Introduction
Epithelial ovarian carcinoma is a significant health
problem in women, accounting for ~160,000 cancer-related
mortalities in 2010 worldwide (1).
Ovarian cancer is especially more common in industrialized nations,
with the exception of Japan (1),
and the risk factors include hormone therapy after menopause, gene
mutations (BRCA1 or BRCA2), tobacco smoking and obesity. The risk
also increases with age but decreases with the number of
pregnancies (2–4). Most ovarian types of cancer are
diagnosed at the advanced stages of disease and lead to a poor
prognosis (5). Despite surgical
resection coupled with systemic chemotherapy and radiotherapy, the
5-year survival rate of ovarian cancer remains ~30%, and most
patients succumb to this disease due to tumor recurrence and
metastasis (6). Thus, our research
efforts on ovarian cancer, as with most other cancer types, should
focus on controlling cancer metastasis, which includes several
essential steps, i.e., tumor cell proliferation, migration,
invasion, adhesion, and formation of metastasis in adjacent or
distant organs or tissues (7,8). At
the gene level, RhoA, a member of the Ras superfamily, acts as a
molecular switch to promote cell mobility and, therefore, to
participate in tumor progression. Specifically, RhoA activation can
lead to a diverse set of biological responses, including cell
motility, proliferation, apoptosis inhibition, cell cycle
progression, invasion, and metastasis of tumor cells (9–12).
Accumulating evidence has shown that RhoA activity is upregulated
in most human carcinogenesis (13,14)
and tumor progression (15,16). Thus, RhoA may be a novel molecular
target for controlling cancer metastasis.
It was previously demonstrated that the negative
regulation of RhoA translation and signaling affected cell
morphogenesis (17), and the
knockdown of RhoA expression using adenovirus-mediated RNA
interference inhibited the proliferation and invasive ability of
lung, gastric and colorectal cancer cells in vitro (18,19).
Thus, in the present study, we utilized a lentivirus-carrying RhoA
short hairpin RNA (shRNA) to knock down RhoA expression in a highly
invasive epithelial ovarian cancer cell line and then assessed the
altered biological behaviors in vitro and in nude mice.
Materials and methods
Cell lines and culture
The human HO8910 epithelial ovarian cancer cell line
and human 293FT embryonic kidney cell line were obtained from the
Zhongshan University Laboratory Animal Center (Guangzhou, China).
HO8910 cells were grown in Roswell Park Memorial Institute
(RPMI)-1640 medium (Invitrogen-Life Technologies, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan,
UT, USA), while 293FT cells were cultured in Dulbecco’s modified
Eagle’s medium supplemented with 4.5 g/l glucose (PAA Laboratories,
Pasching, Austria), 10% heat-inactivated FBS (Hyclone), 100 U/ml
penicillin and 100 µg/ml streptomycin (both from
Invitrogen-Life Technologies) in a humidified incubator containing
5% CO2 at 37°C.
Establishment of stable RhoA-knockdown
(RhoA-KD) tumor cells using a lentivirus-carrying RhoA shRNA
A lentiviral expression vector carrying RhoA shRNA
was obtained from GeneCopoeia (Rockville, MD, USA), and the target
sequence of RhoA cDNA was 5′-GAAGGCAGAGATATGGCAA-3′. A negative
control vector was provided by GeneCopoeia, designated as
Lenti-RhoA-NC. Lenti-RhoA-sh and Lenti-RhoA-NC vectors contained
enhanced green fluorescent protein (eGFP) cDNA. The recombinant and
control lentivirus were then produced via the co-transfection of
293FT cells with the Lenti-Pac™ HIV Expression Packaging kit
(GeneCopoeia), and the virus-containing supernatant was harvested
at 48 and 72 h post infection. To stably establish the
RhoA-knockdown HO8910 subline, cells were grown and infected with
lentivirus at a multiplicity of infection of 50, and the infection
efficiency was directly measured by the detection of eGFP
expression in cells by fluorescence microscopy. The cells were
grown in medium containing 1.5 µg/ml puromycin (Invitrogen)
for 20 days. After reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) and western blot analysis confirmation of
RhoA knockdown, RhoA shRNA-lentivirus-infected cells were named as
RhoA-KD cells, whereas the control Lenti-RhoA-NC-infected cells
were designated as mock cells.
RT-qPCR
Total cell RNA was isolated from ovarian cancer
cells using TRIzol reagent (Invitrogen-Life Technologies) and
reverse transcribed into cDNA using the PrimeScript RT-PCR kit
(Takara Bio Inc., Shiga, Japan), according to the manufacturer’s
instructions. The primers for the human RhoA gene were: forward,
5′-TTCCATCGACAGCCCTGATAGTTTA-3′ and reverse,
5′-CACGTTGGGACAGAAATGCTTG-3′; while GAPDH was used as the internal
control with the primers: forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and
reverse, 5′-TGGTGAAGACGCCAGTGGA-3′. PCR amplification and real-time
fluorescence signaling monitoring was conducted using a Stratagene
fluorescence quantitative PCR instrument according to a previous
study (20).
Protein extraction and western blot
analysis
Cell lysis was performed using RIPA lysis buffer
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), and the
protein concentration was measured using the Bradford method with
the BCA protein assay kit (Pierce Biotechnology, Rockford, IL,
USA). For western blot analysis, equal amounts of protein extracts
(30 µg) were separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and then transferred
onto 0.45-µm polyvinylidene difluoride membranes (Millipore,
Billerica, MA, USA). The membranes were blocked in 5% non-fat dry
milk solution in Tris-based saline Tween-20 (TBST) for 1 h and then
incubated at 4°C overnight with a polyclonal rabbit anti-RhoA
antibody at a dilution of 1:100 (no. sc-179; Santa Cruz
Biotechnology, Inc.) or a rabbit anti-β-actin antibody at a
dilution of 1:1,000 (no. AP0060; Bioworld Technology, Louis Park,
MN, USA) in TBST containing 5% non-fat dry milk, followed by
incubation with a secondary horseradish peroxidase-conjugated
anti-rabbit antibody (no. BS13278; Bioworld Technology) at a
dilution of 1:5,000 in TBST containing 5% non-fat dry milk for 1 h
at room temperature. The target protein bands visualized using an
enhanced chemiluminescence detection system (Pierce Biotechnology)
and quantified using Image J 1.44p software (National Institutes of
Health, Bethesda, MD, USA).
