Introduction
Osteosarcoma, the most common primary malignant bone
cancer in children and young adults, is characterized by potential
spontaneous pulmonary metastasis, strong resistance to chemotherapy
and poor prognosis (1). Although
the 5-year survival rate of osteosarcoma has improved with the
advent of neoadjuvant chemotherapy and vascular-targeted therapy,
impovement in the overall prognosis of osteosarcoma and further
enhancement of survival have not been significantly achieved due to
tumor cell resistance to treatment.
A hypoxic microenvironment which is present in
almost all solid tumors including osteosarcoma, is not only
implicated in tumor pathogenesis and development, but also plays a
vital role in the process of tumor recurrence and metastasis
(2,3). Accumulated evidence has shown that
angiogenesis inhibitors elicit the malignant progression of tumors
to accelerate local invasion and distant metastasis (4,5),
partially due to the induced hypoxic microenvironment (6,7).
However, tumor cells cultured under a hypoxic condition in
vitro are mostly prone to apoptosis or inhibition of
proliferation (8,9). These paradoxical findings pose
pertinent questions as to the probable mechanisms or molecular
events that cause a more aggressive phenotype of tumor cells
resulting from hypoxia in vivo. This may be as some
hypoxia-related genes secreted by tumor tissues protect cells from
deadly hypoxic stimuli, such as hypoxia-inducible factor-1α
(HIF-1α) (10). Therefore,
exploring the association of hypoxia-related genes with tumor
aggressiveness would be valuable for developing novel targeted
therapies for solid tumors (11).
Recently, our research group demonstrated that adrenomedullin
(ADM), known to be one of the hypoxia-regulated peptides, is
overexpressed in human osteosarcoma tissue and is highly associated
with prognosis and disease severity (12). It may become one of the potentially
attractive candidates for targeting osteosarcoma.
ADM is a secreted multifunctional hormone consisting
of 52 amino acids, which belongs to the calcitonin gene-related
peptide (CGRP) family. This peptide utilizes the covalent
receptors, formed between calcitonin receptor-like receptor (CRLR)
and one of the two accessory proteins, receptor activity-modifying
proteins (RAMPs) 2 or 3 (13).
Although ADM was termed for its initial isolation from a human
phaeochromocytoma (14), it is a
ubiquitous peptide synthesized by many normal tissues as well as by
a large variety of human cancers (15,16).
ADM has been reported to exert an anti-apoptotic effect on both
endothelial and tumor cells under certain stress conditions
(15). However, it is unclear
whether ADM confers a protective effect on apoptosis in
osteosarcoma cells under hypoxia.
Apoptosis, cell programmed suicide, plays an
important role in maintaining tissue homeostasis. Impaired
apoptosis by activating pro-apoptotic regulator B-cell lymphoma-2
(Bcl-2) is now considered to play an important role in bone
tumorigenesis (17,18) and lung metastasis (19). Although the effect of ADM on
overexpression of Bcl-2 has been verified in many types of tumor
cells (20,21), the relationship in osteosarcoma has
not been clearly identified.
Therefore, in regards to the close relationship
between overexpression of ADM and osteosarcoma first identified by
our group, the aim of this study was to further ascertain whether
overexpression of ADM is triggered by a hypoxic niche to blunt
apoptosis in osteosarcoma cells via affecting the expression of
Bcl-2, and to identify the possible signaling transduction
pathway.
Materials and methods
Cell culture and MTT assay
F5M2, the highly metastatic potential subline of
human osteosarcoma cell line SOSP-9607, was generously gifted by Dr
B.A. Ma (Fourth Military Medical University, Xi’an, China). F5M2
cells were cultured in RPMI-1640 medium supplemented with 10%
heat-inactivated fetal bovine serum, with 100 U/ml of penicillin
and 100 µg/ml of streptomycin. All of the cells were grown
at 37°C in a humidified atmosphere of 5% CO2. To
establish a hypoxic microenvironment, the exponentially growing
cells were cultured by incubation for 24 h, and then exposed to 200
µM cobalt chloride (CoCl2; Sigma-Aldrich, St.
Louis, MO, USA) with serum deprivation. To determine the effect of
ADM on cell proliferation, the cells were seeded in 96-well plates
at a density of 5×103 cells/well for 24 h, and then
pretreated with different concentrations of ADM (0–500 nM) for
another 24 h with an ADM receptor inhibitor, ADM22-52 (1
µM) (both from Sigma-Aldrich) or left untreated. At the end
of the treatment, 20 µl MTT (2.5 mg/ml; Sigma-Aldrich) was
added and incubation was carried out for another 4 h at 37°C. After
removal of the supernatant, 200 µl dimethyl sufloxide was
added to each well to solubilize the dark blue formazan crystals
that formed in the intact cells. The cell viability was assessed by
measuring the absorbance at 490 nm using an ELISA plate reader
(Bio-Rad, Hercules, CA, USA).
Cell groups
The cells were divided into 3 groups: the control
group (cells cultured in normoxic condition), the hypoxia group
(cells treated with 200 µM CoCl2) and the ADM
group (cells pretreated with the indicated concentrations of ADM
for 1 h, and then treated with 200 µM CoCl2 for
another 24 h).
