Introduction
Protein kinase C (PKC) is a major signaling enzymes
that regulates a variety of cell processes including proliferation,
apoptosis, cell survival and migration (1–3). In
addition, several oncogenes, such as RAS, FOS, MYC and
PKC cooperate during transformation, indicating the
involvement of PKC in tumorigenesis (4–6).
Therefore, PKC has been the subject of extensive studies as a
molecular target for the treatment of various types of cancer.
However, the cofactors involved in the PKC-mediated tumorigenesis
are not clearly defined.
The Aurora kinase family of serine/threonine kinases
has been known to be crucial for cell cycle control (7,8).
Mammalian genomes contain three genes encoding Aurora kinases:
Aurora kinases A, B and C (9).
Dysregulated expression of Aurora kinases is thought to promote
oncogenesis, since an increased expression of Aurora kinases A and
B has been observed in numerous tumor cells (10–12).
Overexpression of Aurora kinase A is sufficient to induce colony
formation in cultured cells and tumors in nude mice, indicating
that Aurora kinase A is important for tumor formation and
progression (13,14). However, few studies have
demonstrated the role and regulation of Aurora kinases in
carcinogenesis.
Matrix metalloproteinases (MMPs) are key regulators
of many physiologic and pathologic cell processes such as wound
healing and cancer metastasis. In cancer invasion, MMPs directly
play a pivotal role in the migration of cancer cells (15). MMPs are now considered to be
promising targets for cancer therapy, and clinical trials have
begun with a large number of synthetic and natural MMP inhibitors
(16). Among MMPs, gelatinases A
(MMP-2) and B (MMP-9) are involved in tumor invasion and metastasis
(17,18). Although MMP-2 is constitutively
expressed in tissues (19), the
induction of MMP-9 was reported to be important for human cancer
cell invasion, particularly breast cancer cells (20–22).
Therefore, the specific inhibition of MMP-9 expression has been
suggested to be a reasonable pharmacological strategy for reducing
the invasive potential of tumor cells (23–27).
MMP-9 can be upregulated by several different growth factors and
inflammatory cytokines (28,29).
Moreover, phorbol esters, the PKC activators, have been identified
as tumor-promoting agents but also strong activators of MMPs
(30), indicating that PKC is
primarily involved in the expression of MMPs in cancer.
In the present study, we first examined whether PKC
regulates Aurora kinases in MCF-7 breast cancer cells.
Subsequently, we investigated whether the inhibition of Aurora
kinases blocks PKC-induced invasion and MMP-9 expression of MCF-7
breast cancer cells. The results showed that Aurora kinase is
activated by PKC and is essential for MMP-9 expression and invasion
of MCF-7 breast cancer cells.
Materials and methods
Cells and reagents
MCF-7 was purchased from ATCC (Manassas, VA, USA).
The cells were cultured in Dulbecco’s modified Eagle’s medium
(DMEM) supplemented with 10% fetal bovine serum (FBS) and 1%
antibiotics at 37°C in a 5% CO2 incubator. Reversine and
Aurora kinase inhibitor were obtained from Calbiochem (St. Louis,
MO, USA). VX-680 was purchased from Selleck (Houston, TX, USA).
12-O-tetradecanoylphorbol-13-acetate (TPA),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
dimethylsulfoxide (DMSO) and anti-β-actin antibodies were obtained
from Sigma (St. Louis, MO, USA). Primary antibodies against Aurora
kinase A and B, phosphorylated (p)-Aurora kinase A, p-Aurora kinase
B, p-IκBα, p-c-Jun, p38, p-p38, JNK, p-JNK, ERK and p-ERK were
purchased from the Cell Signaling Technology (Beverly, MA, USA).
Antibodies against MMP-9, IκBα, p65, PCNA and horseradish
peroxidase (HRP)-conjugated IgG were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). DMEM, FBS and
phosphate-buffered saline (PBS) were obtained from Gibco-BRL
(Gaithersburg, ME, USA). Ninety-six-well plates, 6-well plates, 6
and 10 cm dishes were purchased from SPL Life Sciences (Pocheon,
Gyeonggi, Korea). Matrigel was purchased from BD Biosciences
(Bedford, MA, USA).
Determination of cell viability
A cell viability assay was performed using an MTT
assay. Briefly, the cells were seeded at a density of
3ⅹl04 cells/well and allowed to attach to the plate
surface. After 24 h, the cells were treated with Aurora kinase
inhibitors. After incubation for an additional 24 h, the cells were
washed with PBS, treated with MTT (0.5 mg/ml PBS) and incubated at
37°C for 30 min. Formazan crystals were dissolved with DMSO (100
µl/well) and detected at 570 nm using a microplate reader
(Model 3550; Bio-Rad, Richmond, CA, USA).
Western blot analysis
The cells were lysed with an ice-cold
M-PER® Mammalian Protein Extraction reagent (Pierce
Biotechnology, Rockford, IL, USA). The protein concentration of
each lysate was determined using the Bradford assay (31). Samples (20 µg) were resolved
by electrophoresis on 10% acrylamide gel, and transferred
electrophoretically to Hybond™-PVDF membranes. The membranes were
blocked with 5% bovine serum albumin or 5% skim milk and
subsequently incubated overnight with primary antibodies (1
µg/ml), followed by incubation with secondary antibodies
(HRP-conjugated anti-IgG). ECL reagents (GE Healthcare) were used
as substrates for the detection of peroxidase.
Gelatin zymography assay
The procedure for a gelatin zymography assay was
performed as previously described (32).
Quantitative PCR assay
Total RNA was extracted from cells using a FastPure™
RNA kit (Takara, Shiga, Japan). cDNA was synthesized from 1
µg total RNA using a PrimeScript™ RT reagent kit (Takara).
The mRNA levels were determined by quantitative PCR using the ABI
PRISM 7900 sequence detection system and SYBR-Green (Applied
Biosystems, Foster City, CA, USA). Aurora kinase A and B, and 18S
primers were purchased from SABiosciences (Qiagen Inc., Valencia,
CA, USA). The primers used were: MMP-9 (NM 004994) sense,
CCTGGAGACCTGAGAACCAATCT and antisense, CCA CCCGAGTGTAACCATAGC; and
GAPDH (NM 002046) sense, ATGGAAATCCCATCACCATCTT and antisense,
CGCCCCACTTGATTTTGG. To control for variation in mRNA concentration,
the results were normalized to the housekeeping gene, GAPDH
or 18S. Relative quantification was performed using the comparative
Ct method according to the manufacturer’s instructions.
