural
ingredients or identifying potential sensitizers from a list of
approved drugs in the clinic provide a promising approach for the
development of ABCB1-modulating compounds. Butorphanol belongs to a
prototypically mixed agonist-antagonist opioid analgesic with a
weakly competitive µ-receptor agonist and strong κ-receptor
agonist. Because of its compounded agonist-antagonist functions,
butorphanol owns less incidences of respiratory depression and
pruritus but more balance anesthesia. At present, butorphanol has
been frequently applied in postoperative analgesia, labor analgesia
and cancer analgesia, including malignant leukemia (9–11).
In the present study, we demonstrated that
butorphanol, at a clinically used dose, obviously increased the
chemosensitivity of drug-resistant leukemia cells to conventional
anticancer drugs by directly inhibiting the efflux activity of
ABCB1.
Materials and methods
Chemicals and reagents
Butorphanol was purchased from Jiangsu Hengrui
Pharmaceutical Co. (Jiangsu, China). Doxorubicin (DOX), verapamil
(VRP), cisplatin, vincristine (VCR) and other chemicals were
purchased from Sigma Chemical Co. (St. Louis, MO, USA). Monoclonal
antibodies against ABCB1 (sc-55510) were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
1-(4,5-Dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) was
purchased from Zhongshan Golden Bridge Biotechmology (Beijing,
China).
Cell lines and cell culture
HL60 and HL60/VCR cell lines were purchased from the
institute of Hematology, the Chinese Academy of Medical Sciences
(Tianjin, China). K562 and K562/ADR cell lines were purchased from
the international medical Center of the First Central Hospital of
Tianjin (Tianjin, China). HL60/VCR and K562/ADR cells were
vincristine- and adriamycin-selected ABCB1-overexpressing cells,
respectively (12). All of the cell
lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM)
and RPmi-1640 medium with 10% fetal bovine serum (FBS) and 1%
antibiotic solution (penicillin-streptomycin) at 37°C in a
humidified atmosphere of 5% CO2 (11). All cells were grown in drug-free
culture medium for 2 weeks before the assay.
Cytotoxicity assay
The aim of the MTT assay was to evaluate the
sensitivity of leukemia cells to the drugs. Cells were seeded in
96-well plates and allowed to attach overnight. Afterwards, the
cells were preincubated with or without butorphanol and verapamil
for 1 h, and then various concentrations of chemotherapeutic drugs
were added into the designated wells. After 68 h, MTT was added
into the wells for an additional 4-h incubation after which the
yellow-colored MTT changed into dark-blue formazan crystals
(13). Subsequently, the medium was
discarded, and 120 µl of dimethylsulfoxide (DMSO) was added
to each well. The absorbance was determined at 655 nm using a model
550 microplate reader (Bio-Rad, Hercules, CA, USA). The
concentration required to inhibit cell growth by 50%
(IC50) was calculated from survival curves using the
Bliss methods (14). The
resistance-fold was calculated by dividing the IC50
value for the MDR cells with or without inhibitor by that of the
parental cells without inhibitor.
DOX accumulation
The intracellular DOX accumulation in the
ABCB1-overexpressing cells (HL60/VCR) and their parental sensitive
cells (HL60) was examined by flow cytometry. The logarithmically
growing cells were treated with 1, 2 and 4 µm butorphanol
and 5 µm verapamil at 37°C for 3 h (15). Then DOX (terminal concentration 10
µm) was added to the designated wells followed by incubation
for 30 min and 3 h, respectively. The cells were then collected,
centrifuged and washed twice with cold phosphate-buffered saline
(PBS). Cells were resuspended in 400 ml PBS and then analyzed by
flow cytometry (Cytomics FC 500; Beckman Coulter, USA). Verapamil,
which is known as an ABCB1 inhibitor, was used as a positive
control. The relative values were calculated by dividing the
fluoresence intensity of each measurement by that of the
negative-control cells (16,17).
Western blotting
Cell extracts were collected in cell lysis buffer
for 20 min on ice, and then the cells were centrifuged at 12,000
rpm at 48°C for 15 min. Equal amounts of proteins were resolved by
SDS-PAGE and transferred onto nitrocellulose membranes. Following
blocking in 5% non-fat milk in Tris-buffered saline and Tween-20
(TBST) buffer for 2 h at room temperature, the cells were incubated
with appropriately diluted primary antibodies overnight at 4°C
(18). The membranes were then
washed thrice with TBST buffer and incubated with HRP-conjugated
secondary antibody at a 1:5,000 dilution for 2 h at room
temperature. After being washed thrice with TBST buffer, the
protein antibody complex was visualized using the enhanced
Phototope™-HRP detection kit (Cell Signaling, USA) and exposed to a
Kodak medical X-ray processor (Carestream Health, USA). The
expression of GAPDH was used as a loading control. The protein
expression level was quantified using gray value analysis software
(17).