Detection of gene expression by
RT-PCR
Treated and control cells were collected and washed
with cold phosphate-buffered saline (PBS). Total RNA was extracted
from the cells using RNA Fast 200 reagent (Takara, Dalian, China)
according to the manufacturer’s instructions. The RNA purity and
concentration were assessed by UV-Vis spectroscopy with the Bio-Rad
SmartSpec 3000 system (Bio-Rad) by the ratio of OD readings at
260/280 (1.8). Total RNA (2 mg) was used to synthesize cDNA in a
total volume of 20 µl reaction. cDNA (1 µl) was
amplified in a total volume of 25 µl using the RT-PCR kit
(Takara). Information regarding the sense and antisense primers of
ADM, its covalent receptors and Bcl-2 is listed in Table I. β-actin was examined as an
endogenous control for stable expression. The conditions of RT-PCR
cycling were as follows: 94°C for 5 min, followed by 32 cycles of
94°C for 30 sec, melting temperatures (Table I) for 30 sec, and 72°C for 30 sec
and a final extension at 72°C for 10 min. PCR products were
separated on a 2% agarose gel, and viewed by ethidium bromide
staining under UV light.
 | Table IInformation regarding the primers
used for RT-PCR. |
Table I
Information regarding the primers
used for RT-PCR.
| Gene name | Gene bank no. | Primer sense | Sequence
(5′-3′) | Size (bp) | Melting temperature
(°C) |
|---|
| ADM | NM_001124 | Forward |
AGAAGTGGAATAAGTGG | 295 | 45 |
| Reverse |
TTATCTGTGAACTGGTAG |
| CRLR | NM_005795 | Forward |
CTCCAGCAGAGAGTGTCACC | 205 | 55 |
| Reverse |
TCAAGACCCAGTCCAGCTCT |
| RAMP2 | NM_005854 | Forward |
GATATAGGCGCCCCCACAC | 184 | 58 |
| Reverse |
CTCGTGGGGATTCAGGACAG |
| RAMP3 | NM_005856 | Forward |
TGTCGTGGGCTGCTACTGG | 207 | 58 |
| Reverse |
AGCGTGTCGGTGCGTTTGC |
| Bcl-2 | NM_00633 | Forward |
GTTTCTTGAAGGTTTCCTCGTC | 300 | 58 |
| Reverse |
GGTTTCCTGCTTTCTTGGTG |
| β-actin | NM_001101 | Forward |
ATCGTGCGTGACATTAAGGAGAAG | 179 | 58 |
| Reverse |
AGGAAGGAAGGCTGGAAGAGTG | | |
Detection of apoptotic morphological
features by Hoechst 33342
F5M2 cells (1×105 cells/well) were plated
onto 6-well plates, incubated for 24 h at 37°C, and then pretreated
or not with ADM (100 nM) for 1 h, followed by administration of
CoCl2 (200 µM). The cells were then incubated in
serum-deprived culture for another 24 h. Cells cultured in a
normoxic condition acted as the control group. Three groups of
cells were fixed with 4% paraformaldehyde for 20 min, and then
incubated with Hoechst 33342 (1 µg/ml; Sigma-Aldrich) for 10
min at room temperature. The cells were then washed twice with PBS,
and examined under a fluorescence microscope. The numbers of
apoptotic cells in each group were counted in 10 random fields with
>500 cells.
Apoptosis detection by flow
cytometry
Cells (5×105 cells/well) were plated onto
6-well plates, incubated for 24 h at 37°C, and then individually
treated as mentioned in the above experiment. In each group, the
adherent cells and the cells contained in the supernatant were
harvested gently. The collected cells were washed twice, and
adjusted to a concentration of 1×106 cells/ml with PBS.
The cells were stained by combined application of Annexin V-FITC
and propidium iodide (PI; Dingguo, Beijing, China) to differentiate
apoptotic cells from the viable and necrotic cells. The cells were
added to 200 µl binding buffer, 5 µl (20 mg/l)
Annexin V-FITC and 10 µl (50 mg/l) PI, and then incubated at
room temperature in the dark for 15 min. The cells were then
analyzed by flow cytometry.
Western blot analysis
Treated and control cells were lysed in RIPA protein
extraction solution (Dingguo) in the presence of phosphatase
inhibitors for 1 h at 4°C, followed by centrifugation at 12,000 × g
for 20 min. The BCA protein assay kit was used to determine the
protein concentrations. Protein samples (30 µg/lane) were
separated by SDS-PAGE gels and then electrophoretically transferred
to polyvinylidene fluoride membranes. The membranes were blocked
with 5% fat-free milk in PBST buffer and then incubated with
primary antibodies against β-actin, ADM (1:1,000; Santa Cruz
Biotechnology, Santa Cruz, CA, USA), Bcl-2, extracellular
signal-regulated kinase (ERK) and P-ERK (1:500; Bioworld
Technology, St. Louis Park, MN, USA) overnight at 4°C. The
membranes were then washed and incubated with secondary antibodies
for 2 h (1:10,000; Bioworld Technology). The antigen-antibody
complexes were detected with the ECL chemiluminescence detection
kit. The optical density of the bands was quantified by using
Gel-Pro Analyzer v4.0 (Media Cybernetics, Rockville, MD, USA). The
results were measured by relative band density to that of β-actin
which was detected as the endogenous control.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
software (SPSS Inc., Chicago, IL, USA). All in vitro
experimental data presented represent at least three independent
experiments using samples from a minimum of three separate
isolations and are expressed as means ± SD as indicated and were
analyzed by one-way analysis of variance (ANOVA). A P-value
<0.05 was considered to indicate a statistically significant
result.