Cell viability assay
The altered cell viability after RhoA expression was
knocked down in ovarian cancer cells was assayed by the cell
viability 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium
bromide (MTT) assay as previously described (21). Stable RhoA-knockdown or control
shRNA-infected cells were seeded in 96-well plates at a density of
5×103 cells/well and grown for up to 5 days. At the end
of each experiment, 1 µl of MTT solution (Sigma-Aldrich, St.
Louis, MO, USA) was added into each well and the plates were
incubated for an additional 4-h at 37°C. Then, 100 µl of
dimethyl sulfoxide (DMSO) was added into each well to dissolve
formazan by agitation for 10 min. The absorbance values (A) of
cells were measured at a wavelength of 492 nm using a microplate
reader. The experiments were performed in 5 wells and were repeated
at least three times. The data were summarized as a percentage of
the control using the formula:
(ODRhoA/ODcontrol) × 100, where
ODRhoA and ODcontrol were the optical densities in
RhoA-knockdown and negative control cultures, respectively.
Cell migration and invasion assay
Tumor cell migration and invasion ability were
assayed using a 24-well Transwell chamber system with an
8-µm pore size polycarbonate membrane (Corning Life
Sciences, Corning, NY, USA), as previously described (22,23).
The difference between the tumor cell migration and invasion
ability experiments was that the polycarbonate membrane was either
pre-coated or not with Matrigel (30 µl, diluted at 1:3 with
cell culture medium; BD Biosciences, San Jose, CA, USA). For the
two assays, the cells were starved for 24 h and collected. Cells
(5×104) in 200 µl of growth medium containing
0.5% bovine serum albumin (BSA; Sigma-Aldrich) were then placed in
the upper chamber. The lower chamber was filled with 600 µl
of growth medium containing 20% FBS, and the cells were cultured at
37°C for 48 h. At the end of the experiments, the cells on the
upper surface of the filter were removed using a cotton swab, while
the cells that migrated or invaded through the filter or
Matrigel-coated filter on the lower surface were fixed with 4%
neutral-buffered formalin and stained in 0.01% crystal violet
solution. The cell numbers were counted in 5 fields (up, down,
middle, left and right; magnification x200) for each chamber, and
the results were presented as the mean value ± standard deviation
(SD). The experiment was performed in triplicate and repeated three
times.
Cell adhesion assay
The cell adhesion ability was assayed using a
96-well plate that was pre-coated with 30 µl of Matrigel
(diluted at 1:3 with growth medium). Particularly, cells were
seeded on the plate and incubated in growth medium containing 0.1%
BSA overnight. The following day, the cells were washed three times
with serum-free medium, resuspended at a concentration of
1×106/ml in serum-free medium, seeded into each well
(100 µl/well), and then incubated at 37°C for 1 h.
Subsequently, the detached cells were washed away with
phosphate-buffered saline (PBS), 10 µl of MTT solution was
added to each well containing attached cells, and the plates were
incubated at 37°C for 4 h. DMSO (100 µl) was added into each
well to dissolve formazan, and the absorbance value was measured at
a wavelength of 492 nm using a microplate reader. The data were
summarized and presented as the mean value ± SD, and the tumor cell
adhesion rate was calculated using the following formula: relative
adhesion rate (%) = (A492 of experimental
group/A492 of the control group) × 100%. Each experiment
was performed in triplicate and repeated three times.
Tumor cell intraperitoneal tumorigenicity
assay in nude mice
Athymic nude female BALBC/c mice (4–6 weeks old,
14.96±0.96 g, animal protocol number: 0113061) were obtained from
the Guangdong Medical Laboratory Animal Center (Guangzhou, China)
[registration no. SCXK(YUE)2008–0002], maintained in specific
pathogen-free, temperature-controlled isolation conditions, and fed
with sterilized food and autoclaved water. Animal breeding, care,
and experimental procedures were approved by the Ethics and Human
Committee of Guangzhou Medical University and carried out strictly
in accordance with the related regulations on the use of
experimental animals.
To evaluate tumor cell peritoneal metastasis
ability, 21 athymic nude female BALBC/c mice were randomly assigned
to three groups (n=7 per group): i) RhoA-KD xenograft, ii) mock
xenograft, and iii) parental xenograft groups. Cells in the
logarithmic growth phase were collected and washed twice with PBS.
Cells (5.0×106/mouse) in 200 µl of serum-free
medium with a survival rate of >95% (assessed using trypan blue
staining) were injected into the peritoneal cavity of each mouse.
The mice were monitored every 2 days and sacrificed 28 days after
tumor cell inoculation. The abdominal circumference and ascetic
volume were measured. The number of tumor lesions and disseminated
tumors were counted, and the tumor nodules were excised for
histopathology, RT-qPCR, western blot analysis, immunohistochemical
staining, and terminal deoxynucleotidyltransferase-mediated dUTP
nick end-labeling (TUNEL) assays. The liver, spleen, lung, and
renal tissues were taken and used for the histopathological
analysis of tumor metastasis.
Histopathology and
immunohistochemistry
Mouse tumor tissues were fixed in 4%
paraformaldehyde for 24 h and embedded in paraffin. Tissue sections
(4 µm) were prepared and stained with hematoxylin and eosin.
The consecutive sections were used for immunohistochemical
staining. Specifically, the sections were dewaxed in toluene,
rehydrated, permeabilized in citrate buffer (pH 6.0), and quenched
by 3% H2O2 for 15 min to inhibit any
endogenous peroxidase activities. The sections were then incubated
overnight at 4°C with a polyclonal rabbit anti-RhoA antibody
(diluted at 1:100). The following day, the sections were washed
with PBS three times and then incubated with a biotinylated goat
anti-rabbit IgG (Dako, Glostrup, Denmark), followed by
peroxidase-conjugated streptavidin. The colorimetric reaction was
performed using diaminobenzidine, and the sections were
counterstained with hematoxylin. Immunostained sections were
evaluated by two independent observers who were blinded to the
tissue identity. The staining was scored as positive vs. Negative
when ≥10% tumor cells were positively stained with the polyclonal
rabbit anti-RhoA antibody.
