Introduction
Glioblastoma multiforme (GBM) is the most frequently
occurring and the most aggressive malignant type of primary brain
tumor in the central nervous system (CNS). GBM accounts for 15.6%
of all primary brain tumors and 45.2% of primary malignant brain
tumors. More than 10,000 new patients are diagnosed every year in
the United States (1–4). Current standard therapy for GBM
includes surgery of maximal safe resection followed by radiotherapy
in combination with temo-zolomide (TMZ) for newly diagnosed GBM
(2,3). However, the relative survival
estimates for GBM are very poor; less than 5% of patients survive 5
years post diagnosis (4). The
reasons for the reduced survival include the early invasion of GBM
into the central nervous system, making a surgical cure nearly
impossible, and also the resistance to radiotherapy and T M Z
(5).
Radiation induces a variety of DNA damage, including
oxidized base damage, abasic sites, single-stand breaks (SSBs) and
double-stand breaks (DSBs). TMZ as the first-line drug to treat GBM
exerts its effects mainly via the mutagenic product
O6-methylguanine, a cytotoxic DNA lesion
(6). TMZ can disrupt gene
transcription and induce DSBs as well (7). It has been known that the activity of
DNA repair processes, particularly DSB repair after radiotherapy
and chemotherapy can regulate the response of tumor cells to
treatment (8). The DSBs trigger the
DNA damage response (DDR) mediated by ATM and ATR (the ATM and
Rad3-related) protein kinases. ATM kinase, a member of the
phosphatidylinositol 3-kinase (PI3K)-like kinase family, is
activated by DSBs and then activates more than 700 proteins
involved in cell cycle checkpoints, DNA repair and modulation of
chromatin structure. ATM-dependent H2AX phosphorylation is one of
the earliest signs of DNA damage and is necessary for efficient DSB
repair (9).
Except for ATM and ATR, other signaling transduction
pathways such as PI3K-AKT, nuclear factor-κB (NF-κB), ERK and
transforming growth factor-β (TGFβ) pathways can also mediate
radioresistance and chemoresistance by decreasing apoptosis or
increasing DNA damage repair. In addition, ~40% of patients with
GBM have an amplified EGFR gene which results in constitutive
activation of AKT and ERK (10).
AKT and ERK signaling pathways play vital roles in regulating many
fundamental cellular processes and they are associated with
resistance to treatment in GBM cells (11,12).
β-elemene, a Chinese antitumor medicine extracted
from the plant Curcuma wenyujin, has been isolated as a
mono-meric drug and has a broad-spectrum antitumor effect in
various tumor cells, such as lung (13), breast carcinoma (14), leukemia (15), ovarian cancer (16) and GBM (17). β-elemene can reverse the resistance
to other drugs such as cisplatin (13). β-elemene with low toxicity has been
well accepted by patients and is currently being used in clinical
therapy for tumors, cancerous hydrothorax and ascites via
intravenous injection, cavity or peritoneal perfusion in China.
In the present study, we investigated the roles of
β-elemene in the radiosensitivity and chemosensitivity of GBM
cells. We found that β-elemene significantly inhibited the repair
of DNA damage of GBM cells combined with radiation or TMZ via
interfering with the ATM, AKT and ERK signaling pathways.
Materials and methods
Cell lines and cell culture
The glioblastoma cell lines U87-MG, U251, T98G and
LN229 from the human and C6 from the rat were used in the
experiments. The GBM cells were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA). Cells were cultured
in Dulbecco’s modi-fed Eagle’s medium (DMEM; Gibco, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 100
IU/ml penicillin and 100 μg/ml streptomycin, and grown at 37°C in a
humidified atmosphere with 5% CO2.
Radiation with X-rays was performed using X-320ix
(Precision X-Ray, Inc., North Branford, CO, USA) at a dose rate of
3.38 Gy/min. The cells were treated at room temperature (25–26°C).
The doses used were as follows: 2, 4 and 6 Gy for colony forming, 4
Gy for comet assay and 10 Gy for immunoblotting.