Results
Hypoxia-mediated induction of ADM
expression in F5M2 cells
To investigate whether ADM expression is induced
under a hypoxic condition by CoCl2 (200 µM)
treatment, we measured the levels of ADM mRNA and protein,
respectively through RT-PCR and western blotting in F5M2 cells. The
result showed that the expression of ADM mRNA and protein
was significantly higher under hypoxia than that under normoxia,
and increased in a time-dependent manner under a hypoxic condition
(Fig. 1A and B).
ADM has autocrine/paracrine effects on
F5M2 cells
We then determined whether the receptors of ADM
(CRLR, RAMP2 and RAMP3) were also expressed in the F5M2 cells using
RT-PCR. As shown in Fig. 2, these
receptors were identified as being expressed. To assess whether ADM
affects cell proliferation in an autocrine or paracrine manner, we
monitored cell growth following exogenous ADM administration with
or without ADM22-52 using MTT assays. The results
demonstrated that cell growth was significantly accelerated by ADM
in a dose-dependent manner, but inhibited by the ADM receptor
selective antagonist ADM22-52 (Fig. 3). This confirmed that ADM positively
affects cell growth in an autocrine and/or paracrine manner.
ADM blocks hypoxia-induced apoptosis
To understand the role of ADM in hypoxia-induced
apoptosis of F5M2 cells, the morphological changes noted in the
apoptotic cells were detected by Hoechst 33342 staining. The
apoptotic cells exhibited chromatin condensation and fragment
staining brighter than that of normal cells. In the control and
ADM-treated cells, the nuclei were stained a weak homogeneous blue,
while in the hypoxia-induced cells, bright chromatin condensation
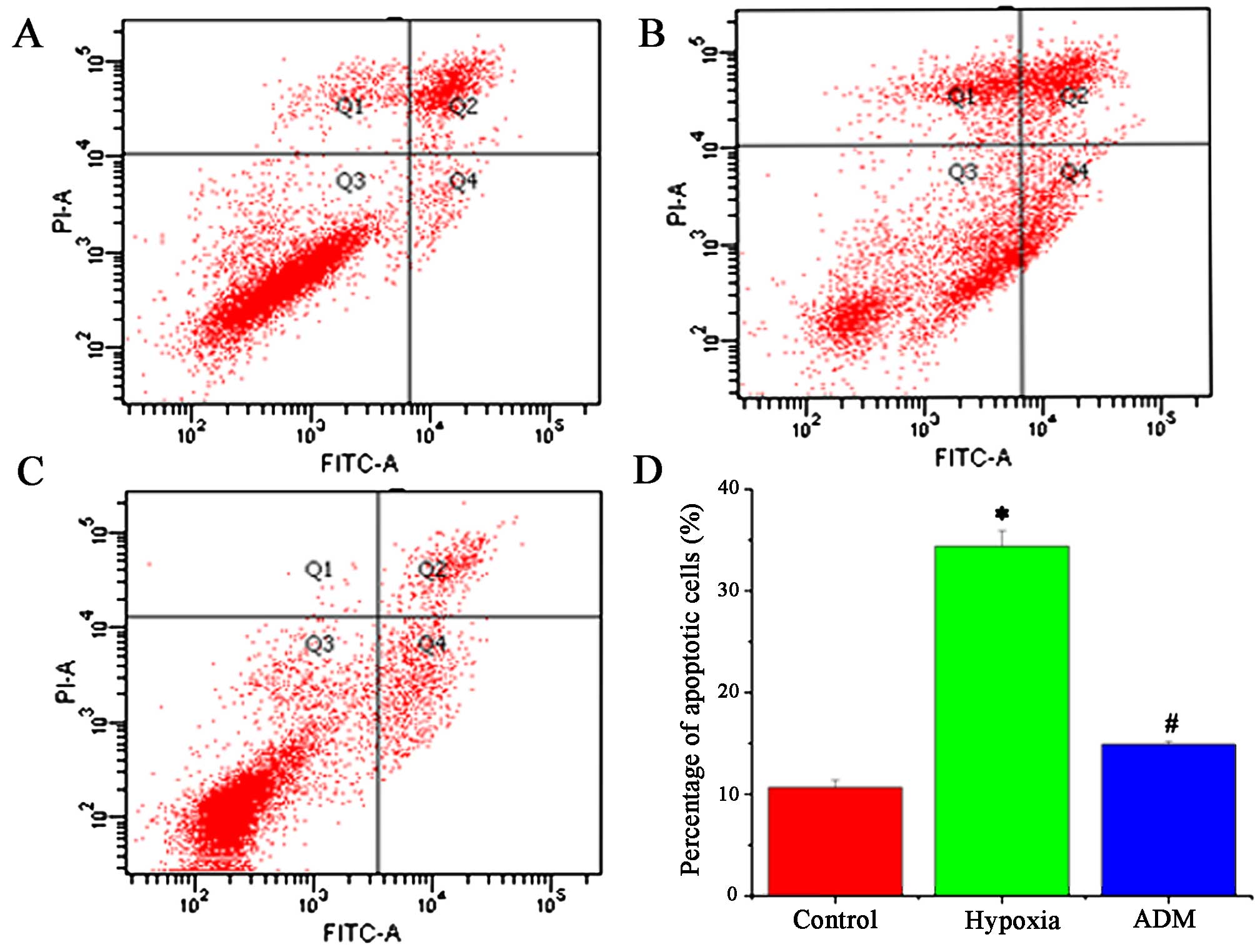
and nuclear fragmentation were observed (Fig. 4A–C). The percentage of apoptotic
cells in the counted fields in the control, hypoxia and ADM group
was 5.2±1.87, 24.8±5.31 and 8.7±2.83, respectively (Fig. 4D). The difference between the
control and ADM group was not statistically significant, yet a
statistically significant difference between the hypoxia group and
the other groups was obtained (P<0.05). The results of flow
cytometry showed that the percentage of apoptotic cells in the
hypoxia group was significantly higher (34.4±1.55) than that of the
control group (10.7±0.71) and ADM group (14.9±0.30) (P<0.05,
Fig. 5).