TUNEL assay
Immunohistochemical detection and quantification of
apoptosis of mouse xenograft tumor tissues were performed using an
In Situ Cell Death Detection kit conjugated with horseradish
peroxidase (Roche Applied Science, Indianapolis, IN, USA),
according to the manufacturer’s instructions. The level of
apoptosis was evaluated by counting the TUNEL-positive cells
(brown-stained). The apoptotic index was determined as the number
of TUNEL-positive cells/total number of cells in five randomly
selected high-power fields (magnification, x400).
Statistical analysis
Data were presented as mean ± SD. Statistical
analysis was performed using SPSS 13.0 statistical software (SPSS,
Inc., Chicago, IL, USA). Differences among different groups were
assessed using one-way analysis of variance, and differences
between two groups were assessed using the Student-Newman-Keuls
(SNK) test. P<0.05 was considered statistically significant.
Results
Stable knockdown of RhoA expression in
ovarian cancer cells using a lentiviruscarrying RhoA shRNA
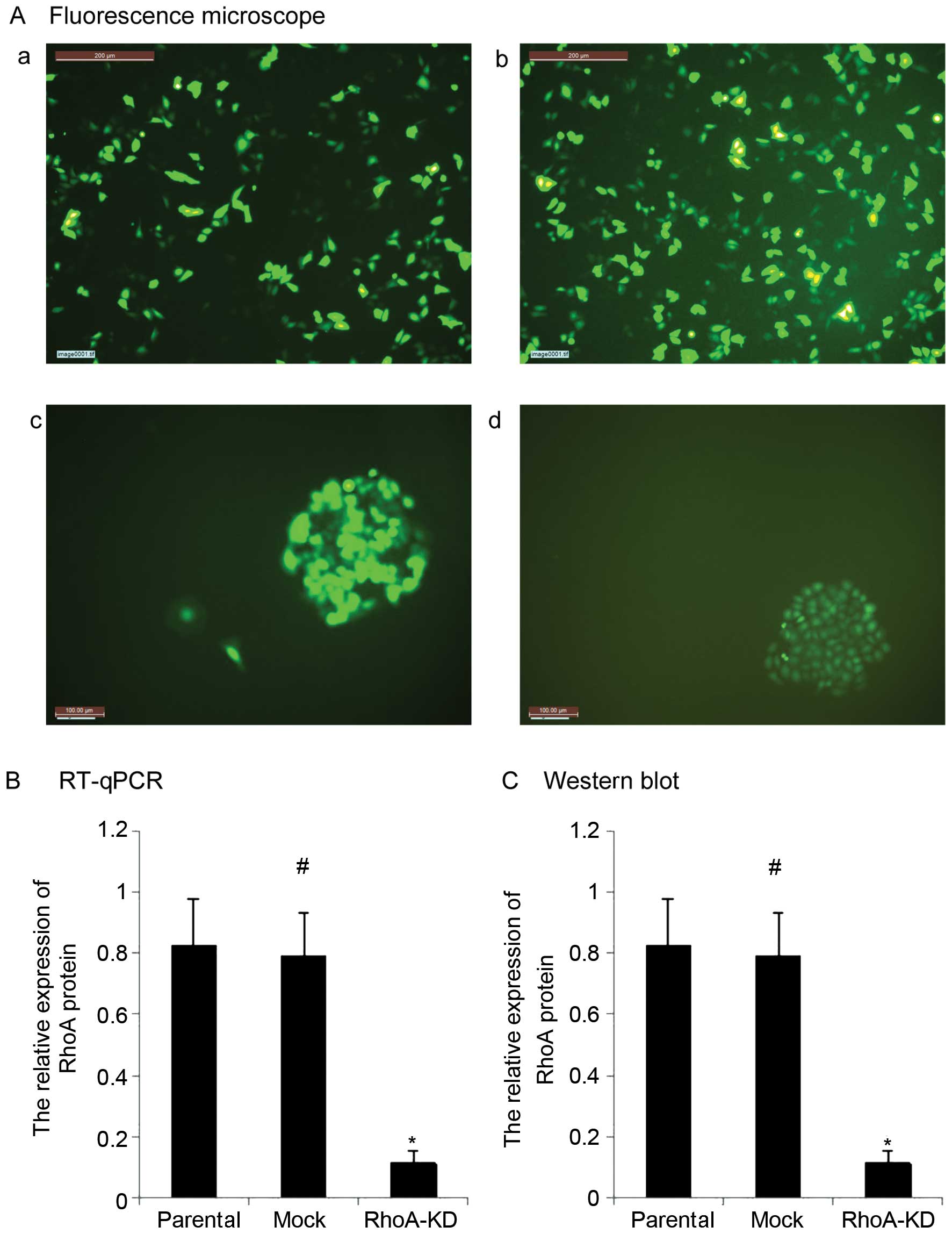
In the present study, we assessed the role of RhoA
knockdown in ovarian cancer cells. HO8910 cells infected with
lentivirus for 48 h showed an infection efficiency of 60% by
fluorescence microscopy (Fig. 1A).
After the cells were cultured in puromycin (1.5
µg/ml)-containing growth medium for 20 days, stable RhoA-KD
and mock ovarian cancer cell populations were successfully
generated (Fig. 1A). Although these
cell populations were cultured and passaged >10 times, the
eGFP-positive expression in the cells remained >98%. The RT-qPCR
and western blot analysis confirmed that the RhoA-KD cells had a
significantly decreased expression of RhoA mRNA and protein
(Fig. 1B and C, P<0.05).
Moreover, there was no difference in the RhoA expression between
parental HO8910 cells and the mock cells (Fig. 1B and C, P>0.05).