Electrophoretic mobility shift assay
(EMSA)
Cells were washed twice, scraped into 1.5 ml of
ice-cold PBS (pH 7.5) and pelleted at 3,000 rpm for 3 min. Cytosol
and nuclear extracts were prepared from cells using the NE-PER
nuclear and cytoplasmic extraction reagents (Pierce Biotechnology).
Activation of AP-1 and NF-κB was analyzed by a gel mobility shift
assay using nuclear extracts. Oligonucleotides containing the
κ-chain (κB, 5′-CCGGTTAACAGAGGGGGCTTTCCGAG-3′) and AP-1
(5′-CGCTTGATGAGTCAGCCGGAA-3′) binding site were synthesized and
used as probes for the gel retardation assays. Complementary
strands were annealed and labeled with [α-32P]dCTP.
Labeled oligonucleotides (10,000 cpm), 10 µg of nuclear
extracts and binding buffer [10 mM Tris-HCl, pH 7.6, 500 mM KCl, 10
mM EDTA, 50% glycerol, 100 ng poly(dI•dC), 1 mM DTT] were then
incubated for 30 min at room temperature in a final volume of 20
µl. Reaction mixtures were analyzed by electrophoresis on 4%
polyacrylamide gels in a 0.5X Tris-borate buffer (pH 8.3). The gels
were dried and examined by autoradiography. Specific binding was
demonstrated through competition with a 50-fold excess of cold κB
or AP-1 oligonucleotide.
Invasion assay
The invasion assay was carried out in 24-well
chambers (8-µm pore size) coated with Matrigel diluted in
DMEM (52 µl/cm2 of growth surface) according to
the manufacturer’s instructions (BD Biosciences). Matrigel coating
was re-hydrated in 0.5 ml DMEM for 30 min immediately prior to
experiments. Cells (2×105) were added to the upper
chamber and chemoattractant was added to the bottom well.
Conditioned medium (0.5 ml) was added to the lower compartment of
the invasion chamber. After 24 h incubation, the cells on the upper
side of the chamber were removed using cotton swabs, and migrated
cells were fixed and stained with toluidine blue solution. Invading
cells were counted in five random areas of the membrane with the
assistance of a light microscope.
Migration assay
The migration assay was carried out in 24-well
chambers (8-µm pore size). Cells (2×105) were
added to the upper chamber and chemoattractant was added to the
bottom well. Conditioned medium (0.5 ml) was added to the lower
compartment of the chamber. After a 24-h incubation, the cells on
the upper side of the chamber were removed using cotton swabs, and
migrated cells were fixed and stained with toluidine blue solution.
Invading cells were counted in five random areas of the membrane
with the assistance of a light microscope.
RNA interference
Aurora kinase-specific small interfering RNA (siRNA)
and negative control siRNA were purchased from Bioneer (Daejeon,
Korea). Transfection of MCF-7 cells was performed according to the
manufacturer’s instructions (Amaxa GmbH, Cologne, Germany).
Briefly, cells (3×105) were collected and re-suspended
in 100 µl of MCF-7 nucleofector solution. The cell
suspensions were mixed with 100 pmol siRNA, placed in a sterile
electroporation cuvette, and subjected to program P-020 with the
Nucleofector II (Amaxa GmbH).
Preparation of the lentivirus
miR RNAi Aurora kinase A and B lentiviral vectors
were produced using the BLOCK-iT™ Pol II miR RNAi Expression system
protocol (Invitrogen, Carlsbad, CA, USA). Single-stranded DNA
oligos (Aurora kinase A and B) were created by the BLOCK-iT™ RNAi
Express system (Invitrogen). As a control, LacZ Oligo sequences
were provided by the RNAi expression system kit. Lentivirus was
produced by the ViraPower™ Lentiviral Expression systems protocol
(Invitrogen).
Statistical analysis
Data are presented as means ± SE from three
individual experiments performed in triplicate replicates.
Statistical data analysis was performed using one-way ANOVA program
and Student’s t-test. Differences with p<0.05 were considered to
indicate a statistically significant result.
Results
Activation of PKC increases the
phosphorylation of Aurora kinase A and B in MCF-7 cells
Previous studies have shown that PKC strongly
induces invasion in MCF-7 cells (33,34).
To examine whether PKC activates Aurora kinases or regulates the
expression of these kinases, we determined levels of phosphorylated
Aurora kinases (p-AURKs) and total protein levels of the kinases
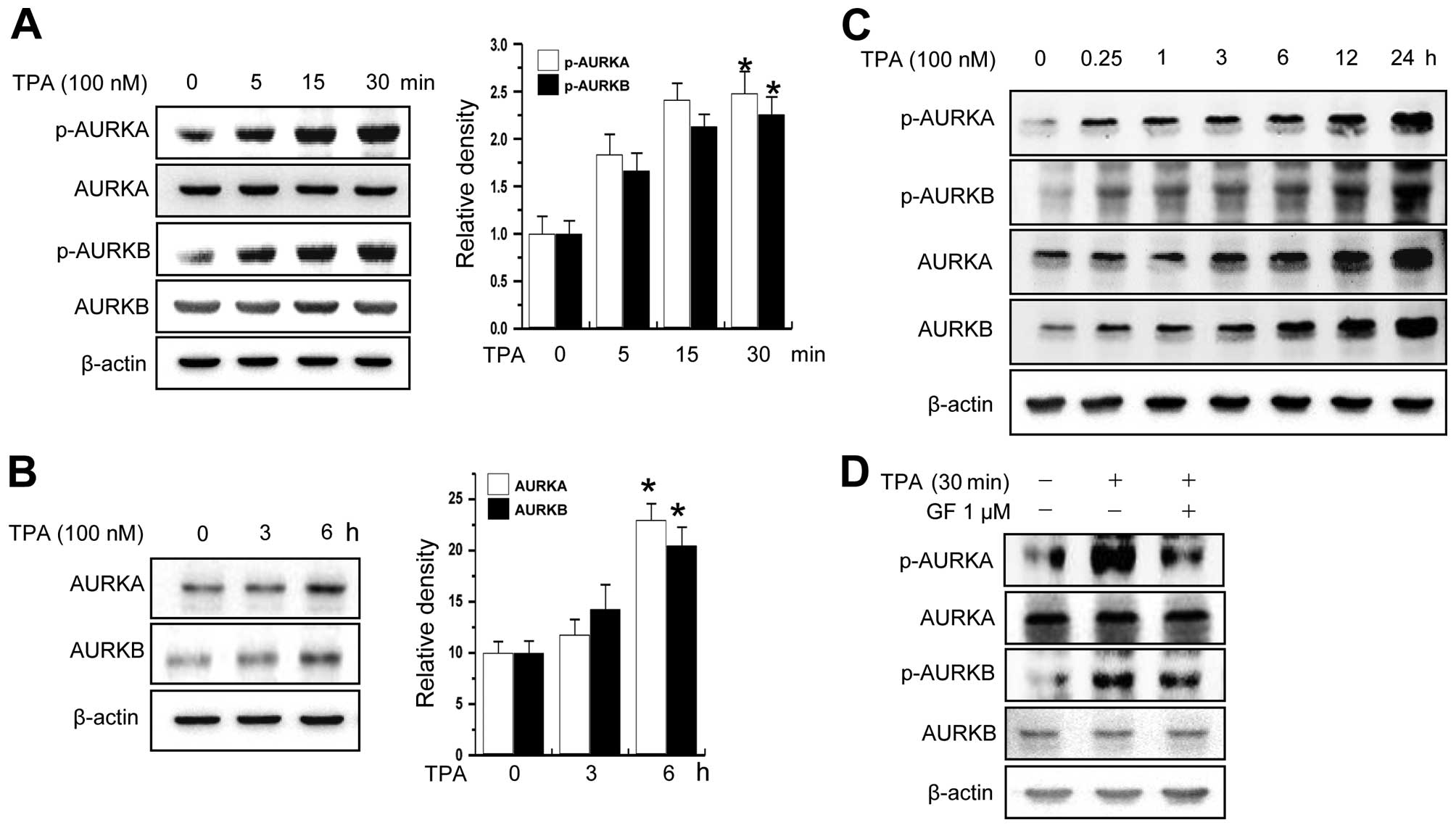
using MCF-7 cells as a model system. As shown in Fig. 1A and B, treatment of cells with TPA,
a PKC activator, increased the levels of total protein and
phosphorylation of AURKA and AURKB in a time-dependent manner. The
phosphorylation of AURKA and AURKB was constantly increased for 24
h (Fig. 1C). Furthermore, we
confirmed that AURKA and B activation is dependent on PKC using PKC
inhibitor (Fig. 1D).