Involvement of the MEK/ERK1/2 pathway in
the upregulation of Bcl-2 by ADM
To clarify the mechanism underlying the suppressive
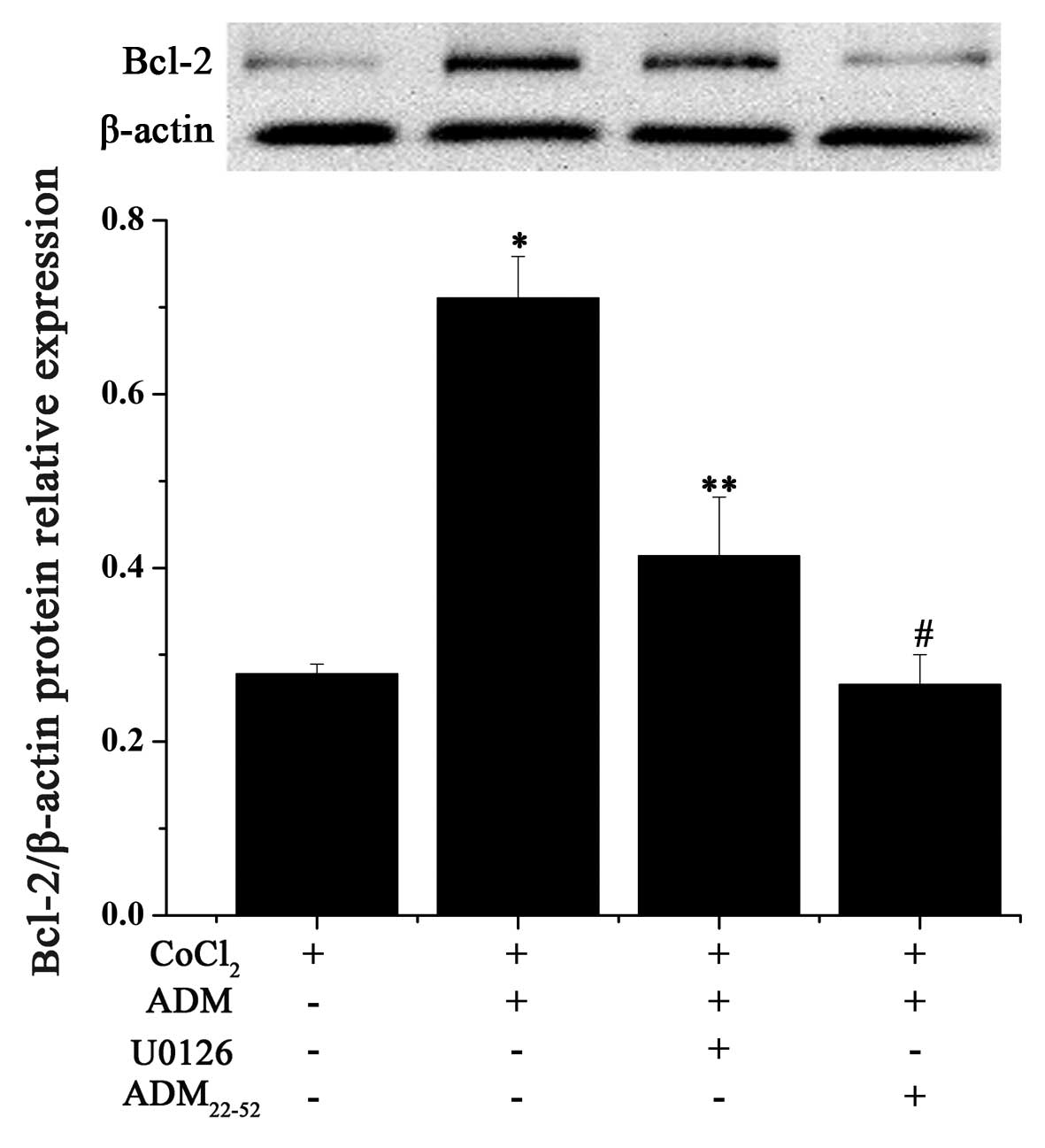
effect of ADM on hypoxia-induced apoptosis, expression of Bcl-2 was
examined by RT-PCR and western blot analysis. ADM pretreatment
resulted in the upregulation of Bcl-2 mRNA, regardless of a
normoxia or hypoxia condition (Fig.