Immunofluorescence staining
For immunocytochemical analysis, the cells were
seeded in 24-well plates, and butorphanol at 4 µM was added
to the wells after overnight culture. After 72 h of incubation, the
cells were washed with PBS and fixed with 4% paraformaldehyde for
15 min at room temperature and then rinsed with PBS three times
(19). The cells were treated with
a monoclonal antibody against ABCB1 (1:500) (Sigma Chemical Co.)
and incubated overnight. Alexa Fluor 488 goat anti-mouse IgG was
added and cultured for 1 h. Immunofluorescence images were captured
with an inverted microscope (Olympus IX70; Olympus, Center Valley,
PA, USA) with IX-FLA fluorescence and a CCD camera (20).
Docking simulation
All docking calculations were performed using the
‘Extra Precision’ (XP) mode of glide program v5.5 (Schrödinger,
Inc., New York, NY, USA, 2009) and the default parameters. The top
scoring pose-ABCB1 complex was then subjected to energy
minimization using MacroModel program v9.7 using the OPLS-AA force
field and used for graphical analysis. All computations were
carried out on a Dell Precision 470n dual processor with Linux OS
(Red Hat Enterprise WS 4.0) (21).
Statistical analysis
All experiments were repeated at least three times,
and the Student’s t-test was used to determine differences.
Statistical significance was determined at p<0.05.
Results
Reversal effects of butorphanol on
ABCB1-overexpressing leukemia cells
In order to investigate the reversal effects of
butorphanol, we initially evaluated its cytotoxicity. The results
are shown in Fig. 1A and B. The
intrinsic cytotoxic effect of butorphanol on various cell lines was
examined by MTT assay. Notably, the results showed that butorphanol
at 4 µM had no obviously cytotoxic effect on all cell lines
used in the assay, and >85% cells survived. The IC50
values were 1.76±0.09, 1.64±0.1, 1.89±0.14 and 1.43±0.09
µmol/l for the K562, K562/ADR, HL60 and HL60/VCR cell lines,
respectively. Thus, butorphanol at 4 µm was chosen as the
maximum working concentration for further reversal assay.
Based on the above concentration, the
IC50 values of DOX, VCR and cisplatin in resistant cells
(HL60/VCR and K562/ADR) and sensitive cells (HL60 and K562) without
or combined with different concentrations of butorphanol,
respectively, are provided in Tables
I and II. Butorphanol, at 4
µM, obviously decreased the IC50 values of ABCB1
substrates DOX and VCR in the ABCB1-overexpressing HL60/VCR and
K562/ADR cells, while butorphanol did not change the
IC50 values of these chemotherapeutic drugs in the
parental HL60 and K562 cells. Meanwhile, the reversal effects were
similar to verapamil at 5 µM which is a well-known ABCB1
inhibitor. Yet, butorphanol did not affect the IC50
values of cisplatin in both the sensitive and resistant cells.
Cisplatin is not a substrate of ABCB1. These results showed that
butorphanol at 4 µm was able to reverse resistance to DOX
and VCR in the HL60/VCR and K562/ADR cells. Yet, in the parental
HL60 and K562 cells, the IC50 values of DOX and VCR were
not significantly different in the presence or absence of
buotorphanol. Thus, our results suggest that butorphanol sensitizes
ABCB1-overexpressing cells to chemotherapeutic agents which are
ABCB1 substrates.
 | Table IEffect of butorphanol on reversing
ABCB1-mediated MDR to doxorubicin (DOX), verapamil, cisplatin and
vincristine (VCR) in the sensitive (K562) and drug-resistant
(K562/ADR) cells. |
Table I
Effect of butorphanol on reversing
ABCB1-mediated MDR to doxorubicin (DOX), verapamil, cisplatin and
vincristine (VCR) in the sensitive (K562) and drug-resistant
(K562/ADR) cells.