Reagents
β-elemene which was obtained from the National
Institutes for Food and Drug Control (NIFDC; Beijing, China) was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis,
MO, USA) at 20 mg/ml as a stock solution. Temozolomide
(Sigma-Aldrich) was dissolved in DMSO at 100 mM as a stock
solution. The stock solutions were diluted immediately before each
experiment. For precise assays, DMSO was added to a control sample
at the same concentration as the treated samples.
Cell proliferation assay
Cell viability was detected by methyl-thiazolyl
tetrazolium (MTT) assay. The cells were cultured in a 96-well plate
at a concentration of 5,000 cells/100 μl/well. After
incubation for 24 h, the cells were treated with the indicated
concentrations of different drugs for 48 h. Then 10 μl of
0.5 mg/ml MTT (Sigma-Aldrich) was added to each well, and the
mixture was incubated at 37°C for 4 h. The culture medium was
replaced with 150 μl DMSO to dissolve the formazan crystals.
The absorbance of each well was determined at 490 nm by a plate
reader (Perkin-Elmer, Waltham, MA, USA). Three replicate wells were
designed for each sample.
Drug synergy was determined by combination index
(CI) from the MTT assay data. The ratio CI was calculated as
follows: CI = survival (combination)/survival (ELE) × survival
(TMZ). If CI was <0.8, the combination was considered
synergistic, if it was between 0.8 and 1.2, the combination was
considered additive, and if CI was >1.2 the combination was
considered antagonistic (18).
Colony forming assay
The effects of elemene combined with radiation or
TMZ on GBM cell survival were assessed by colony forming assay. For
radiation, the cells were incubated with or without elemene (40
μg/ml) for 24 h, and then exposed to X-rays at doses of 0, 2, 4 and
6 Gy. After irradiation, the cells were trypsinized and seeded in a
6-well plate at the indicated numbers with fresh medium. For TMZ
treatment, the cells were seeded in a 6-well plate at 500
cells/well for 24 h, and then treated with the indicated
concentrations of drugs for 48 h; the medium was replaced with
fresh medium and cultured for 14-20 days. After 14-20 days,
colonies of >50 cells were scored as survivors. Three replicate
dishes were used for each treatment.
Comet assay
After the indicated treatment, GBM cells were
trypsinized and washed with ice-cold PBS. Next, the cells (1.
5×104/10 μl) were embedded in 120 μl of low-melting
point agarose (0.5% in PBS at 37°C) onto an agarose-coated (1.5% in
PBS) slide. Then the slides were submersed for at least 1 h in
pre-cooled lysis buffer (2.5 M NaCl, 100 mM EDTA, 10 mM Tris-HCl,
pH 7.5) with 1% Triton X-100 and 10% DMSO. The slides were
denatured for 20 min at 4°C in pre-cooled elec-trophoresis buffer
(300 mM NaOH, 1 mM EDTA) to allow unwinding of the DNA and run at
25 v, 300 mA for 20 min at 4°C. The slides were coated with drops
of neutralization buffer (0.4 M Tris-HCl, pH 7.5), and allowed to
sit for 5 min for three times. The dried slides were stained with
ethidium bromide (2 μg/ml) and images were captured using a Leica
microscope. Comet Assay Software Project (CASP) was used to record
the percentage of DNA in the tail for each cell. The assay was
completed three separate times evaluating 50 cells/experiment.
Immunoblotting
Cells harvested after radiation and drug treatments
were lysed on ice in RIPA buffer with PMSF. The buffer included 25
mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% Triton X-100, 1% sodium
deoxycholate and 0.1% SDS. Protein concentrations were determined
using the BCA protein assay kit (Thermo Fisher Scientific, Waltham,
MA, USA). Samples that included the same amounts of protein were
boiled for 5 min in Laemmli’s buffer and separated by 8–12%
SDS-PAGE, followed by electroblotting on polyvinyli-dene fluoride
membranes. The blots were incubated with the following antibodies
under conditions recommended by the manufacturers. The primary
antibodies against p-ERK, ERK, p-AKT, AKT, γH2AX, p-ATM and GAPDH
(Cell Signaling Technology, Beverly, MA, USA) were used.