6A). Treatment of F5M2 cells with CoCl2 (200
µM) for 24 h decreased the intracellular Bcl-2 production,
while pretreatment with ADM (1, 10 and 100 nM) for 1 h before
addition of CoCl2 markedly attenuated these effects in a
concentration-dependent manner (Fig.
6B). These data revealed that ADM increased expression of Bcl-2
at both the mRNA and protein levels.
In order to determine whether the MEK/ERK1/2
signaling pathway is involved in the inhibition of apoptosis by ADM
in hypoxia-induced F5M2 cells, we detected the phosphorylation of
ERK1/2 by western blotting. After F5M2 cells were treated with
CoCl2 (200 µM) for 30 min, the phosphorylation of
ERK1/2 was significantly decreased compared with the control group.
However, pretreatment of cells with ADM for 1 h reversed this
effect in a dose-dependent manner (Fig.
7). The result indicates that MEK/ERK1/2 is involved in the
signaling pathway of ADM.
In order to ascertain the role of MEK/ERK1/2 in the
ADM-induced overexpression of Bcl-2, a selective inhibitor of MEK
(U0126) was used in the F5M2 culture conditions (Fig. 8). When U0126 was co-administered
with ADM, Bcl-2 expression was partially hindered. Moreover, the
combination of ADM with ADM22-52 also had the same
effect on the significant downregulation of Bcl-2 expression
compared with the effect of U0126. These results collectively imply
that ADM can simulate Bcl-2 expression through its receptors and
partially the MEK/ERK1/2 pathway in F5M2 cells.
Discussion
The tumor hypoxic microenvironment, induced by the
rapid growth of tumor cells or vascular-targeted therapy, plays a
dual contradictory role in regards to the survival of cancer cells.
It starves tumors causing cell death, yet meanwhile, more malignant
cell clones evolve to resist treatment and diminish apoptotic
potential and accelerate metastasis (6). In such a hypoxic niche, it may be
plausible that hypoxia-regulated molecules are triggered and
recruited resulting in the development of a more aggressive
phenotype. It has been confirmed that ADM is one of the
hypoxia-induced peptides found in a variety of human cancers as
well as in cell lines (22,23), and plays an anti-apoptotic role. Our
initial experiment with osteosarcoma clearly demonstrated that ADM
was overexpressed in human osteosarcoma tissue, and is correlated
with the degree of malignancy and metastasis of osteosarcoma
(12). However, the effect of ADM
on apoptosis in osteosarcoma cells and the possible mechanism have
not yet been elucidated.
In order to address this issue, the present study
aimed to establish a hypoxic microenvironment. In our study, the
hypoxic niche was mimicked by addition of CoCl2 to the
cell culture, since this simulation is similar to hypoxia in
vivo, with identical signal transduction and transcription
regulation (8). Since osteosarcoma
has a high propensity to spontaneous pulmonary metastasis, we chose
the highly metastatic potential subline of the human osteosarcoma
cell line SOSP-9607, F5M2, as the research objective which was
confirmed to achieve a 100% spontaneous pulmonary metastasis rate
in an in vivo orthotopic transplantation assay (24). It is significantly important to
study osteosarcoma cell lines with high metastatic potential.
Under hypoxia stress mimicked by CoCl2,
the ADM mRNA and protein expression levels in F5M2 cells
were increased in a time-dependent manner, indicating that ADM was
secreted by osteosarcoma cells themselves, and therefore could
interact with the receptors expressed on cancer cells or cells in
the tumor microenvironment, such as endothelial or vascular smooth
muscle cells (15). mRNA levels of
the co-receptors of ADM, CLRL and RAMPs
(2,3) were expressed in the F5M2 cells.
Meanwhile, the exogenous administration of ADM significantly
increased cancer cell proliferation in a dose-dependent manner, and
this effect was inhibited by its receptor antagonist
(ADM22-52). These data implied that ADM exerted an
effect on osteosarcoma cells through an autocrine or/and paracrine
loop, and inhibition of the interaction of tumor cell-secreted ADM
with its receptors reduced the cellular functions. This conclusion
is in accordance with previous research in other forms of cancers,
such as pancreatic cancer (25) and
hepatocellular carcinoma (HCC) (22).
It is now widely known that apoptosis is a critical
factor for maintaining tissue homeostasis, and impaired apoptosis
is now recognized to be a key step in tumor development (17,18).
Since ADM is induced by hypoxia in osteosarcoma cells, whether
death or survival of cells is induced by administration of ADM
remains unclear. Martinez et al (26) reported that overexpression of ADM in
human breast cancer T47D cells induced higher levels of proteins
involved in oncogenic signaling pathways and lower levels of
pro-apoptotic proteins, indicating that ADM causes resistance to
apoptosis. Similarly, Chen et al (27) also confirmed that silencing of the
ADM gene in HO8910 ovarian cancer cells inhibited cell
proliferation and stimulated apoptosis. Some authors believe that
the extracellular stress conditions or/and types of tumor cell
lines determine the effect of ADM. Abasolo et al (23) demonstrated that ADM exerted a
protective effect against apoptosis in prostate cancer DU-145 and
PC-3 cells, but not in LNCaP cells after serum deprivation.