PKC-induced phosphorylation of Aurora
kinase A and B is mediated by MAPK
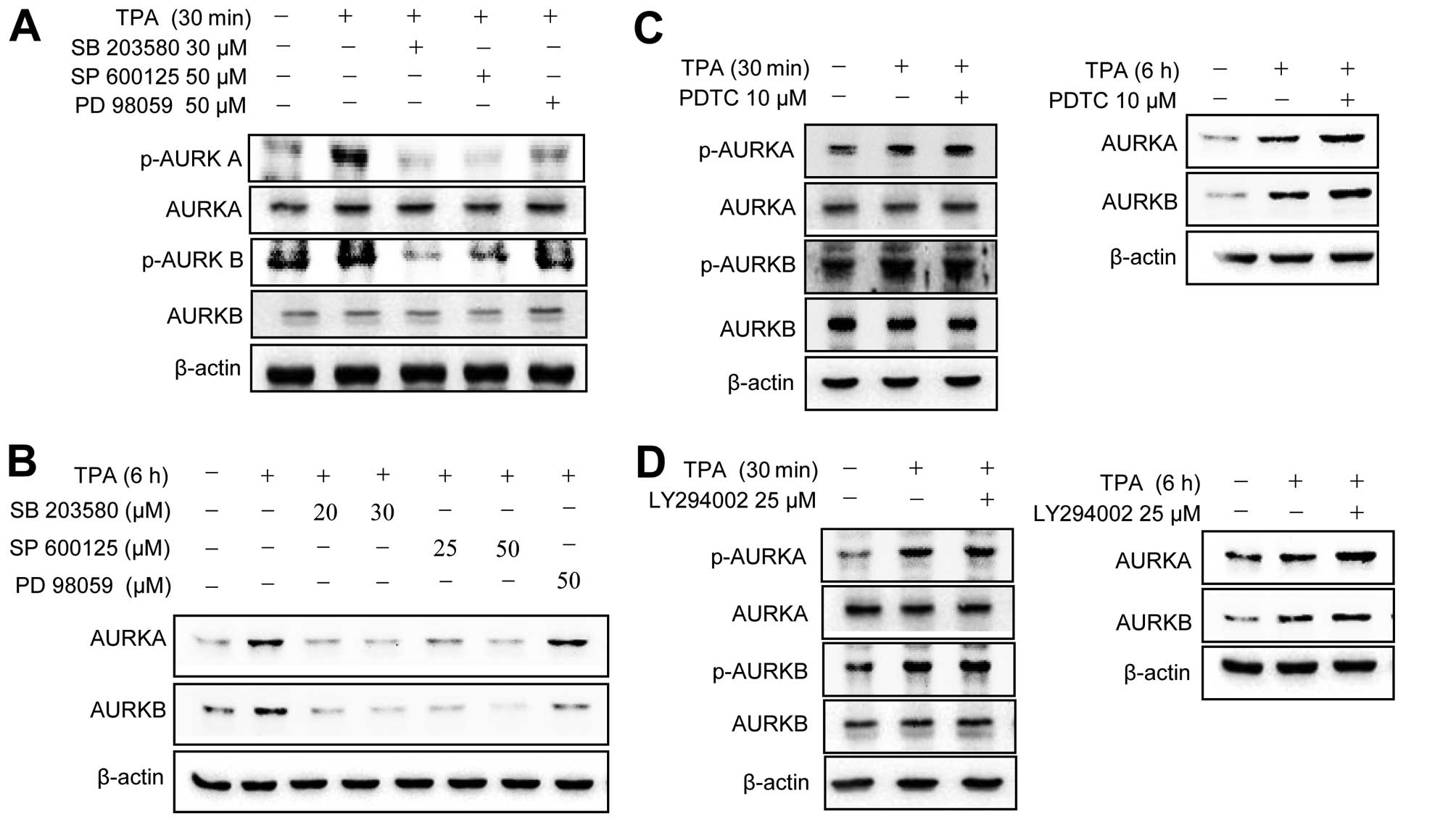
To investigate the molecular basis of the
TPA-induced activation of Aurora kinases, MCF-7 cells were
pretreated with various pharmacological inhibitors of cell
signaling pathways, including MAPK, NF-κB and phosphatidylinositol
3-kinase (PI3K). The TPA-induced phosphorylation (Aurora kinase A,
Th288; Aurora kinase B, Th232) of Aurora kinases A and B was
abrogated by MAPK inhibitors (Fig.
2A). TPA upregulated total protein levels of Aurora kinases
were blocked by MAPK inhibitors (Fig.
2B). By contrast, NF-κB and PI3K inhibitors did not block the
TPA-induced phosphorylation/upregulation of Aurora kinase A and B
expression (Fig. 2C and D). These
results indicated that TPA induces the phosphorylation of Aurora
kinases through a MAPK-mediated pathway in MCF-7 cells.