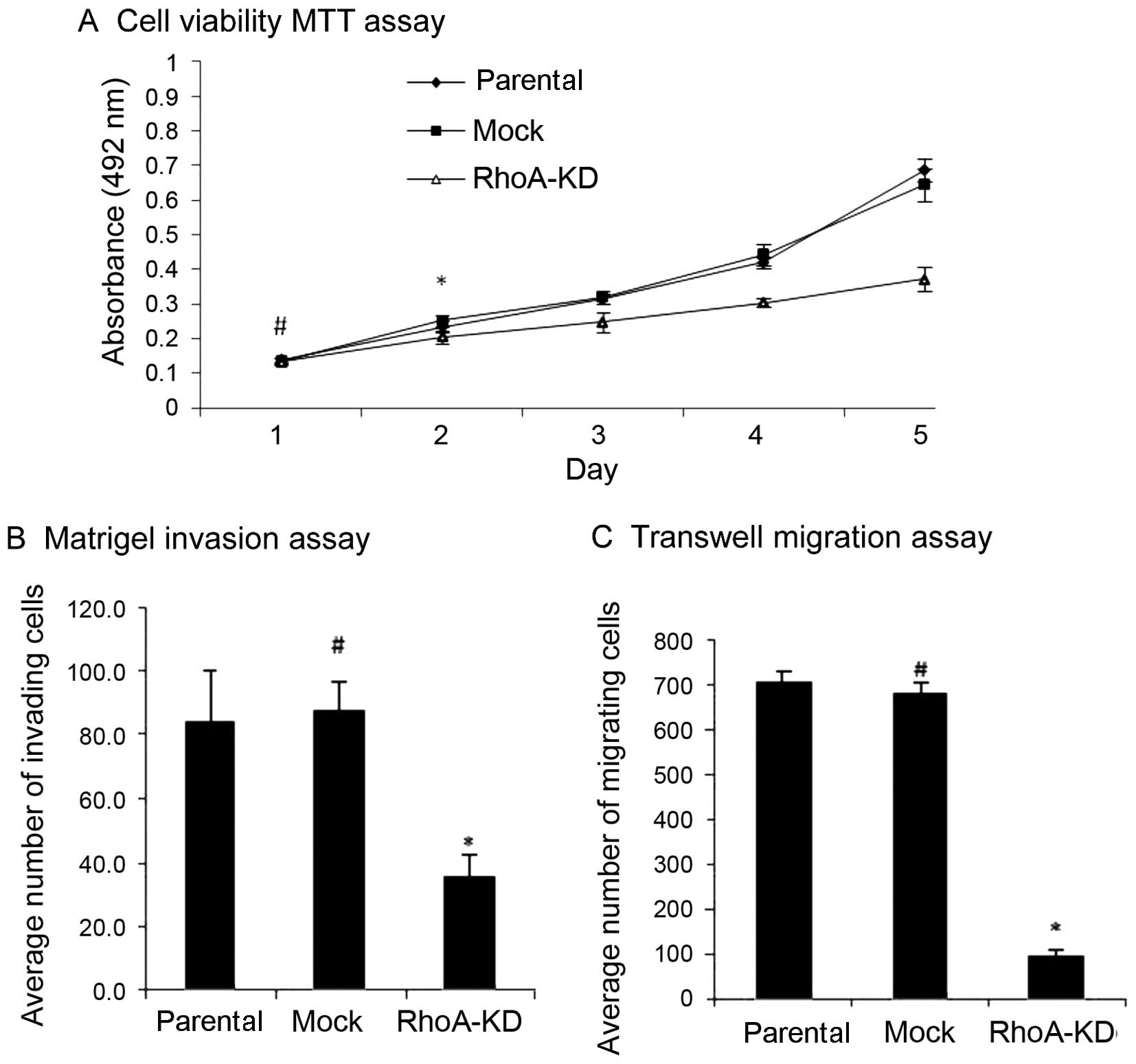
Effects of RhoA knockdown on the
regulation of HO8910 cell biological behaviors in vitro
After the stable RhoA-knockdown ovarian cancer cell
population was obtained, we first assayed the effects of RhoA
silencing on the regulation of tumor cell viability. As shown in
Fig. 2A, although there was not a
statistical difference in the cell viability among the parental,
mock, and RhoA-KD cell cultures on day 1 (P>0.05), the cell
viability of the RhoA-KD cultures was significantly attenuated
between day 2 and 5 (P<0.05). By contrast, there was no
difference in cell viability between the parental HO8910 and mock
tumor cells (P>0.05). Moreover, the tumor cell migration and
invasion assays showed that the knockdown of RhoA expression
reduced the tumor cell migration and invasion abilities compared
with the mock and parental HO8910 cells (P<0.05), whereas the
parental HO8910 and mock cells showed similar migration and
invasion abilities (Fig. 2B and C,
P>0.05).
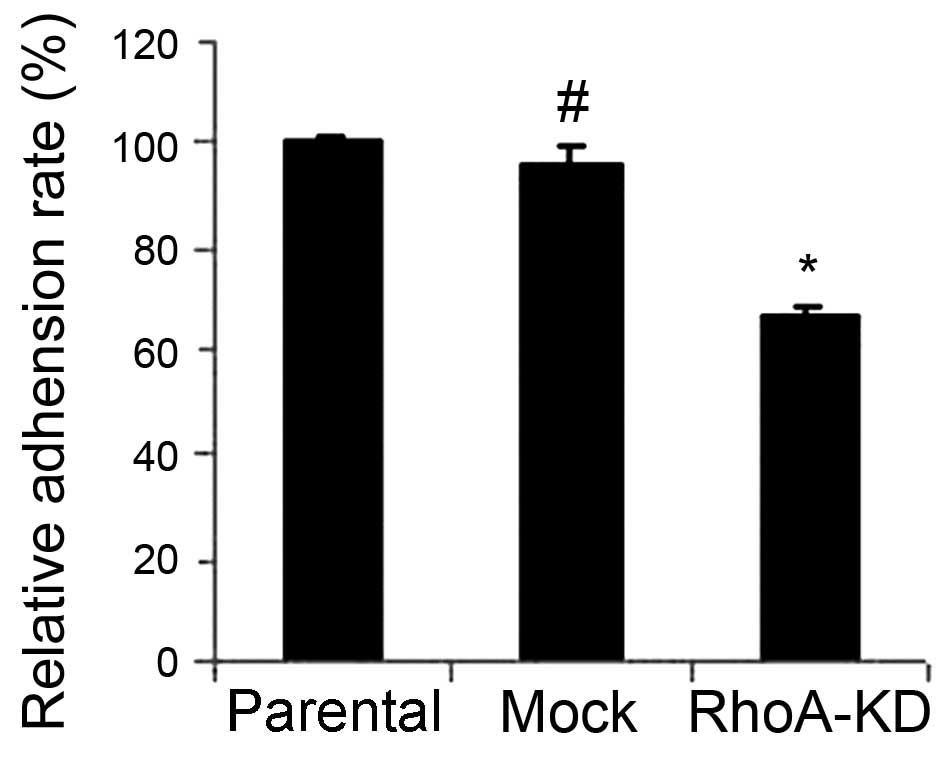
Cancer cell adhesion to the peritoneum is a crucial
process and the initial step during ovarian cancer peritoneal
metastasis. Therefore, we assessed the effects of the knockdown of
RhoA expression on the regulation of tumor cell adhesion. As shown
in Fig. 3, the tumor cell adhesion
was markedly decreased after RhoA knockdown compared to that of the
parental HO8910 and mock cells (P<0.05). However, the mock and
parental HO8910 cells showed similar cell adhesion activities
(P>0.05).