Notably, when PC-3 and LNCaP cells were treated with etoposide to
induce apoptosis, ADM played an anti-apoptotic role, but not in
DU-145 cells. Our findings showed that ADM significantly hindered
apoptosis under a CoCl2-induced hypoxic microenvironment
in F5M2 cells. This process was confirmed by Hoechst 33342 and
Annexin V-FITC/PI staining assay. This anti-apoptotic effect of ADM
is in agreement with observation by Oehler et al in
endometrial cancer cells under the same stress condition (21). These results imply that the
overexpression of ADM by a hypoxic stimulus exerts a protective
effect from tumor apoptosis via interacting with its acceptors.
Bcl-2 is a proto-oncogene that prevents the
apoptosis of various cancer cell types from many apoptotic stimuli,
such as hypoxia (28).
Overexpression of Bcl-2 is frequently detected in malignant tumors
and is related to poor prognosis (29). Downregulation of Bcl-2 expression
increased cellular apoptosis of osteosarcoma cells and sensitized
them to chemotherapeutic drugs (30). Li et al (20) identified that Bcl-2 and ADM were
co-expressed in bulky invasive squamous carcinoma. This implies
that ADM and Bcl-2 act as useful prognostic markers in selecting
tumor cells resistant to apoptosis and in promoting malignant
progression.
However, whether ADM produces Bcl-2 or the latter
resulted in ADM should be verified. To clarify their relationship,
Oehler et al (21) observed
that the addition of ADM to the culture of endometrial cancer cells
led to a 6-fold increase in Bcl-2 mRNA and a 5-fold increase in
Bcl-2 protein expression. Our present results in osteosarcoma cells
also demonstrated that ADM induced Bcl-2 mRNA expression,
regardless of a hypoxic or a normoxic condition. Meanwhile, ADM
also increased expression of Bcl-2 protein in a
concentration-dependent manner. The data demonstrated that ADM
facilitated the anti-apoptotic signaling and hindered the cells
from undergoing apoptosis by upregulation of Bcl-2.
Intracellular levels of ERK1/2 are usually
correlated with a broad array of cellular functions including
apoptosis, proliferation, survival, malignant transformation of
cells, and other biological responses (31). Activation of ERK1/2 has been
reported to achieve anti-apoptosis, and ultimately leads to
cellular transformation and tumorigenesis by upregulation of Bcl-2
(32). Previous studies have
demonstrated that ADM mediated-signaling transduction differs
between cell types (15,33). Uzan et al (34) showed that ADM acted as a survival
factor to exhibit an anti-apoptotic effect in osteoblastic cells
via the MEK/ERK pathway. Chen et al (27) reported that silencing of the ADM
gene stimulated apoptosis through downregulation of ERK1/2 and
Bcl-2 expression. In contrast, Park et al (22) did not detect the activation of any
other mitogen-activated protein kinases (MAPKs) or anti-apoptotic
signaling in HCC cells following ADM treatment. Abasolo et
al (23) found that P-ERK1/2
levels in PC-3 cells overexpressing ADM were lower than levels in
parental PC-3 cells. In our study, we found that ADM increased the
phosphorylation of ERK1/2 in CoCl2-mediated hypoxia
induction in osteosarcoma F5M2 cells. In addition, the effect of
upregulation of Bcl-2 induced by ADM was partially attenuated by
concomitant treatment with the specific MEK inhibitor (U0126) or
the selective ADM receptor antagonist (ADM22-52
fragment). These data suggest that i) ADM acts as a survival factor
in F5M2 cells via interacting with its receptors CRLR-RAMP2 and
CRLR-RAMP3; and ii) ADM activates the MEK/ERK1/2 pathway to
upregulate Bcl-2 expression to abrogate the apoptotic effect
induced by a hypoxic microenvironment.
All of the above findings imply that synergetically
targeting the ADM/ADM acceptors/ERK1/2/Bcl-2 pathway may provide a
potential strategy to promote the apoptosis of osteosarcoma cells,
yet further research must be undertaken to elucidate the more
detailed mechanisms in regards to other signaling transduction
pathways, other apoptotic pathways and experiments in vivo.
For example, compared with the inhibitory effect of U0126, the
inhibitory effect of ADM22-52 on the upregulation
function of Bcl-2 by ADM was more significant. Therefore, there may
be other signaling pathways involved in the effect on apoptosis by
ADM. As known, in addition to ERK1/2, JNK (c-Jun N-terminal kinase)
and p38 MAPK constitute the MAPK family that contributes to diverse
cellular apoptosis or survival processes (35,36).
Phosphatidylinositol 3′kinase (PI3K)/Akt pathway is also involved
in cell survival via the negative regulation of the Bcl-2 homology
domain (BH3)-only proteins (37).
Meanwhile, cAMP/protein kinase A (PKA) also mediates apoptosis via
regulating pro-apoptotic factor Bcl-2-interacting mediator of cell
death (BIM) (38). Although these
signaling transduction pathways are activated by ADM in vascular
endothelial, smooth muscle or cancer cells (15,16,39),
whether the apoptosis is still mediated by these pathways through
the overexpression of ADM has been unclear and should be further
elucidated. Secondly, in our study, Bcl-2 was chosen as an
indicator of apoptosis, and it is verified to be one product of the
anti-apoptotic ADM target gene. However, the effect of ADM on
expression of the pro-survival proteins (such as Bcl-xL and Bcl-w),
the pro-apoptotic proteins (such as Bax, Bak, Bad, Bmf and Bid),
caspase activation and pathways to apoptosis should be determined
further (18), as inhibition of
apoptosis by Bcl-2 is related to its capacity to interact with
these anti- or pro-apoptotic proteins (18,37).