Horseradish peroxidase-conjugated anti-mouse or anti-rabbit (Cell
Signaling Technology) was used as the secondary antibodies. Singles
were detected using ChemiDoc™ XRS+ Imaging System (Bio-Rad
Laboratories, Hercules, CA, USA).
Results
β-elemene inhibits the proliferation of
GBM cells and enhances the radiosensitivity in different cell
lines
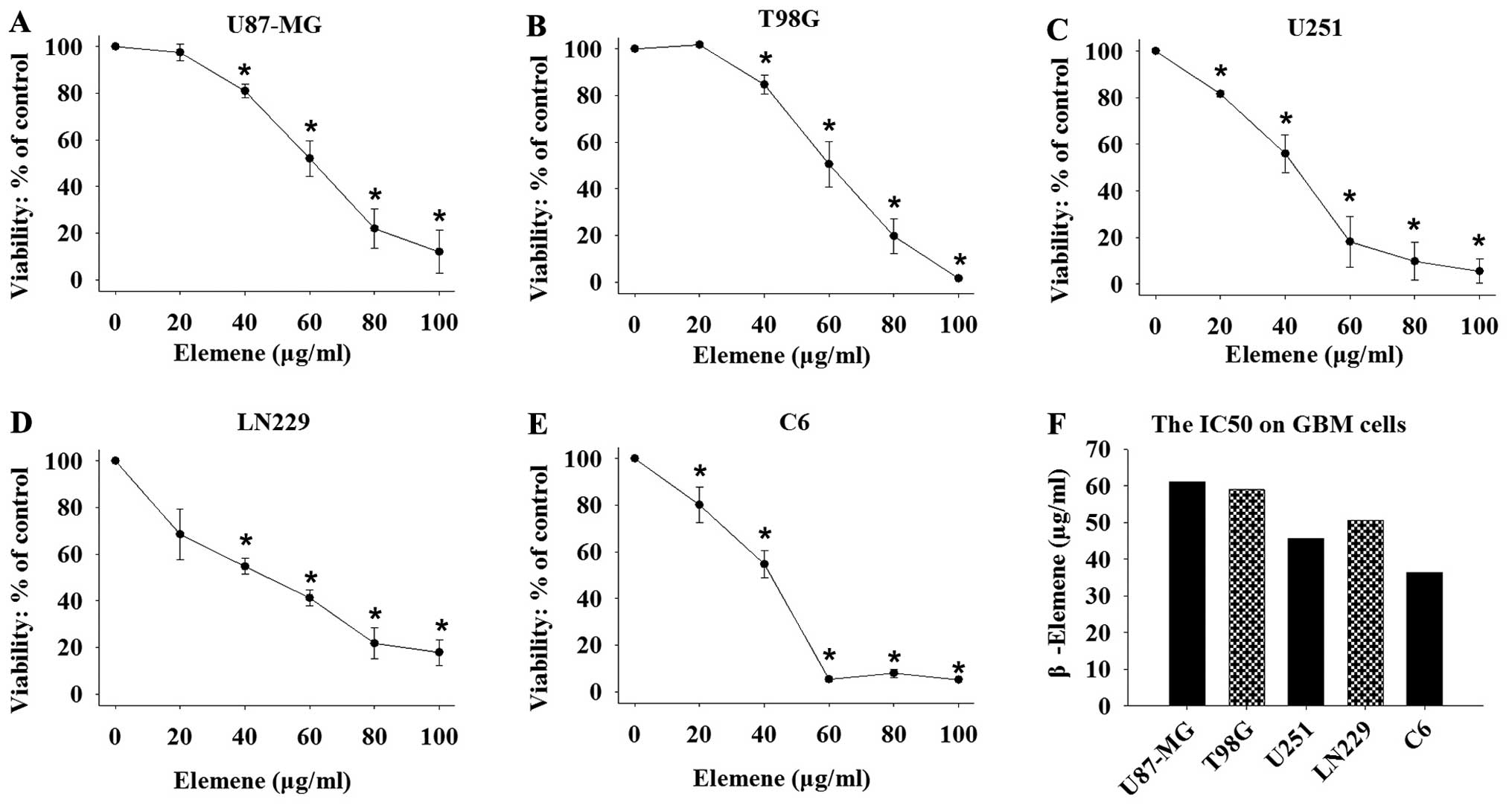
We used MTT assay to evaluate the effects of
β-elemene on the proliferation of GBM cells. The human GBM cell
lines U87-MG, T98G, U251, LN229 and the rat GBM cell line C6 were
used in the examination. Significant inhibition of cell
proliferation was observed in each cell line following treatment
with different concentrations of β-elemene at 0, 20, 40, 60, 80 and
100 μg/ml for 24 h (Fig. 1A–E).