| Treatment | IC50 ±
SDa (µM) (resistance fold)
|
|---|
| K562 | K562/ADR |
|---|
| DOX | 0.154±0.015
(1.00)b | 7.412±0.117
(48.13) |
| +Butor 1
(µM) | 0.149±0.008
(0.97) | 3.32±0.094
(21.58)c |
| +Butor 2 | 0.155±0.014
(1.01) | 2.32±0.037
(15.04)c |
| +Butor 4 | 0.146±0.001
(0.95) | 1.482±0.021
(9.63)c |
| +Verapamil 5 | 0.153±0.011
(0.99) | 1.457±0.018
(9.42)c |
| VCR | 0.030±0.002
(1.00)b | 3.252±0.381
(108.4) |
| +Butor 1
(µM) | 0.029±0.001
(0.98) | 1.47±0.172
(49.03)c |
| +Butor 2 | 0.029±0.001
(0.98) | 1.08±0.105
(36.00)c |
| +Butor 4 | 0.031±0.002
(1.03) | 0.62±0.081
(20.68)c |
| +Verapamil 5 | 0.029±0.001
(0.97) | 0.61±0.085
(20.08)c |
| Cisplatin | 1.63±0.174
(1.00)b | 2.35±0.481
(1.43) |
| +Butor 1
(µM) | 1.52±0.165
(0.93) | 2.40±0.427
(1.47) |
| +Butor 2 | 1.63±0.152
(1.00) | 2.34±0.445
(1.44) |
| +Butor 4 | 1.60±0.149
(0.98) | 2.27±0.385
(1.39) |
| +Verapamil 5 | 1.55±0.161
(0.95) | 2.28±0.392
(1.30) |
 | Table IIEffect of butorphanol on reversing
ABCB1-mediated MDR to doxorubicin (DOX), verapamil, cisplatin and
vincristine (VCR) in the sensitive (HL60) and drug-resistant
(HL60/VCR) cells. |
Table II
Effect of butorphanol on reversing
ABCB1-mediated MDR to doxorubicin (DOX), verapamil, cisplatin and
vincristine (VCR) in the sensitive (HL60) and drug-resistant
(HL60/VCR) cells.
| Treatment | IC50 ±
SDa (µM) (resistance
folds)
|
|---|
| HL60 | HL60/VCR |
|---|
| DOX | 67±11.0
(1.00)b | 2,532.6±151
(37.8) |
| +Butor 1
(µM) | 65±9.0 (0.97) | 1,326.6±232
(19.8)c |
| +Butor 2 | 69±10.0 (1.03) | 703.2±33.5
(10.5)c |
| +Butor 4 | 60±8.0 (0.92) | 506.5±35.6
(7.56)c |
| +Verapamil 5 | 62±8.2 (0.93) | 482.4±22.57
(7.2)c |
| VCR | 2.7±0.8
(1.00)b | 63.8±8.5
(23.4) |
| +Butor 1
(µM) | 2.58±0.75
(0.95) | 28.43±3.80
(10.53)c |
| +Butor 2 | 2.63±0.87
(0.97) | 19.44±2.65
(7.2)c |
| +Butor 4 | 2.85±0.61
(1.06) | 12.96±1.53
(4.8)c |
| +Verapamil 5 | 2.78±0.70
(1.03) | 10.55±2.19
(3.9)c |
| Cisplatin | 1,374±90.15
(1.00)b | 1,701±94.16
(1.23) |
| +Butor 1
(µM) | 1,410±102.60
(1.02) | 1,649±92.54
(1.20) |
| +Butor 2 | 1,319±88.29
(0.96) | 1,662±92.30
(1.21) |
| +Butor 4 | 1,370±85.41
(1.00) | 1,648±90.75
(1.20) |
| +Verapamil 5 | 1,358±190.13
(0.99) | 1,607.6±90.57
(1.17) |
Increased DOX accumulation by butorphanol
in the HL60/VCR cells
To explore the potential mechanism of butorphanol in
HL60/VCR and K562/ADR cells, we examined the intracellular
accumulation levels of DOX in the HL60/VCR cells and counterpart
HL60 cells, respectively. As shown in Fig. 2A and B, in the absence of
butorphanol, the fluorescence of DOX was lower in the resistant
cells than that in the sensitive cells. Yet, combined with 1, 2 or
4 µM butorphanol, accumulation levels of DOX in the HL60/VCR
cells was obviously increased by 2.3-, 3.47- and 5.91-fold,
respectively. The intracellular accumulation effect of DOX with 4
µM of butorphanol was similar to that with 5 µM of
verapamil. However, neither butorphanol nor verapamil affected the
intracellular levels of DOX in the HL60 cells. These results
indicated that butorphanol increased the intracellular accumulation
of DOX in the HL60/VCR cells in a concentration-dependent
manner.