Aurora kinases are involved in the
PKC-induced invasion of MCF-7 cell
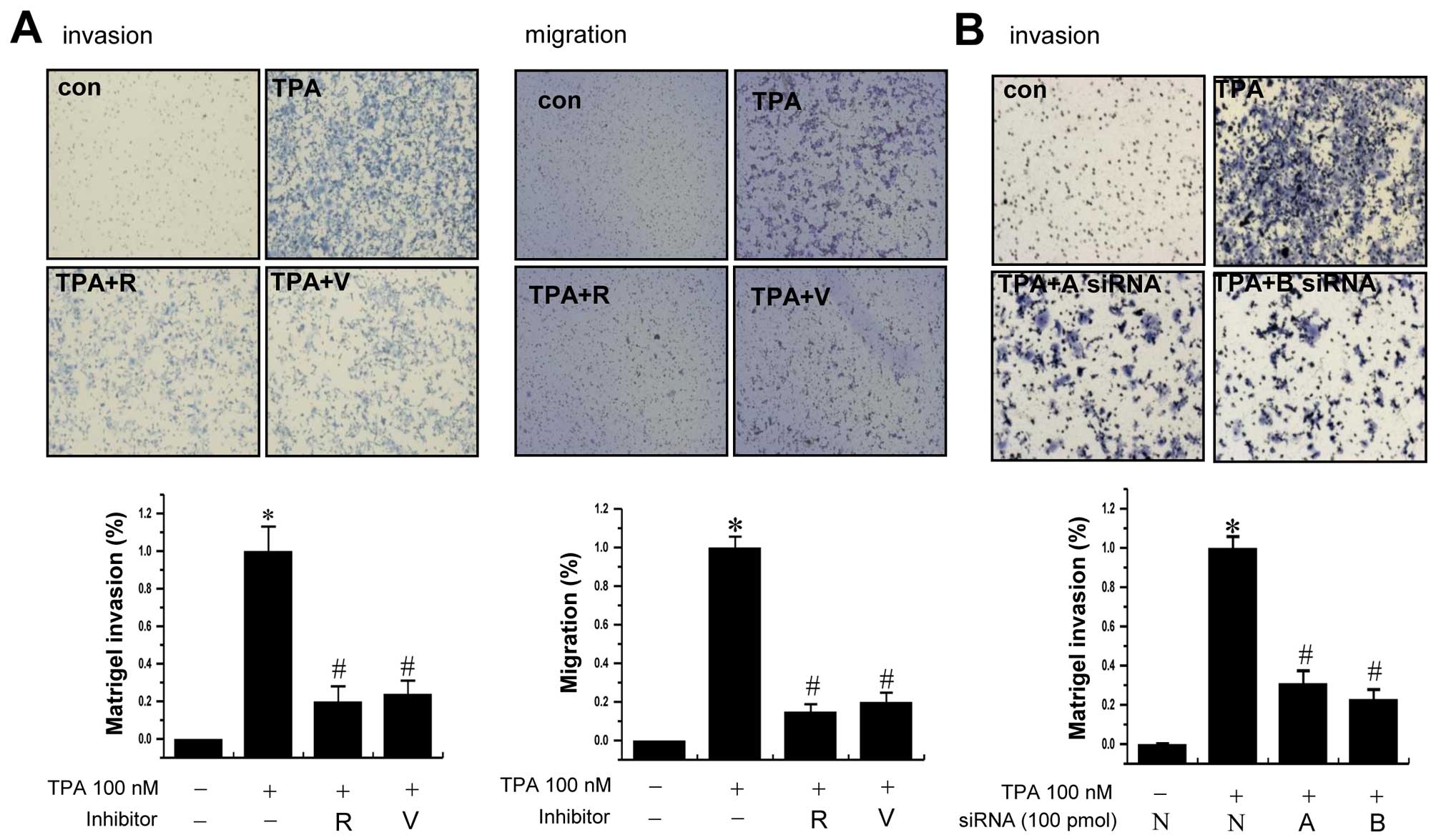
To examine whether AURKs are involved in the
invasion of MCF-7 cells, we used Aurora kinase inhibitors
(reversine and VX-680) and AURK A- and B-specific siRNAs. Treatment
of cells with TPA significantly increased the invasion/migration of
MCF-7 cells compared to that of control cells. The TPA-induced
increase of cell invasion/migration was significantly reduced by
pretreatment with the inhibitors of Aurora kinases (Fig. 3A). Supporting the results,
transfection of cells with Aurora kinase A or B specific siRNA also
reduced the TPA-induced invasion of cells (Fig. 3B).
Activation of Aurora kinases by TPA
upregulates the expression of MMP-9
MMP-9 is a key enzyme for degrading type IV
collagen, which is a major component of the basement membrane,
therefore MMP-9 plays a critical role in cancer cell invasion.