Effects of RhoA knockdown on the
regulation of HO8910 cell biological behaviors in nude mice
We assessed the in vivo effect of RhoA
silencing in nude mice. We found that 10 days after tumor cell
inoculation, the mice with parental or mock cell injections
exhibited gradually decreased activity and appeared sluggish, but
their abdominal circumference was obviously increased. By contrast,
the nude mice with the RhoA-KD cell injection were generally in
good condition and their abdominal circumference was gradually
increased compared to the remaining two groups (Fig. 4A, P<0.05). Moreover, when the
mice were sacrificed at 4 weeks post-inoculation, the tumor
formation rate was 71.4% (5/7) in the RhoA-KD group compared to
100% (7/7) in the remaining two groups. The volume of ascites,
number of tumor lesions, total number of disseminated tumor
nodules, and tumor weight were all signifi-cantly smaller in the
RhoA-KD group than in the remaining two groups (Fig. 4B, P<0.05). However, there was no
statistically significant difference in tumor growth between the
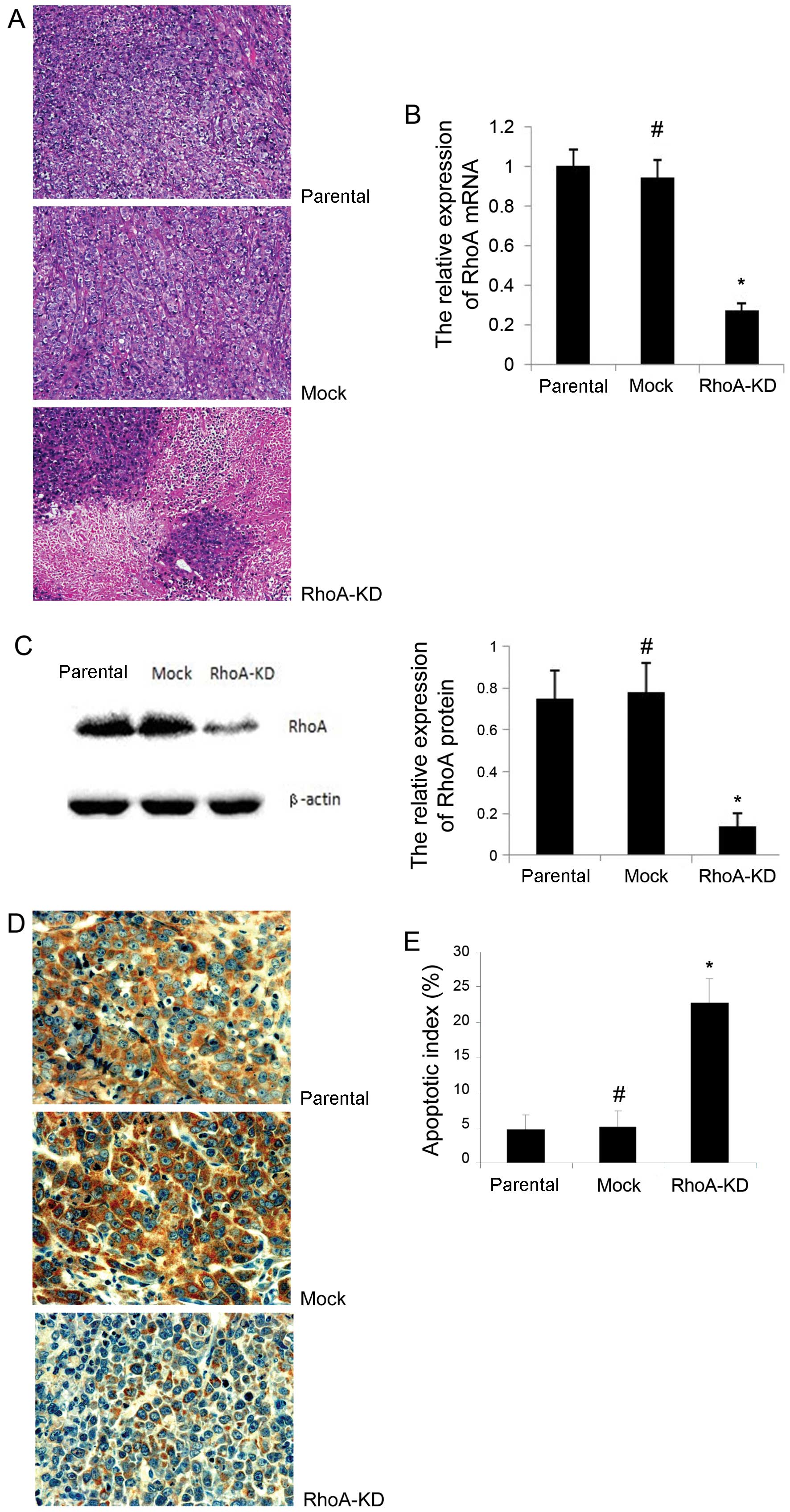
parental and mock groups (P>0.05). In addition, as shown in
Fig. 5A, there were more necrotic
and apoptotic regions found in tumors with RhoA knockdown than in
the parental and mock groups. We performed a TUNEL assay to detect
the apoptotic level in these tumor xenografts and found that the
apoptotic index was significantly higher in the RhoA-KD group than
in the parental and mock groups (Fig.
5E, P<0.05). However, there was no statistically significant
difference observed between the parental and mock groups (Fig. 5E, P>0.05).