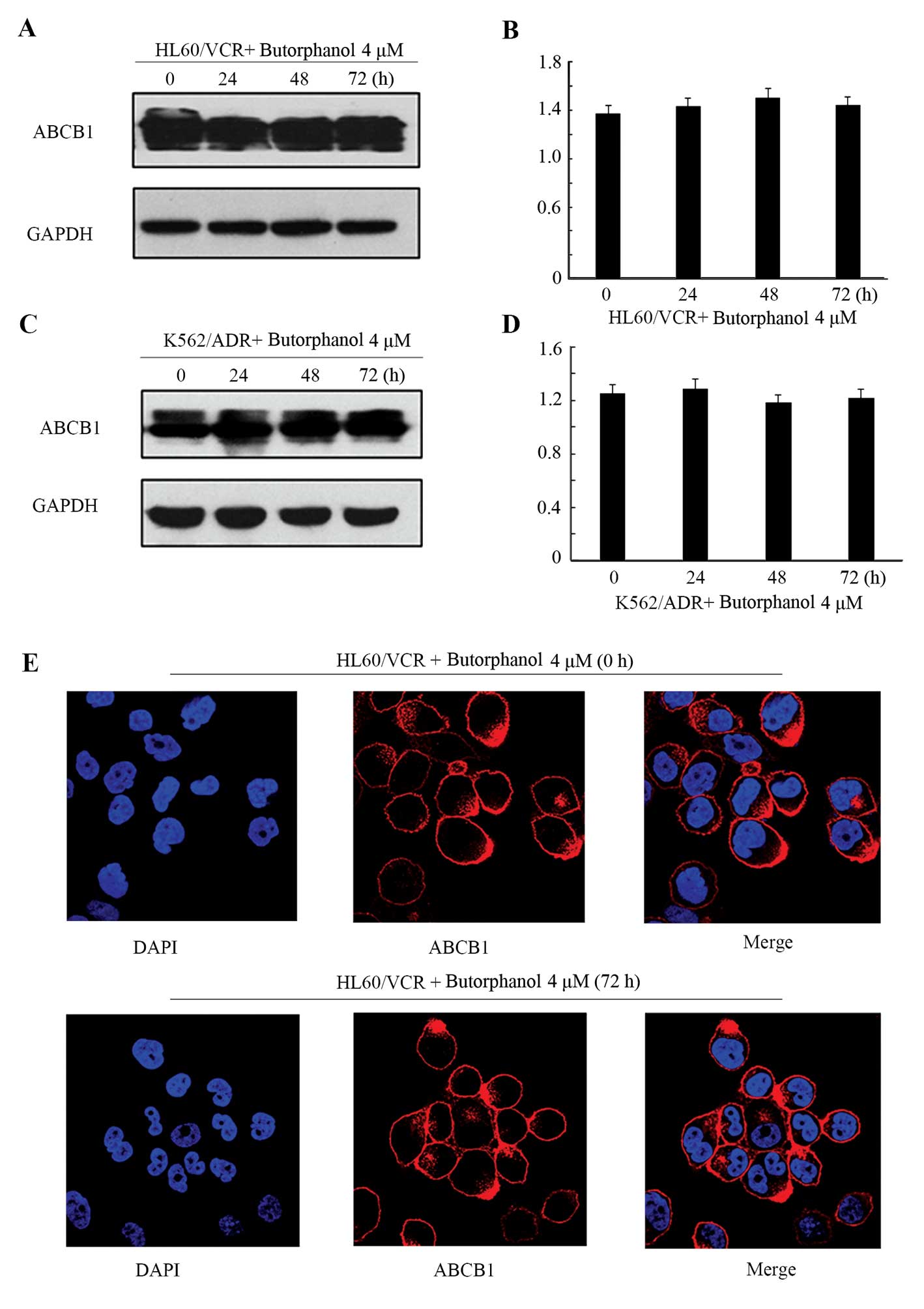
Effect of butorphanol on the expression
levels and cellular localizations of ABCB1
To evaluate whether butorphanol alters the protein
expression of ABCB1, we examined the protein levels of ABCB1
following treatment with butorphanol at 4 µm for 0, 24, 48
and 72 h in the HL60/VCR and K562/ADR cells. The protein levels of
ABCB1 were not significantly altered after incubation with
butorphanol at 4 µM for up to 72 h (Fig. 3A–D). In addition, we also tested the
localization of ABCB1 in the HL60/VCR cells in the presence of
butorphanol when treated with the drug for up to 72 h. There was no
obvious modulation in regards to the surface localization of ABCB1
between treatment with or without butorphanol in the HL60/VCR cells
(Fig. 3E). These results indicated
that the reversal of ABCB1-mediated MDR by butorphanol was neither
through altered expression nor translocation of ABCB1.
Model of molecular docking for
butorphanol binding with ABCB1
To predict the binding conformation of the large
cavity of the transmembrane region of ABCB1, we used a homology
model of human ABCB1 to explore the potential binding mode with
Glide docking software. There are three binding sites in the
homology model of ABCB1: ABCB1-QZ59-RRR (site-1), ABCB1-QZ59-SSS
(site-2) and ABCB1-verapamil (site-3). The binding energy data
(Glide scores for butorphanol at site-1 to site-3) suggest that
site-1 is the most suitable one. The predicted docking conformation
of butorphanol with the large hydrophobic drug binding cavity
(site-1) of human ABCB1 is shown in Fig. 4A and B. The 3.14-morphinan group is
stabilized into a hydrophobic pocket formed by residues IIe340,
Met68, Phe75, Tyr307, Phe339, IIe343, Phe951, Phe981 and Val987.