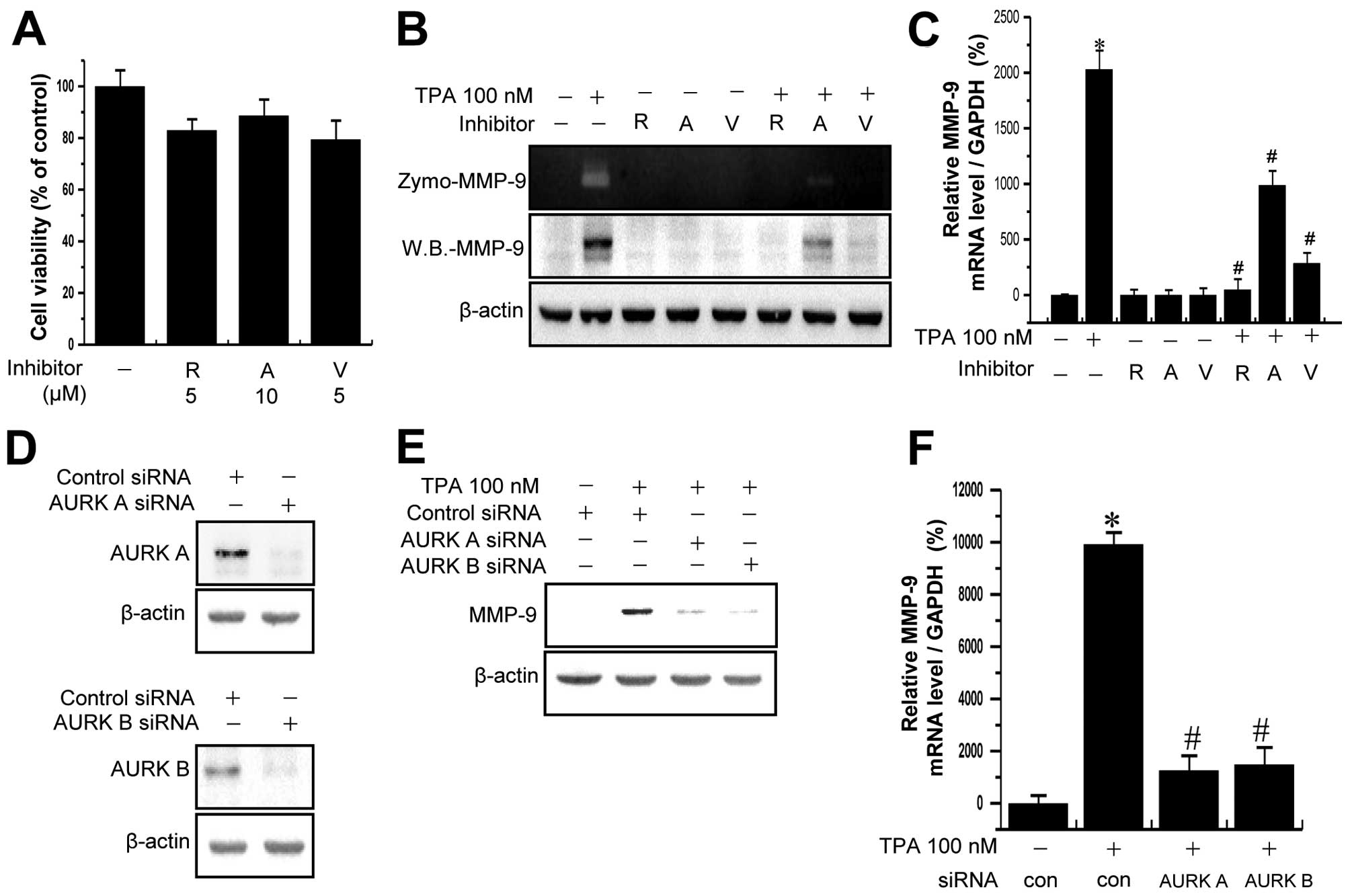
Treatment of MCF-7 cells with the Aurora kinase inhibitors did not
lead to a significant change in cell viability, as measured by the
MTT assay at the indicated concentrations and incubation lengths
(Fig. 4A). To determine whether
Aurora kinases are involved in TPA-induced MMP-9 expression, MCF-7
cells were pretreated with Aurora kinase inhibitors for 1 h and
then additionally treated with TPA for 24 h. As shown in Fig. 4B, treatment of MCF-7 cells with TPA
significantly increased MMP-9 expression and secretion. The
TPA-induced upregulation of MMP-9 expression and secretion in MCF-7
cells was significantly suppressed by pretreatment with Aurora
kinase inhibitors. RT-PCR analysis revealed that TPA increased the
levels of MMP-9 mRNA in MCF-7 cells and that Aurora kinase
inhibitors blocked the TPA-induced upregulation of MMP-9 mRNA
expression (Fig. 4C). To confirm
the result, we performed siRNA-mediated gene silencing of Aurora
kinases in MCF-7 cells. As shown in Fig. 4D, transfection of MCF-7 cells with
Aurora kinase A- and B-targeting siRNA markedly reduced the protein
levels of Aurora kinases. The siRNA-mediated silencing of Aurora
kinase A and B markedly decreased TPA-mediated increases in MMP-9
mRNA expression and protein levels (Fig. 4E and F). To determine the off-target
effect, infected MCF-7 cells by miR RNAi-AURK A and B lentivirus
were treated with TPA. The TPA-induced upregulation of MMP-9
expression in MCF-7 cells was significantly suppressed by miR
RNAi-AURK A and B lentivirus (Fig. 4G
and H). These results indicated that Aurora kinases serve as
one of the underlying mechanisms for PKC-induced MMP-9
expression.
Suppression of Aurora kinases A and B
inhibits the TPA-induced activation of NF-κB and AP-1 transcription
factor
The MMP-9 promoter has been reported to contain
NF-κB and AP-1 binding sites. These transcription factors are
centrally involved in the induction of MMP-9 gene expression by TPA
(27,32). Therefore, we examined whether the
Aurora kinase-mediated upregulation of MMP-9 expression was nvolved
NF-κB or AP-1. MCF-7 cells were pretreated with Aurora kinase
inhibitors for 1 h and then treated with TPA (100 nM) for 3 h, and
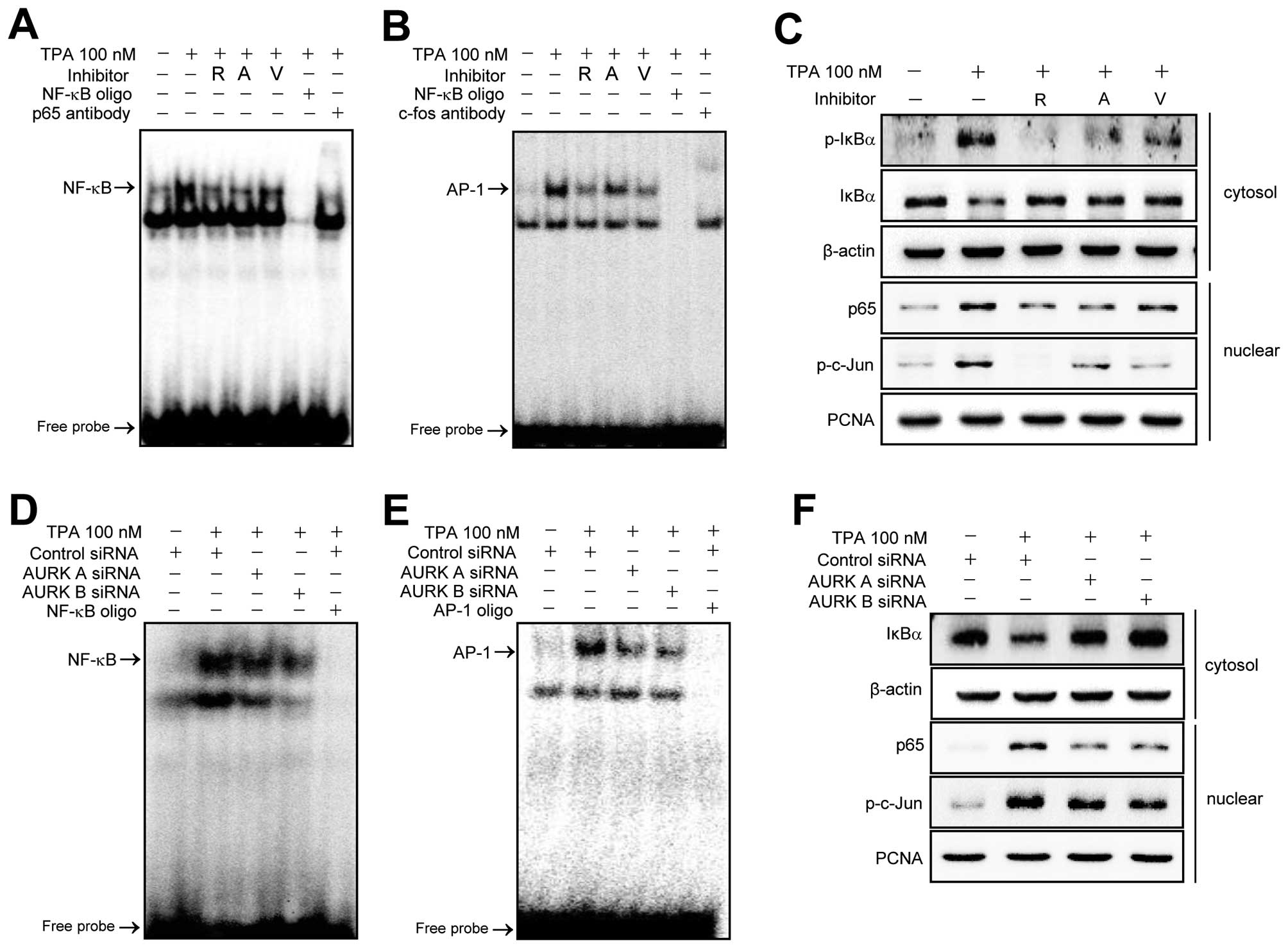
the nuclear extracts were analyzed by EMSA. As shown in Fig. 5A and B, NF-κB and AP-1 DNA binding
activities were markedly increased by TPA. The TPA-induced
increases of DNA binding were significantly blocked by Aurora
kinase inhibitors. Additionally, the nuclear translocation of the
p65 subunit of NF-κB and the phosphorylation status of c-Jun a
subunit of AP-1 were analyzed by western blotting (Fig. 5C). Activation of NF-κB is known to
be regulated by IκBα phosphorylation and degradation (33,35).
Therefore, we examined the effect of Aurora kinase inhibitors on
the TPA-induced phosphorylation and degradation of IκBα by western
blotting. As shown in Fig. 5C
Aurora kinase inhibitors suppressed the TPA-induced phosphorylation
and degradation of IκBα. Moreover, in Aurora kinase A and B
knockdown cells, NF-κB and AP-1 DNA binding activities, the nuclear
translocation of p65 and degradation of IκBα and phosphorylation of
c-Jun by TPA were significantly blocked (Fig. 5D–F, respectively).
Discussion
PKCs have been shown to be a transforming oncogene,
and PKC-mediated oncogenic activity is linked to its ability to
promote cell invasion (2). However,
the mechanisms by which PKC signals cell invasion remain elusive.
The present study has shown that TPA increases the phosphorylation
of Aurora kinase A and B through MAPK, a major PKC downstream