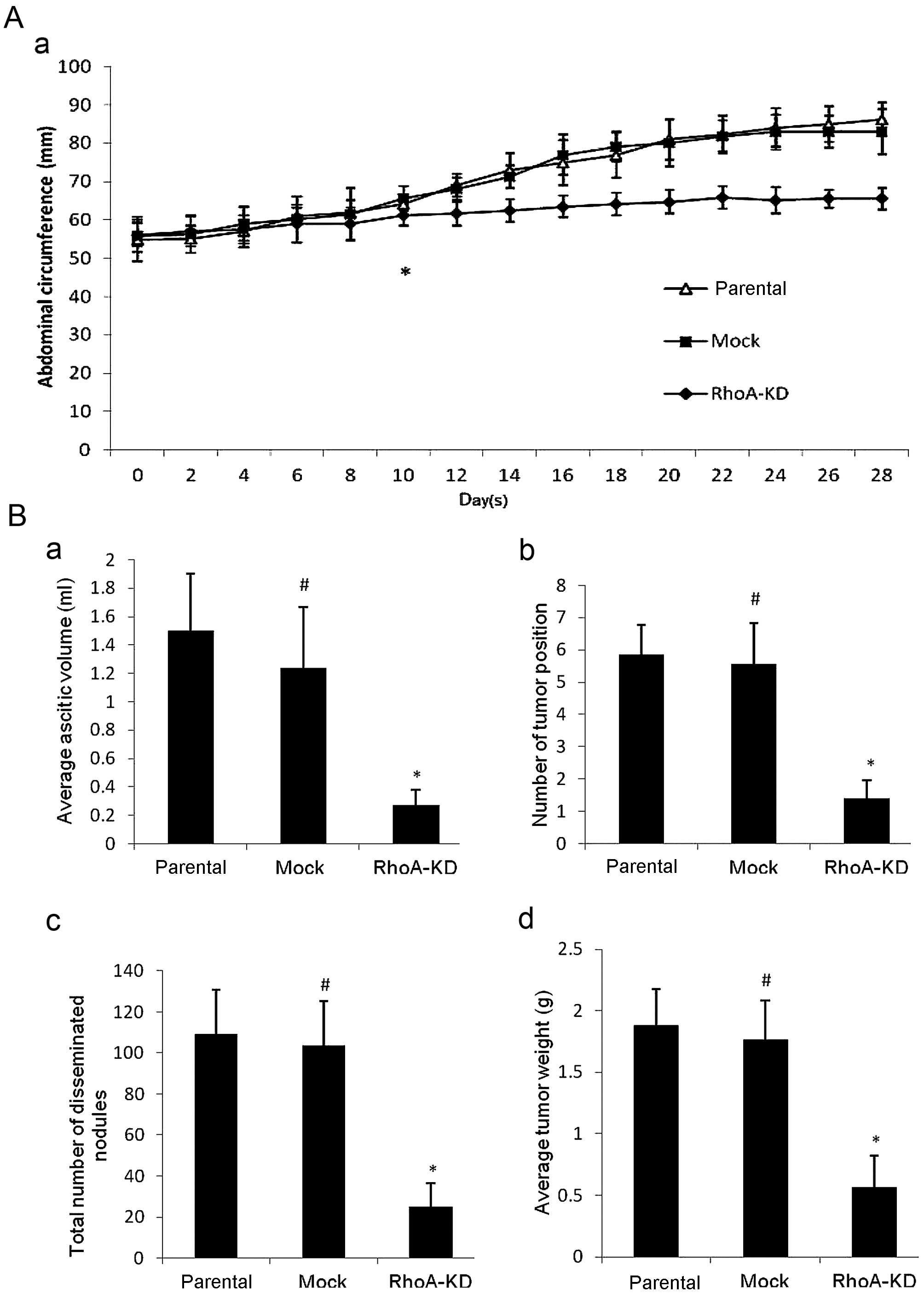 | Figure 4Effects of RhoA knockdown on the
regulation of tumor xenograft formation and growth in a nude mouse
intraperitoneal tumorigenicity model. (A) Measurement of abdominal
circumferences of nude mice. (a) Abdominal circumference growth
curve was plotted against time elapsed (P<0.05, from this point
onwards). The results show that the abdominal circumference from
the RhoA-KD-bearing mice was much smaller than that of the parental
and mock groups (P<0.05), whereas there was no significantly
difference between the mock and parental groups (P>0.05). (B)
Measurement of tumor burdens. Tumor cells were disseminated in the
abdominal cavities of the three groups of nude mice (data not
shown). Numerous tumor nodules were disseminated on the peritoneal
surface, stomach, diaphragm, colic omentum, mesentery, liver,
spleen or kidney of the parental and mock groups, whereas few tumor
nodules were shown on the peritoneal surface and around the radix
of mesentery in the RhoA-KD group. (a) Volume of ascites. (b)
Number of tumor-disseminated position. (c) Total number of tumor
nodules. (d) Tumor weight. These parameters were shown to be
significantly reduced in the RhoA-KD group compared to the parental
and mock groups (P<0.05). Data are presented as means ± SD.
*P<0.05 vs. parental and mock groups;
#P>0.05 vs. parental cells. RhoA-KD,
RhoA-knockdown. |
We confirmed RhoA knockdown in the mouse tumor
xenografts using RT-qPCR, western blot analysis and immunos-taining
of RhoA mRNA and protein, respectively. The results showed that the
RhoA mRNA level was significantly reduced in the RhoA-KD group,
compared to the parental and mock groups (Fig. 5B, P<0.05). Similarly, the RhoA
protein level was significantly reduced in the RhoA-KD group
compared with the parental and mock groups (Fig. 5C and D, P<0.05). However, there
was no significant difference of RhoA expression between the
parental and mock groups (P>0.05).
Discussion
Epithelial ovarian cancer is frequently diagnosed at
the advanced stages of disease. Thus, it is a lethal gynecological
malignancy and only 5–30% of ovarian cancer patients survive for 5
years (6,24). Tumor peritoneal metastasis occurs
most frequently (6,24). In the present study, we targeted
RhoA in ovarian cancer cells to further assess the role of RhoA
knockdown in the regulation of ovarian cancer cell biological
behaviors in vitro and in a nude mouse model. We found that
RhoA knockdown significantly reduced tumor cell viability,
migration, invasion, and adhesion abilities in vitro and
inhibited tumor cell settling in the abdominal cavity and tumor
formation in nude mice. The residual tumor xenografts showed
necrosis and apoptosis after RhoA knockdown. The results of the
present study confirmed RhoA activity in ovarian cancer. Future
studies may target RhoA activity as a novel strategy to treat
ovarian cancer patients.