Third, according to our research, the strategy targeting the
ADM/ADM receptor/ERK1/2/Bcl-2 pathway should also be confirmed by
in vivo experiments to induce the apoptosis of tumors and
inhibit pulmonary metastasis.
In conclusion, we observed that ADM expression is
increased in human osteosarcoma F5M2 cells under a hypoxic
microenvironment. Treatment with ADM significantly blunts
hypoxic-induced apoptosis. In an in vitro experiment, the
expression of Bcl-2 was increasingly induced by administration of
ADM, and was also reversed by an inhibitor of MEK, or a specific
receptor antagonist of ADM. These results showed that ADM inhibits
hypoxic-induced apoptosis in osteosarcoma cells by upregulating the
expression of Bcl-2 partially through activation of the MEK/ERK1/2
signaling pathway. Therefore, targeting the ADM/ADM
acceptors/ERK1/2/Bcl-2 pathway may provide a potential strategy to
induce the apoptosis of osteosarcoma cells and provide cancer
patients with maximal survival benefit.
References
|
1
|
Cleton-Jansen AM, Anninga JK, Briaire-de
Bruijn IH, Romeo S, Oosting J, Egeler RM, Gelderblom H, Taminiau AH
and Hogendoorn PC: Profiling of high-grade central osteosarcoma and
its putative progenitor cells identifies tumourigenic pathways. Br
J Cancer. 101:1909–1918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeng W, Wan R, Zheng Y, Singh SR and Wei
Y: Hypoxia, stem cells and bone tumor. Cancer Lett. 313:129–136.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao N, Sun BC, Sun T, Ma YM, Zhao XL, Liu
ZY, Dong XY, Che N, Mo J and Gu Q: Hypoxia-induced vasculogenic
mimicry formation via VE-cadherin regulation by Bcl-2. Med Oncol.
29:3599–3607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pàez-Ribes M, Allen E, Hudock J, Takeda T,
Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D and Casanovas O:
Antiangiogenic therapy elicits malignant progression of tumors to
increased local invasion and distant metastasis. Cancer Cell.
15:220–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason
GA, Christensen JG and Kerbel RS: Accelerated metastasis after
short-term treatment with a potent inhibitor of tumor angiogenesis.
Cancer Cell. 15:232–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loges S, Mazzone M, Hohensinner P and
Carmeliet P: Silencing or fueling metastasis with VEGF inhibitors:
Antiangiogenesis revisited. Cancer Cell. 15:167–170. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nurwidya F, Takahashi F, Minakata K,
Murakami A and Takahashi K: From tumor hypoxia to cancer
progression: The implications of hypoxia-inducible factor-1
expression in cancers. Anat Cell Biol. 45:73–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dai ZJ, Gao J, Ma XB, Yan K, Liu XX, Kang
HF, Ji ZZ, Guan HT and Wang XJ: Up-regulation of hypoxia inducible
factor-1α by cobalt chloride correlates with proliferation and
apoptosis in PC-2 cells. J Exp Clin Cancer Res. 31:282012.
View Article : Google Scholar
|
|
9
|
Knowles HJ, Schaefer KL, Dirksen U and
Athanasou NA: Hypoxia and hypoglycaemia in Ewing’s sarcoma and
osteosarcoma: Regulation and phenotypic effects of
hypoxia-inducible factor. BMC Cancer. 10:3722010. View Article : Google Scholar
|
|
10
|
El Naggar A, Clarkson P, Zhang F, Mathers
J, Tognon C and Sorensen PH: Expression and stability of hypoxia
inducible factor 1α in osteosarcoma. Pediatr Blood Cancer.
59:1215–1222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bao B, Ahmad A, Kong D, Ali S, Azmi AS, Li
Y, Banerjee S, Padhye S and Sarkar FH: Hypoxia induced
aggressiveness of prostate cancer cells is linked with deregulated
expression of VEGF, IL-6 and miRNAs that are attenuated by CDF.
PLoS One. 7:e437262012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai X, Ma W, He XJ and Jha RK: Elevated
expression of adrenomedullin is correlated with prognosis and
disease severity in osteosarcoma. Med Oncol. 30:3472013. View Article : Google Scholar
|
|
13
|
Hay DL, Walker CS and Poyner DR:
Adrenomedullin and calcitonin gene-related peptide receptors in
endocrine-related cancers: Opportunities and challenges. Endocr
Relat Cancer. 18:C1–C14. 2011. View Article : Google Scholar
|
|
14
|
Kitamura K, Kangawa K, Kawamoto M, Ichiki
Y, Nakamura S, Matsuo H and Eto T: Adrenomedullin: A novel
hypotensive peptide isolated from human pheochromocytoma. Biochem
Biophys Res Commun. 192:553–560. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nikitenko LL, Fox SB, Kehoe S, Rees MC and
Bicknell R: Adrenomedullin and tumour angiogenesis. Br J Cancer.