molecule. Our results also showed that AURKs are involved in the
PKC-induced invasion of breast cancer cells, MMP-9 expression, and
activation of NF-κB and AP-1. These findings suggest that Aurora
kinases play central roles in cancer invasion.
Aurora kinase A has been shown to be overexpressed
in a high proportion of invasive breast carcinomas, preinvasive
carcinomas and even proliferative benign breast disease (BBDs)
(36–38), suggesting that Aurora kinase A has
an important role in human mammary tumor metastasis. Aurora kinases
A and B promote inappropriate cellular mobility (39,40), a
characteristic of invasion and metastasis of tumor cells. RNA
interference targeting Aurora kinase A reduces the migration
ability of human hepatic cancer cells, and Aurora kinase A has also
been shown to enhance collagen-induced cell migration (41). However, the PKC-mediated expression
of Aurora kinases has not been studied. Our results showed that PKC
signaling upregulates the expression of Aurora kinases.
PKC regulates various cell functions, including
signal transduction and gene expression (42). Thus, alterations in PKC signaling
leads to malignant transformation and tumor progression.
Overexpression of several PKCs has been reported in malignant
breast tissue and breast cancer cell lines (43). As the major cell receptor for
phorbol esters such as TPA, PKC acts as an important mediator for
the transcriptional regulation of growth factor-responsive MMP
genes (30). Notably, in the
present study, the results reveal that activation of PKC with TPA
increases phosphorylation of Aurora kinase A and B. TPA-induced
phosphorylation (Aurora kinase A, Th288; Aurora kinase B, Th232) of
Aurora kinases A and B were abrogated only by MAPK inhibitors.
Supporting these observations, Oktay et al have shown that
c-Jun N-terminal kinase, JNK functions upstream of Aurora kinase B
(44). These findings suggest that
PKC/MAPK signaling pathways activates Aurora kinases in MCF-7
cells.
Activation of PKCs has been shown to be correlated
with the MMP-9 expression in breast cancer cells (20). MMP-9 is believed to be an important
enzyme in tumor invasion due to its ability to degrade collagen
(15,18). Numerous studies have shown that TPA
stimulates the synthesis and secretion of MMP-9 in MCF-7 cells
(45,46). In the present study, we found that
inhibition of Aurora kinases strongly suppressed TPA-induced MMP-9
expression/secretion. The findings suggest that activation of
Aurora kinases is necessary for the PKC-mediated induction of MMP-9
gene expression.
Nuclear transcriptional factors, AP-1 and NF-κB are
involved in the upregulation of MMP-9 expression (47,48).
AP-1 is a sequence-specific transcriptional factor composed of Jun,
Fos and ATF family proteins, and is induced by multiple stimuli
such as TPA (49). In the present
study, suppression of Aurora kinases inhibited the TPA-mediated
activation of NF-κB and AP-1. Recently, Briassouli et al
have shown that Aurora kinase A regulates NF-κB signaling through
IκBα phosphorylation (50).
Collectively, these observations indicate that Aurora kinases play
central roles in PKC-induced AP-1 and NF-κB activation, and MMP-9
expression.
In conclusion, the present study provides evidence
that Aurora kinases A and B mediate PKC-MAPK signal to AP-1 and
NF-κB with increasing MMP-9 expression and invasion of cancer
cells. Tot the best of our knowledge, this is the first study
showing that PKC regulates the activity of Aurora kinases, and
provides insight into the molecular mechanism. Therefore, we
suggest that Aurora kinases are key molecules in PKC-induced
invasion in breast cancer cells.