Moreover, the 17-cyclobutylmethyl formed hydrophobic bond
interacted with the side chain of Phe339 and was stabilized through
hydrophobic contact with the side chain of Met64, Phe335 and
Ile330.
Discussion
In the present study, we first demonstrated that
butorphanol, a kind of opioid analgesics, owns potentially
antineoplastic function via reversal of ABCB1-mediated MDR in
leukemia cell lines.
Over the past three decades, obvious developments
have been achieved in the treatment options for leukemia and
improvements in the clinical effects of anticancer drugs have been
noted. Following standard first-line treatment, adults with
leukemia have a 60–90% chance of attaining a completely hematologic
remission (22). Unfortunately, the
estimated 5-year survival of 40% is extremely low, since most
patients subsequently suffer relapse after withdrawal of cytotoxic
agents. Moreover, there is no effective therapy for patients with
relapse (23). MDR has been known
as the mainly contributing factor to treatment failure, and its
involvement has become a primary cause of mortality and a
formidable impediment to achieving long-term remission in leukemia
(24). The universally recognized
mechanism of MDR may be the overexpression of ABC transporters
which can weaken the cytotoxic effect of chemotherapeutic agents
via transferring drugs to the cell exterior (25). A clinical meta-analysis of 1,826
patients found that an increased ABCB1 level in leukemia patients
confers a worse response to conventional anticancer drugs (26). Recent studies have also identified
that leukemia cells with high levels of ABCB1 produce stubborn
resistance to imatinib which represents a standard regimen for the
first-line treatment of leukemia (27). Thus, ABCB1 may be a potential target
for successful chemotherapy of leukemia and a combination of ABCB1
inhibitors together with the chemotherapeutic regimen may be an
effective strategy.
Over the last decade, a large number of ABCB1
inhibitors have been developed and are categorized into three
generations based on sequential refinements in pharmacodynamic
properties (28). Yet, most are
limited in regards to their application in the clinic due to
serious side-effects and unexpected interaction affecting
pharmacokinetics (29). Therefore,
the development of safe and effective ABCB1 inhibitors to overcome
MDR is critical. Identification of previously approved drugs for
use as chemotherapeutic sensitizers is a more efficient method
which has been discussed broadly.
In the present study, we demonstrated that
butorphanol reversed ABCB1-mediated MDR in leukemia cell lines at
clinical doses by promoting the penetration of chemotherapeutic
agents into tumor cells, increasing their bioavailability and
achieving a better treatment outcome in leukemia cells. As a type
of synthetic opioid, butorphanol is applied to alleviate moderate
to serious pain, such as intraoperative, postoperative and
malignant trauma pain (30).
Butorphanol is used to produce an intrinsic function in the
treatment of leukemia (31,32). Based on these finding, butorphanol
has the ability to overcome MDR in parallel with the suppression of
pain in leukemia patients.
To evaluate the sufficient concentration and
cytotoxicity of butorphanol, we identified the maximum reversing
concentration of butorphanol for combination treatment with
antineoplastic drugs by MTT assay (Fig.
1A and B) (33). The results of
the MTT assay showed that butorphanol at 4 µM did not
restrain the growth of the cells used in the present study. The
results of colony formation assay were consistent with the MTT
assay (data not shown). Thus, butorphanol itself had no cytotoxic
effect on the leukemia cells. As shown in Tables I and II, butorphanol had no additive or
synergistic antitumor effect in sensitive cells, but in the
resistant cells, butorphanol markedly increased the sensitivity to
DOX and VCR of the HL60/VCR and K562/ADR cells, respectively.
Compared with the saline group, the resistance fold was effectively
reduced to 7.5-fold by 4 µM butorphanol; the resistance rate
was decreased by 76.4% compared with the group without butorphanol.
The reversal effect of butorphanol was achieved in a dose-dependent
manner.