RhoA is a member of the Ras gene superfamily of
small GTPases. The biological function of RhoA is controlled by the
cycling between the active GTP-bound and inactive GDP-bound states
(25). Early evidence has shown
that RhoA is able to modulate cell adhesion, contraction, mobility
and degradation of the extracellular matrix. However, more recent
studies have clearly demonstrated that RhoA affects cell
proliferation, angiogenesis, gene expression and tumor cell
invasion and metastasis (26,27).
For example, Rho proteins must be present for cells to progress
through the G1 phase of the cell cycle (28). In experimental models of
carcinogenesis, aberrant RhoA activation induced cell growth,
transformation, invasion and metastasis (29–32).
Molecularly, RhoA regulates the activities of multiple
transcription factors, such as ROCK, Cdc42 and Rac1, most of which
are involved in cancer (10). The
upregulation of RhoA expression or activity has been associated
with tumor progression (15),
whereas the downregulation of RhoA expression or activity has been
shown to promote apoptosis of gastric cancer cells and inhibit the
growth and invasion of gastric cancer cells in vitro
(19). Furthermore, factors such as
epidermal growth factor and lysophosphatidic acid, are able to
activate RhoA protein (29,32), further indicating the role of RhoA
in tumorigenesis.
A previous study has shown that RhoA expression was
significantly higher in metastatic omentum than in ovarian cancer,
benign tumors, and normal fallopian tube epithelium and is
associated with poor tumor differentiation and advanced stages of
ovarian cancer (33). In addition,
an in vitro study has demonstrated that inhibition of the
Rho/ROCK pathway enhanced the efficacy of cisplatin in human
ovarian cancer cells (34). By
contrast, Rho expression was higher in patients who did not respond
to chemotherapy (35). In the
present study, the results on RhoA knockdown in ovarian cancer
cells in vitro supported the previous findings (15,19).
Our results using the nude mouse intraperitoneal tumorigenicity
model specifically showed that RhoA knockdown was able to suppress
tumor formation and growth in the abdominal cavity, which is the
site where ovarian cancer frequently spreads. A recent review
indicated that RhoA is a therapeutic target for ovarian cancer
(36), while another study showed
that the over-expression of RhoA enhanced peritoneal dissemination
and that RhoA suppression with lovastatin may be a useful therapy
for ovarian cancer (37).
In the present study, shRNA, made of a tight hairpin
turn of RNA sequences, was used to silence the target gene
expression via RNA interference, which is a well-established
technique to inhibit gene expression, typically by causing the
destruction of specific mRNA molecules with high efficiency and
specificity as well as low toxicity (38). In recent years, lentivirus-mediated
shRNA has been used successfully in vitro and in vivo
and has shown great promise in the field of cancer therapy
(39). Thus, in the present study,
we used this technique to knock down RhoA expression in ovarian
cancer HO8910 cells, a highly invasive human ovarian cancer cell
line with a high level of RhoA expression (17). The results have shown that this
lentivirus may silence RhoA expression with high efficiency.
However, our results are preliminary and a proof-of-principle study
as the side effects of this lentivirus in vivo were not
assessed. In addition, we did not examine any molecular events
after RhoA knockdown. Thus, future studies should focus on the
underlying mechanisms of the antitumor effects in RhoA-knockdown
tumor cells.
Acknowledgments
This study was supported in part by a grant from the
Scientific and Technological program of Guangdong Province, China
(no. 2010B031600185). This manuscript has been edited and proofread
by Medjaden Bioscience Limited.
References
|
1
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: a systematic analysis for the Global
Burden of Disease Study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moyer VA: USA Preventive Services Task
Force: Screening for ovarian cancer: USA Preventive Services Task
Force reaffirmation recommendation statement. Ann Intern Med.
157:900–904. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vo C and Carney ME: Ovarian cancer
hormonal and environmental risk effect. Obstet Gynecol Clin North
Am. 34:687–700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hunn J and Rodriguez GC: Ovarian cancer:
etiology, risk factors, and epidemiology. Clin Obstet Gynecol.
55:3–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gilbert L, Basso O, Sampalis J, Karp I,
Martins C, Feng J, Piedimonte S, Quintal L, Ramanakumar AV and
Takefman J; DOvE Study Group: Assessment of symptomatic women for
early diagnosis of ovarian cancer: results from the prospective
DOvE pilot project. Lancet Oncol. 13:285–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hennessy BT, Coleman RL and Markman M:
Ovarian cancer. Lancet. 374:1371–1382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu J, Shao S, Song Y, Zhao J, Dong Y, Gong
L and Yang P: Hepatocyte growth factor induces invasion and
migration of ovarian cancer cells by decreasing the expression of
E-cadherin, beta-catenin, and caveolin-1. Anat Rec (Hoboken).
293:1134–1139. 2010. View
Article : Google Scholar
|
|
8
|
Fader AN and Rose PG: Role of surgery in
ovarian carcinoma. J Clin Oncol. 25:2873–2883. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gur S, Kadowitz PJ and Hellstrom WJ:
RhoA/Rho-kinase as a therapeutic target for the male urogenital
tract. J Sex Med. 8:675–687. 2011. View Article : Google Scholar
|
|
10
|
Rathinam R, Berrier A and Alahari SK: Role
of Rho GTPases and their regulators in cancer progression. Front
Biosci (Landmark Ed). 16:2561–2571. 2011. View Article : Google Scholar
|
|
11
|
Kwiatkowska A and Symons M: Signaling
determinants of glioma cell invasion. Adv Exp Med Biol.
986:121–141. 2013. View Article : Google Scholar
|
|
12
|
Oh HK, Sin JI, Choi J, Park SH, Lee TS and
Choi YS: Overexpression of CD73 in epithelial ovarian carcinoma is
associated with better prognosis, lower stage, better
differentiation and lower regulatory T cell infiltration. J Gynecol
Oncol. 23:274–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takami Y, Higashi M, Kumagai S, Kuo PC,
Kawana H, Koda K, Miyazaki M and Harigaya K: The activity of RhoA
is correlated with lymph node metastasis in human colorectal
cancer. Dig Dis Sci. 53:467–473. 2008. View Article : Google Scholar
|
|
14
|
Zhao X, Lu L, Pokhriyal N, Ma H, Duan L,
Lin S, Jafari N, Band H and Band V: Overexpression of RhoA induces
preneo-plastic transformation of primary mammary epithelial cells.