Abbreviations:
|
PKC
|
protein kinase c
|
|
TPA
|
12-O-tetradecanoyl-phorbol-13-acetate
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
NF-κB
|
nuclear factor-κB
|
|
AP-1
|
activator protein-1
|
|
MAPKs
|
mitogen activator protein kinases
|
Acknowledgments
The present study was supported by the Korea Breast
Cancer Foundation, and the National Research Foundation of Korea
(NRF) grant funded by the Korea government (MEST) (nos.
2011-0030716 and 2010-0012716).
References
|
1
|
Sumagin R, Robin AZ, Nusrat A and Parkos
CA: Activation of PKCβII by PMA facilitates enhanced epithelial
wound repair through increased cell spreading and migration. PLoS
One. 8:e557752013. View Article : Google Scholar
|
|
2
|
Dumont JA and Bitonti AJ: Modulation of
human melanoma cell metastasis and adhesion may involve integrin
phosphorylation mediated through protein kinase C. Biochem Biophys
Res Commun. 204:264–272. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakagawa Y: Artificial analogs of
naturally occurring tumor promoters as biochemical tools and
therapeutic leads. Biosci Biotechnol Biochem. 76:1262–1274. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Way D, Smith S, Sivendran S, Chie L,
Kanovsky M, Brandt-Rauf PW, Chung DL, Michl J and Pincus MR: A
protein kinase C inhibitor induces phenotypic reversion of
ras-transformed pancreatic cancer cells and cooperatively blocks
tumor cell proliferation with an anti-ras peptide. Cancer Chemother
Pharmacol. 49:429–437. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu WS, Lin JK and Wu FY: Differential
induction of c-fos and c-jun proto-oncogenes and AP-1 activity by
tumor promoter 12-O-tetradecanoyl phorbol 13-acetate in cells at
different stages of tumor promotion in vitro. Oncogene.
7:2287–2294. 1992.PubMed/NCBI
|
|
6
|
Xie Z, Zeng X, Waldman T and Glazer RI:
Transformation of mammary epithelial cells by
3-phosphoinositide-dependent protein kinase-1 activates
beta-catenin and c-Myc, and down-regulates caveolin-1. Cancer Res.
63:5370–5375. 2003.PubMed/NCBI
|
|
7
|
Bischoff JR and Plowman GD: The
Aurora/Ipl1p kinase family: Regulators of chromosome segregation
and cytokinesis. Trends Cell Biol. 9:454–459. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan F, Sun C, Perumal M, Nguyen QD,
Bavetsias V, McDonald E, Martins V, Wilsher NE, Raynaud FI, Valenti
M, et al: Mechanism of action of the Aurora kinase inhibitor
CCT129202 and in vivo quantification of biological activity. Mol
Cancer Ther. 6:3147–3157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nigg EA: Mitotic kinases as regulators of
cell division and its checkpoints. Nat Rev Mol Cell Biol. 2:21–32.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tatsuka M, Katayama H, Ota T, Tanaka T,
Odashima S, Suzuki F and Terada Y: Multinuclearity and increased
ploidy caused by overexpression of the aurora-and Ipl1-like
midbody-associated protein mitotic kinase in human cancer cells.
Cancer Res. 58:4811–4816. 1998.PubMed/NCBI
|
|
11
|
Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW,
Sahin A, Brinkley BR and Sen S: Tumour amplified kinase STK15/BTAK
induces centrosome amplification, aneuploidy and transformation.
Nat Genet. 20:189–193. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Katayama H, Ota T, Jisaki F, Ueda Y,
Tanaka T, Odashima S, Suzuki F, Terada Y and Tatsuka M: Mitotic
kinase expression and colorectal cancer progression. J Natl Cancer
Inst. 91:1160–1162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bischoff JR, Anderson L, Zhu Y, Mossie K,
Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, et
al: A homologue of Drosophila aurora kinase is oncogenic and
amplified in human colorectal cancers. EMBO J. 17:3052–3065. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sen S, Zhou H and White RA: A putative
serine/threonine kinase encoding gene BTAK on chromosome 20q13 is
amplified and overexpressed in human breast cancer cell lines.
Oncogene. 14:2195–2200. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato H and Seiki M: Regulatory mechanism
of 92 kDa type IV collagenase gene expression which is associated
with invasiveness of tumor cells. Oncogene. 8:395–405.
1993.PubMed/NCBI
|
|
16
|
Mannello F, Tonti G and Papa S: Matrix
metalloproteinase inhibitors as anticancer therapeutics. Curr
Cancer Drug Targets. 5:285–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nabeshima K, Inoue T, Shimao Y and
Sameshima T: Matrix metalloproteinases in tumor invasion: Role for
cell migration. Pathol Int. 52:255–264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Poulsom R, Hanby AM, Pignatelli M, Jeffery
RE, Longcroft JM, Rogers L and Stamp GW: Expression of gelatinase A
and TIMP-2 mRNAs in desmoplastic fibroblasts in both mammary
carcinomas and basal cell carcinomas of the skin. J Clin Pathol.
46:429–436. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu JF, Crépin M, Liu JM, Barritault D and
Ledoux D: FGF-2 and TPA induce matrix metalloproteinase-9 secretion
in MCF-7 cells through PKC activation of the Ras/ERK pathway.
Biochem Biophys Res Commun. 293:1174–1182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao J, Xiong S, Klos K, Nguyen N, Grijalva
R, Li P and Yu D: Multiple signaling pathways involved in
activation of matrix metalloproteinase-9 (MMP-9) by heregulin-beta1
in human breast cancer cells. Oncogene. 20:8066–8074. 2001.
View Article : Google Scholar
|
|
22
|
Nagai S, Nakamura M, Yanai K, Wada J,
Akiyoshi T, Nakashima H, Ohuchida K, Sato N, Tanaka M and Katano M:
Gli1 contributes to the invasiveness of pancreatic cancer through
matrix metalloproteinase-9 activation. Cancer Sci. 99:1377–1384.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SO, Jeong YJ, Kim M, Kim CH and Lee
IS: Suppression of PMA-induced tumor cell invasion by capillarisin
via the inhibition of NF-kappaB-dependent MMP-9 expression. Biochem
Biophys Res Commun. 366:1019–1024. 2008. View Article : Google Scholar
|
|
24
|
Kondraganti S, Mohanam S, Chintala SK, Kin
Y, Jasti SL, Nirmala C, Lakka SS, Adachi Y, Kyritsis AP, Ali-Osman
F, et al: Selective suppression of matrix metalloproteinase-9 in
human glioblastoma cells by antisense gene transfer impairs
glioblastoma cell invasion. Cancer Res. 60:6851–6855. 2000.
|
|
25
|
Thangapazham RL, Passi N and Maheshwari
RK: Green tea polyphenol and epigallocatechin gallate induce
apoptosis and inhibit invasion in human breast cancer cells. Cancer
Biol Ther. 6:1938–1943. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi Q, Lu N, Wang XT, Gu HY, Yang Y, Liu W,
Li C, You QD and Guo QL: Anti-invasive effect of gambogic acid in
MDA-MB-231 human breast carcinoma cells. Biochem Cell Biol.