The obvious results induced us to investigate the
reversal mechanism of butorphanol in the leukemia cells. We tested
the drug accumulation of DOX in the HL60/VCR cells. The result of
flow cytometric analyses demonstrated that butorphanol
significantly increased the intracellular accumulation of Dox
(Fig. 2A and B). Similar effects
were not found in the parental HL60 cells (15). These observations were consistent
with previous studies (34). Some
studies have identified that there are two main ways leading to the
reversal of MDR, reducing ABCB1 expression or inhibiting the efflux
function of ABCB1 (35). Therefore,
we examined the ABCB1 expression by treating the
ABCB-overexpressing cells with butorphanol at 4 µM for 24,
48 and 72 h, respectively. The results showed that butorphanol did
not alter the ABCB1 protein expression in the HL60/VCR and K562/ADR
cells (Fig. 3A–D). In addition,
there was no alteration in ABCB1 protein expression in the plasma
membranes of the HL60/VCR cells after treatment with butorphanol
(Fig. 3E). A number of recent
studies have corroborated that numerous ABC modulators directly
inhibit the pump function of ABC transporters without changing
their protein expression levels, such as motesanib (36), AST1306 (37) and tivozanib (38). Notably, Hamabe et al reported
that morphine, fentanyl and methadone can increase ABCB1 ATPase
activity, and their concentration-responses are bell-shaped
(39). Gonzalez et al also
demonstrated that the ATPase activity was obviously higher in cells
with low concentration of morphine than cells with morphine
tolerance (40). As an analogue of
morphine, the structure and function of butorphanol are similar to
morphine; thus, it may also have similar effects. Butorphanol at
low concentrations competitively binds to the sites of ABCB1,
leaving few sites for transporter substrates. When its
concentration is beyond a threshold, butorphanol directly inhibits
ATPase activity (7). Thus, we
conclude that butorphanol potentiated the sensitivity of anticancer
agents in HL60/VCR and K562/ADR cells through directly inhibiting
the efflux function of ABCB1.
To further understand the mechanisms underlying the
ABCB1 inhibitor butorphanol, we tested the docking simulation of
butorphanol and ABCB1 (21).
Through docking score, we identified the most appropriate binding
site. Electrostatic interaction of NH may form correct and stable
conformations in the hydrophobic pocket of ABCB1. Cyclobutylmethyl
and morphinan provide the predominant pharmacophoric features for
docking at the ABCB transporter. Considering the above findings,
butorphanol may be a better candidate as an ABCB1 inhibitor in
leukemia cells via providing a more effective and suitable method
to prevent and correct ABCB1-mediated MDR, simultaneously
suppressing cancer-related pain and enhancing the quality of life
of these patients.
In conclusion, the discovery of butorphanol offers a
potential method of the pharmacologic downregulation of
ABCB1-mediated MDR in HL60/VCR and K562/ADR cells in vitro.
These results suggest that butorphanol may be a new candidate as an
ABCB1 inhibitor and may be combined with conventional anticancer
drugs to overcome MDR. Butorphanol may also simultaneously improve
the pain threshold of patients.
However, before butorphanol can be administered to
leukemia patients further investigations are needed. Yet, the
present study provides an important clue to explore new functions
of drugs that have been previously used in the clinic.
References
|
1
|
Cooper SL and Brown PA: Treatment of
pediatric acute lymphoblastic leukemia. Pediatr Clin North Am.
62:61–73. 2015. View Article : Google Scholar :
|
|
2
|
Lussana F and Rambaldi A: Role of
allogeneic hematopoietic stem cell transplantation in adult
patients with acute lymphoblastic leukemia. Mediterr J Hematol
Infect Dis. 6:e20140652014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
te Boekhorst PA, de Leeuw K, Schoester M,
Wittebol S, Nooter K, Hagemeijer A, Löwenberg B and Sonneveld P:
Predominance of functional multidrug resistance (MDR-1) phenotype
in CD34+ acute myeloid leukemia cells. Blood.
82:3157–3162. 1993.PubMed/NCBI
|
|
4
|
Zhang BB, Xuan C, Deng KF, Wu N and Lun
LM: Association between the MDR1 gene variant C3435T and risk of
leukaemia: A meta-analysis. Eur J Cancer Care. 22:617–625. 2013.
View Article : Google Scholar
|
|
5
|
Lage H: An overview of cancer multidrug
resistance: A still unsolved problem. Cell Mol Life Sci.
65:3145–3167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saneja A, Khare V, Alam N, Dubey RD and
Gupta PN: Advances in P-glycoprotein-based approaches for
delivering anticancer drugs: Pharmacokinetic perspective and
clinical relevance. Expert Opin Drug Deliv. 11:121–138. 2014.
View Article : Google Scholar
|
|
7
|
Gillet JP, Efferth T and Remacle J:
Chemotherapy-induced resistance by ATP-binding cassette transporter
genes. Biochim Biophys Acta. 1775:237–262. 2007.PubMed/NCBI
|
|
8
|
Shi Z, Tiwari AK, Shukla S, Robey RW, Kim
IW, Parmar S, Bates SE, Si QS, Goldblatt CS, Abraham I, et al:
Inhibiting the function of ABCB1 and ABCG2 by the EGFR tyrosine
kinase inhibitor AG1478. Biochem Pharmacol. 77:781–793. 2009.