Cancer Res. 69:483–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Friesland A, Zhao Y, Chen YH, Wang L, Zhou
H and Lu Q: Small molecule targeting Cdc42-intersectin interaction
disrupts Golgi organization and suppresses cell motility. Proc Natl
Acad Sci USA. 110:1261–1266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Tang LD, Tang WX and Ng SW:
Expression and significance of RhoA protein in epithelial ovarian
cancer cells. J Endocr Surg. 3:147–153. 2008.In Chinese.
|
|
17
|
Xing L, Yao X, Williams KR and Bassell GJ:
Negative regulation of RhoA translation and signaling by hnRNP-Q1
affects cellular morphogenesis. Mol Biol Cell. 23:1500–1509. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimada T, Nishimura Y, Nishiuma T,
Rikitake Y, Hirase T and Yokoyama M: Adenoviral transfer of rho
family proteins to lung cancer cells ameliorates cell proliferation
and motility and increases apoptotic change. Kobe J Med Sci.
53:125–134. 2007.PubMed/NCBI
|
|
19
|
Wang H, Zhao G, Liu X, Sui A, Yang K, Yao
R, Wang Z and Shi Q: Silencing of RhoA and RhoC expression by RNA
interference suppresses human colorectal carcinoma growth in vivo.
J Exp Clin Cancer Res. 29:1232010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li QQ, Skinner J and Bennett JE:
Evaluation of reference genes for real-time quantitative PCR
studies in Candida glabrata following azole treatment. BMC Mol
Biol. 13:222012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ali HM, Urbinati G, Raouane M and
Massaad-Massade L: Significance and applications of nanoparticles
in siRNA delivery for cancer therapy. Expert Rev Clin Pharmacol.
5:403–412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kelly P, Stemmle LN, Madden JF, Fields TA,
Daaka Y and Casey PJ: A role for the G12 family of heterotrimeric G
proteins in prostate cancer invasion. J Biol Chem. 281:26483–26490.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kelly P, Moeller BJ, Juneja J, Booden MA,
Der CJ, Daaka Y, Dewhirst MW, Fields TA and Casey PJ: The G12
family of heterotrimeric G proteins promotes breast cancer invasion
and metastasis. Proc Natl Acad Sci USA. 103:8173–8178. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Basu Roy UK, Henkhaus RS, Loupakis F,
Cremolini C, Gerner EW and Ignatenko NA: Caveolin-1 is a novel
regulator of K-RAS-dependent migration in colon carcinogenesis. Int
J Cancer. 133:43–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nikonova E, Tsyganov MA, Kolch W, Fey D
and Kholodenko BN: Control of the G-protein cascade dynamics by GDP
dissociation inhibitors. Mol Biosyst. 9:2454–2462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Struckhoff AP, Rana MK and Worthylake RA:
RhoA can lead the way in tumor cell invasion and metastasis. Front
Biosci (Landmark Ed). 16:1915–1926. 2011. View Article : Google Scholar
|
|
27
|
Fiordalisi JJ, Keller PJ and Cox AD: PRL
tyrosine phosphatases regulate rho family GTPases to promote
invasion and motility. Cancer Res. 66:3153–3161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Olson MF, Ashworth A and Hall A: An
essential role for Rho, Rac, and dc42 GTPases in cell cycle
progression through G1. Science. 269:1270–1272. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burridge K and Wennerberg K: Rho and Rac
take center stage. Cell. 116:167–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gómez del Pulgar T, Benitah SA, Valerón
PF, Espina C and Lacal JC: Rho GTPase expression in tumourigenesis:
evidence for a significant link. BioEssays. 27:602–613. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qiu RG, Chen J, McCormick F and Symons M:
A role for Rho in Ras transformation. Proc Natl Acad Sci USA.
92:11781–11785. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sahai E and Marshall CJ: RHO-GTPases and
cancer. Nat Rev Cancer. 2:133–142. 2002. View Article : Google Scholar
|
|
33
|
Chen S, Wang J, Gou WF, Xiu YL, Zheng HC,
Zong ZH, Takano Y and Zhao Y: The involvement of RhoA and Wnt-5a in
the tumorigenesis and progression of ovarian epithelial carcinoma.
Int J Mol Sci. s14:24187–24199. 2013. View Article : Google Scholar
|
|
34
|
Ohta T, Takahashi T, Shibuya T, Amita M,
Henmi N, Takahashi K and Kurachi H: Inhibition of the Rho/ROCK
pathway enhances the efficacy of cisplatin through the blockage of
hypoxia-inducible factor-1α in human ovarian cancer cells. Cancer
Biol Ther. 13:25–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Canet B, Pons C, Espinosa I and Prat J:
Ovarian clear cell carcinomas: RHO GTPases may contribute to
explain their singular biologic behavior. Hum Pathol. 42:833–839.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gest C, Mirshahi P, Li H, Pritchard LL,
Joimel U, Blot E, Chidiac J, Poletto B, Vannier JP, Varin R, et al:
Ovarian cancer: Stat3, RhoA and IGF-IR as therapeutic targets.
Cancer Lett. 317:207–217. 2012. View Article : Google Scholar
|
|
37
|
Horiuchi A, Kikuchi N, Osada R, Wang C,
Hayashi A, Nikaido T and Konishi I: Overexpression of RhoA enhances
peritoneal dissemination: RhoA suppression with lovastatin may be
useful for ovarian cancer. Cancer Sci. 99:2532–2539. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boudreau RL and Davidson BL: Generation of
hairpin-based RNAi vectors for biological and therapeutic
application. Methods Enzymol. 507:275–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khatri N, Rathi M, Baradia D, Trehan S and
Misra A: In vivo delivery aspects of miRNA, shRNA and siRNA. Crit
Rev Ther Drug Carrier Syst. 29:487–527. 2012. View Article : Google Scholar : PubMed/NCBI
|