86:386–395. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chung TW, Moon SK, Chang YC, Ko JH, Lee
YC, Cho G, Kim SH, Kim JG and Kim CH: Novel and therapeutic effect
of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma
cells: Complete regression of hepatoma growth and metastasis by
dual mechanism. FASEB J. 18:1670–1681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fischoeder A, Meyborg H, Stibenz D, Fleck
E, Graf K and Stawowy P: Insulin augments matrix
metalloproteinase-9 expression in monocytes. Cardiovasc Res.
73:841–848. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kondapaka SB, Fridman R and Reddy KB:
Epidermal growth factor and amphiregulin up-regulate matrix
metalloproteinase-9 (MMP-9) in human breast cancer cells. Int J
Cancer. 70:722–726. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mackay AR, Ballin M, Pelina MD, Farina AR,
Nason AM, Hartzler JL and Thorgeirsson UP: Effect of phorbol ester
and cytokines on matrix metalloproteinase and tissue inhibitor of
metalloproteinase expression in tumor and normal cell lines.
Invasion Metastasis. 12:168–184. 1992.PubMed/NCBI
|
|
31
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Noh EM, Chung EY, Youn HJ, Jung SH, Hur H,
Lee YR and Kim JS: Cis-guggulsterone inhibits the IKK/NF-κB
pathway, whereas trans-guggulsterone inhibits MAPK/AP-1 in MCF-7
breast cancer cells: Guggulsterone regulates MMP-9 expression in an
isomer-specific manner. Int J Mol Med. 31:393–399. 2013.
|
|
33
|
Karin M: How NF-kappaB is activated: The
role of the IkappaB kinase (IKK) complex. Oncogene. 18:6867–6874.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Krappmann D and Scheidereit C: A pervasive
role of ubiquitin conjugation in activation and termination of
IkappaB kinase pathways. EMBO Rep. 6:321–326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arenzana-Seisdedos F, Turpin P, Rodriguez
M, Thomas D, Hay RT, Virelizier JL and Dargemont C: Nuclear
localization of I kappa B alpha promotes active transport of
NF-kappa B from the nucleus to the cytoplasm. J Cell Sci.
110:369–378. 1997.PubMed/NCBI
|
|
36
|
Tanaka T, Kimura M, Matsunaga K, Fukada D,
Mori H and Okano Y: Centrosomal kinase AIK1 is overexpressed in
invasive ductal carcinoma of the breast. Cancer Res. 59:2041–2044.
1999.PubMed/NCBI
|
|
37
|
Miyoshi Y, Iwao K, Egawa C and Noguchi S:
Association of centrosomal kinase STK15/BTAK mRNA expression with
chromosomal instability in human breast cancers. Int J Cancer.
92:370–373. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Goepfert TM, Adigun YE, Zhong L, Gay J,
Medina D and Brinkley WR: Centrosome amplification and
overexpression of aurora A are early events in rat mammary
carcinogenesis. Cancer Res. 62:4115–4122. 2002.PubMed/NCBI
|
|
39
|
Nguyen HG, Chinnappan D, Urano T and Ravid
K: Mechanism of Aurora-B degradation and its dependency on intact
KEN and A-boxes: Identification of an aneuploidy-promoting
property. Mol Cell Biol. 25:4977–4992. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu JC, Chen TY, Yu CT, Tsai SJ, Hsu JM,
Tang MJ, Chou CK, Lin WJ, Yuan CJ and Huang CY: Identification of
V23RalA-Ser194 as a critical mediator for
Aurora-A-induced cellular motility and transformation by small pool
expression screening. J Biol Chem. 280:9013–9022. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guan Z, Wang XR, Zhu XF, Huang XF, Xu J,
Wang LH, Wan XB, Long ZJ, Liu JN, Feng GK, et al: Aurora-A, a
negative prognostic marker, increases migration and decreases
radiosensitivity in cancer cells. Cancer Res. 67:10436–10444. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dekker LV and Parker PJ: Protein kinase C
- a question of specificity. Trends Biochem Sci. 19:73–77. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Urtreger AJ, Kazanietz MG and Bal de Kier
Joffé ED: Contribution of individual PKC isoforms to breast cancer
progression. IUBMB Life. 64:18–26. 2012. View Article : Google Scholar
|
|
44
|
Oktay K, Buyuk E, Oktem O, Oktay M and
Giancotti FG: The c-Jun N-terminal kinase JNK functions upstream of
Aurora B to promote entry into mitosis. Cell Cycle. 7:533–541.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Woo MS, Jung SH, Kim SY, Hyun JW, Ko KH,
Kim WK and Kim HS: Curcumin suppresses phorbol ester-induced matrix
metalloproteinase-9 expression by inhibiting the PKC to MAPK
signaling pathways in human astroglioma cells. Biochem Biophys Res
Commun. 335:1017–1025. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Noh EM, Youn HJ, Jung SH, Han JH, Jeong
YJ, Chung EY, Jung JY, Kim BS, Lee SH, Lee YR, et al: Cordycepin
inhibits TPA-induced matrix metalloproteinase-9 expression by
suppressing the MAPK/AP-1 pathway in MCF-7 human breast cancer
cells. Int J Mol Med. 25:255–260. 2010.PubMed/NCBI
|
|
47
|
Bond M, Fabunmi RP, Baker AH and Newby AC:
Synergistic upregulation of metalloproteinase-9 by growth factors
and inflammatory cytokines: An absolute requirement for
transcription factor NF-kappa B. FEBS Lett. 435:29–34. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Takahra T, Smart DE, Oakley F and Mann DA:
Induction of myofibroblast MMP-9 transcription in three-dimensional
collagen I gel cultures: Regulation by NF-kappaB, AP-1 and Sp1. Int
J Biochem Cell Biol. 36:353–363. 2004. View Article : Google Scholar
|
|
49
|
Lee SO, Jeong YJ, Im HG, Kim CH, Chang YC
and Lee IS: Silibinin suppresses PMA-induced MMP-9 expression by
blocking the AP-1 activation via MAPK signaling pathways in MCF-7
human breast carcinoma cells. Biochem Biophys Res Commun.
354:165–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Briassouli P, Chan F, Savage K, Reis-Filho
JS and Linardopoulos S: Aurora-A regulation of nuclear
factor-kappaB signaling by phosphorylation of IkappaBalpha. Cancer
Res. 67:1689–1695. 2007. View Article : Google Scholar : PubMed/NCBI
|