View Article : Google Scholar
|
|
9
|
Du BX, Song ZM, Wang K, Zhang H, Xu FY,
Zou Z and Shi XY: Butorphanol prevents morphine-induced pruritus
without increasing pain and other side effects: A systematic review
of randomized controlled trials. Can J Anaesth. 60:907–917. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parikh GP, Veena SR, Vora K, Parikh B and
Joshi A: Comparison of epidural butorphanol versus epidural
morphine in postoperative pain relief. Middle East J Anaesthesiol.
22:371–376. 2014.PubMed/NCBI
|
|
11
|
Shah B, Raichandani Y and Misra A:
Development and evaluation of oral osmotic pump of butorphanol
tartrate. Pharm Dev Technol. 19:868–880. 2014. View Article : Google Scholar
|
|
12
|
Guo HQ, Zhang GN, Wang YJ, Zhang YK,
Sodani K, Talele TT, Ashby CR Jr and Chen ZS: β-elemene, a compound
derived from Rhizoma zedoariae, reverses multidrug resistance
mediated by the ABCB1 transporter. Oncol Rep. 31:858–866. 2014.
|
|
13
|
Xie X, Yin J, Jia Q, Wang J, Zou C, Brewer
KJ, Colombo C, Wang Y, Huang G and Shen J: Quercetin induces
apoptosis in the methotrexate-resistant osteosarcoma cell line
U2-OS/MTX300 via mitochondrial dysfunction and dephosphorylation of
Akt. Oncol Rep. 26:687–693. 2011.PubMed/NCBI
|
|
14
|
Bonelli MA, Fumarola C, Alferi RR, La
monica S, Cavazzoni A, Galetti M, Gatti R, Belletti S, Harris AL,
Fox SB, et al: Synergistic activity of letrozole and sorafenib on
breast cancer cells. Breast Cancer Res Treat. 124:79–88. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao T, Song Y, Liu B, Qiu Q, Jiao L, Li
Y, Huang W and Qian H: Reversal of P-glycoprotein-medicated
multidrug resistance by LBM-A5 in vitro and a study of its
pharmacokinetics in vivo. Can J Physiol Pharmacol. 93:33–38. 2015.
View Article : Google Scholar
|
|
16
|
Zhou WJ, Zhang X, Cheng C, Wang F, Wang
XK, Liang YJ, To KK, Zhou W, Huang HB and Fu LW: Crizotinib
(PF-02341066) reverses multidrug resistance in cancer cells by
inhibiting the function of P-glycoprotein. Br J Pharmacol.
166:1669–1683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu LW, Zhang YM, Liang YJ, Yang XP and Pan
QC: The multidrug resistance of tumour cells was reversed by
tetrandrine in vitro and in xenografts derived from human breast
adenocarcinoma MCF-7/adr cells. Eur J Cancer. 38:418–426. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SK, Jung WH and Koo JS: Differential
expression of enzymes associated with serine/glycine metabolism in
different breast cancer subtypes. PLoS One. 9:e1010042014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Samuel S, Beljanski V, Van Grevenynghe J,
Richards S, Ben Yebdri F, He Z, Nichols C, Belgnaoui SM, Steel C,
Goulet ML, et al: BCL-2 inhibitors sensitize therapy-resistant
chronic lymphocytic leukemia cells to VSV oncolysis. Mol Ther.
21:1413–1423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aggarwal H, Aggarwal A, Hunter WJ III,
Yohannes P, Khan AU and Agrawal DK: Expression of leukemia/lymphoma
related factor (LRF/Pokemon) in human benign prostate hyperplasia
and prostate cancer. Exp Mol Pathol. 90:226–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Duan S, Chu C, Xu J, Zeng G, Lam AK,
Zhou J, Yin Y, Fang D, Reynolds MJ, et al: Melaleuca alternifolia
concentrate inhibits in vitro entry of influenza virus into host
cells. Molecules. 18:9550–9566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berman E, Heller G, Santorsa J, McKenzie
S, Gee T, Kempin S, Gulati S, Andreeff M, Kolitz J, Gabrilove J, et
al: Results of a randomized trial comparing idarubicin and cytosine
arabinoside with daunorubicin and cytosine arabinoside in adult
patients with newly diagnosed acute myelogenous leukemia. Blood.
77:1666–1674. 1991.PubMed/NCBI
|
|
23
|
Pulte D, Gondos A and Brenner H: Expected
long-term survival of patients diagnosed with acute myeloblastic
leukemia during 2006–2010. Ann Oncol. 21:335–341. 2010. View Article : Google Scholar
|
|
24
|
Vargas JR, Stanzl EG, Teng NN and Wender
PA: Cell-penetrating, guanidinium-rich molecular transporters for
overcoming efflux-mediated multidrug resistance. Mol Pharm.