94:1–7. 2006. View Article : Google Scholar
|
|
16
|
Dai X, Ma W, Jha RK and He X:
Adrenomedullin and its expression in cancers and bone. A literature
review. Front Biosci (Elite Ed). 2:1073–1080. 2010. View Article : Google Scholar
|
|
17
|
Kaseta MK, Khaldi L, Gomatos IP,
Tzagarakis GP, Alevizos L, Leandros E, Papagelopoulos PJ and
Soucacos PN: Prognostic value of bax, bcl-2, and p53 staining in
primary osteosarcoma. J Surg Oncol. 97:259–266. 2008. View Article : Google Scholar
|
|
18
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: Roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferrari S, Bertoni F, Zanella L, Setola E,
Bacchini SP, Alberghini M, Versari M and Bacci G: Evaluation of
P-glyco-protein, HER-2/ErbB-2, p53, and Bcl-2 in primary tumor and
metachronous lung metastases in patients with high-grade
osteosarcoma. Cancer. 100:1936–1942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z, Takeuchi S, Ohara N and Maruo T:
Paradoxically abundant expression of Bcl-2 and adrenomedullin in
invasive cervical squamous carcinoma. Int J Clin Oncol. 8:83–89.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oehler MK, Norbury C, Hague S, Rees MC and
Bicknell R: Adrenomedullin inhibits hypoxic cell death by
upregulation of Bcl-2 in endometrial cancer cells: A possible
promotion mechanism for tumour growth. Oncogene. 20:2937–2945.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park SC, Yoon JH, Lee JH, Yu SJ, Myung SJ,
Kim W, Gwak GY, Lee SH, Lee SM, Jang JJ, et al: Hypoxia-inducible
adrenomedullin accelerates hepatocellular carcinoma cell growth.
Cancer Lett. 271:314–322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abasolo I, Montuenga LM and Calvo A:
Adrenomedullin prevents apoptosis in prostate cancer cells. Regul
Pept. 133:115–122. 2006. View Article : Google Scholar
|
|
24
|
Chen X, Yang TT, Wang W, Sun HH, Ma BA, Li
CX, Ma Q, Yu Z and Fan QY: Establishment and characterization of
human osteosarcoma cell lines with different pulmonary metastatic
potentials. Cytotechnology. 61:37–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ramachandran V, Arumugam T, Hwang RF,
Greenson JK, Simeone DM and Logsdon CD: Adrenomedullin is expressed
in pancreatic cancer and stimulates cell proliferation and invasion
in an autocrine manner via the adrenomedullin receptor, ADMR.
Cancer Res. 67:2666–2675. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martínez A, Vos M, Guédez L, Kaur G, Chen
Z, Garayoa M, Pío R, Moody T, Stetler-Stevenson WG, Kleinman HK, et
al: The effects of adrenomedullin overexpression in breast tumor
cells. J Natl Cancer Inst. 94:1226–1237. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen P, Pang X, Zhang Y and He Y: Effect
of inhibition of the adrenomedullin gene on the growth and
chemosensitivity of ovarian cancer cells. Oncol Rep. 27:1461–1466.
2012.PubMed/NCBI
|
|
28
|
Shimizu S, Eguchi Y, Kosaka H, Kamiike W,
Matsuda H and Tsujimoto Y: Prevention of hypoxia-induced cell death
by Bcl-2 and Bcl-xL. Nature. 374:811–813. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu X, Cai ZD, Lou LM and Zhu YB:
Expressions of p53, c-MYC, BCL-2 and apoptotic index in human
osteosarcoma and their correlations with prognosis of patients.
Cancer Epidemiol. 36:212–216. 2012. View Article : Google Scholar
|
|
30
|
Zhao Y, Zhang CL, Zeng BF, Wu XS, Gao TT
and Oda Y: Enhanced chemosensitivity of drug-resistant osteosarcoma
cells by lentivirus-mediated Bcl-2 silencing. Biochem Biophys Res
Commun. 390:642–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ramos JW: The regulation of extracellular
signal-regulated kinase (ERK) in mammalian cells. Int J Biochem
Cell Biol. 40:2707–2719. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Atabakhsh E and Schild-Poulter C: RanBPM
is an inhibitor of ERK signaling. PLoS One. 7:e478032012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deville JL, Salas S, Figarella-Branger D,
Ouafik L and Daniel L: Adrenomedullin as a therapeutic target in
angiogenesis. Expert Opin Ther Targets. 14:1059–1072. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Uzan B, Villemin A, Garel JM and Cressent
M: Adrenomedullin is anti-apoptotic in osteoblasts through CGRP1
receptors and MEK-ERK pathway. J Cell Physiol. 215:122–128. 2008.
View Article : Google Scholar
|
|
35
|
Vacotto M, Coso O and Fiszer de Plazas S:
Programmed cell death and differential JNK, p38 and ERK response in
a prenatal acute hypoxic hypoxia model. Neurochem Int. 52:857–863.
2008. View Article : Google Scholar
|
|
36
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hers I, Vincent EE and Tavaré JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zambon AC, Wilderman A, Ho A and Insel PA:
Increased expression of the pro-apoptotic protein BIM, a mechanism
for cAMP/protein kinase A (PKA)-induced apoptosis of immature T
cells. J Biol Chem. 286:33260–33267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nagaya N, Mori H, Murakami S, Kangawa K
and Kitamura S: Adrenomedullin: Angiogenesis and gene therapy. Am J
Physiol Regul Integr Comp Physiol. 288:R1432–R1437. 2005.
View Article : Google Scholar : PubMed/NCBI
|