11:2553–2565. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nori A and Kopecek J: Intracellular
targeting of polymer-bound drugs for cancer chemotherapy. Adv Drug
Deliv Rev. 57:609–636. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng Q, Wu H, Yu Q, Kim DH, Lipton JH,
Angelini S, Soverini S, Vivona D, Takahashi N and Cao J: ABCB1
polymorphisms predict imatinib response in chronic myeloid leukemia
patients: A systematic review and meta-analysis. Pharmacogenomics
J. 15:127–134. 2015. View Article : Google Scholar
|
|
27
|
Kosztyu P, Bukvova R, Dolezel P and
Mlejnek P: Resistance to daunorubicin, imatinib, or nilotinib
depends on expression levels of ABCB1 and ABCG2 in human leukemia
cells. Chem Biol Interact. 219:203–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shaffer BC, Gillet JP, Patel C, Baer MR,
Bates SE and Gottesman MM: Drug resistance: Still a daunting
challenge to the successful treatment of AML. Drug Resist Updat.
15:62–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krishna R, St-Louis M and Mayer LD:
Increased intracellular drug accumulation and complete
chemosensitization achieved in multidrug-resistant solid tumors by
co-administering valspodar (PSC 833) with sterically stabilized
liposomal doxorubicin. Int J Cancer. 85:131–141. 2000. View Article : Google Scholar
|
|
30
|
Golianu B, Krane EJ, Galloway KS and
Yaster M: Pediatric acute pain management. Pediatr Clin North Am.
47:559–587. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Osipova NA, Petrova VV, Novikov GA, Ziai
GR and Mel’nikova ZL: Synthetic analgesic moradol at various stages
of surgical treatment of patients with cancer. Anesteziol
Reanimatol. 2:56–58. 1990.In Russian.
|
|
32
|
Nakadate H, Endoh M, Satake A, Sida S,
Hatayama Y, Hatae Y, Takeda T, Wada I, Itho T and Kidoguchi T:
Continuous infusion of butorphanol for pain in children with
cancer. Gan To Kagaku Ryoho. 16:3495–3498. 1989.In Japanese.
PubMed/NCBI
|
|
33
|
Shi Z, Tiwari AK, Shukla S, Robey RW,
Singh S, Kim IW, Bates SE, Peng X, Abraham I, Ambudkar SV, et al:
Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug
resistance. Cancer Res. 71:3029–3041. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sodani K, Patel A, Anreddy N, Singh S,
Yang DH, Kathawala RJ, Kumar P, Talele TT and Chen ZS: Telatinib
reverses chemotherapeutic multidrug resistance mediated by ABCG2
efflux transporter in vitro and in vivo. Biochem Pharmacol.
89:52–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang XK, To KK, Huang LY, Xu JH, Yang K,
Wang F, Huang ZC, Ye S and Fu LW: Afatinib circumvents multidrug
resistance via dually inhibiting ATP binding cassette subfamily G
member 2 in vitro and in vivo. Oncotarget. 5:11971–11985.
2014.PubMed/NCBI
|
|
36
|
Wang YJ, Kathawala RJ, Zhang YK, Patel A,
Kumar P, Shukla S, Fung KL, Ambudkar SV, Talele TT and Chen ZS:
Motesanib (AMG706), a potent multikinase inhibitor, antagonizes
multidrug resistance by inhibiting the efflux activity of the
ABCB1. Biochem Pharmacol. 90:367–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang H, Wang YJ, Zhang YK, Wang DS,
Kathawala RJ, Patel A, Talele TT, Chen ZS and Fu LW: AST1306, a
potent EGFR inhibitor, antagonizes ATP-binding cassette subfamily G
member 2-mediated multidrug resistance. Cancer Lett. 350:61–68.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang D, Kathawala RJ, Chufan EE, Patel A,
Ambudkar SV, Chen ZS and Chen X: Tivozanib reverses multidrug
resistance mediated by ABCB1 (P-glycoprotein) and ABCG2 (BCRP).
Future Oncol. 10:1827–1841. 2014. View Article : Google Scholar
|
|
39
|
Hamabe W, Maeda T, Fukazawa Y, Kumamoto K,
Shang LQ, Yamamoto A, Yamamoto C, Tokuyama S and Kishioka S:
P-glycoprotein ATPase activating effect of opioid analgesics and
their P-glycoprotein-dependent antinociception in mice. Pharmacol
Biochem Behav. 85:629–636. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gonzalez LG, Masocha W, Sánchez-Fernández
C, Agil A, Ocaña M, Del Pozo E and Baeyens JM: Changes in
morphine-induced activation of cerebral Na+,
K+-ATPase during morphine tolerance: Biochemical and
behavioral consequences. Biochem Pharmacol. 83:1572–1581. 2012.
View Article : Google Scholar : PubMed/NCBI
|