Cell viability was calculated as a percentage of the control, and
the median inhibitory concentration (IC50) was
calculated from the growth inhibition curves fitted to the data.
The results showed that β-elemene caused a dose-dependent
inhibition with a half-maximal inhibitory effect on GBM cell growth
at 61.02 μg/ml for U87-MG, 59 μg/ ml for T98G, 55.66
μg/ml for U251, 55.95 μg/ml for LN229 and 36.4
μg/ml for C6 cells (Fig.
1F).
Then the human GBM cell lines U87-MG, T98G and U251
were used to study the effects of β-elemene on radiotherapy, and
the surviving fractions (SFs) of the colony formation assays are
shown in Fig. 2A–C. The cells were
pretreated with β-elemene at 40 μg/ml for 24 h, exposed to
radiation at 0, 2, 4 and 6 Gy, and then the cells were trypsinized
and seeded in a 6-well plate and cultured for 14–20 days. The
curves of the SFs were fitted by the multi-target single-hit model.
For each cell line, radiosensitivity was significantly increased
after cells were pretreated with β-elemene. Fig. 2D shows the representative images of
the colony formation after treatment with radiation at 4 Gy alone
or in combination with β-elemene.
 | Figure 2β-elemene enhances the
radiosensitivity of GBM cells. Human GBM U87-MG, T98G, U251 cells
were pretreated without or with β-elemene (40 μg/ml) for 24
h, and exposed to different doses of radiation at 0, 2, 4 and 6 Gy.
Then the cells were seeded in a 6-well plate as 250, 250, 500 and
1,000/well corresponding to 0, 2, 4 and 6 Gy (A–C) or 500/well (D).
(A–C) The surviving fraction curves were fitted using the
multi-target single-hit model. (D) The colony formation assay was
carried out as described in Materials and methods. The results are
expressed as the mean ± SEM of at least three independent
experiments. *P<0.05, group treated with β-elemene vs. untreated
group. |
In addition, we analyzed the difference between the
groups treated with and without β-elemene following radiotherapy by
calculating the SFs at 2 Gy (SF2), quasi-threshold dose (Dq) and
doses of radiation for 37% survival (D37) (Table I). The data showed that β-elemene
was more effective in the T98G cells with a DER of 1.38±0.315.
Taking all the factors into consideration, β-elemene significantly
radiosensitized the GBM cells.
 | Table ISurvival fractions at 2 Gy (SF2),
quasi-threshold dose (Dq) and doses for 37% survival (D37). |
Table I
Survival fractions at 2 Gy (SF2),
quasi-threshold dose (Dq) and doses for 37% survival (D37).
| Cell lines | Control | ELE | P-value |
|---|
| SF2
| |
| U87-MG | 0.84±0.009 | 0.66±0.051 | 0.026a |
| T98G | 0.89±0.017 | 0.67±0.010 | <0.01a |
| U251 | 0.83±0.041 | 0.65±0.019 | 0.019a |
| Dq
| |
| Control | ELE | P-value |
| U87-MG | 2.55±0.023 | 1.95±0.164 | 0.022a |
| T98G | 2.94±0.068 | 1.97±0.051 | <0.01a |
| U251 | 2.60±0.226 | 1.67±0.131 | 0.024a |
| D37
| |
| Control | ELE | DER |
| U87-MG | 3.44±0.100 | 2.84±0.221 | 1.21±0.063 |
| T98G | 4.26±0.849 | 3.09±0.104 | 1.38±0.315 |
| U251 | 3.98±0.409 | 3.41±0.192 | 1.17±0.101 |
β-elemene enhances the radiation-induced
DNA damage of GBM cells
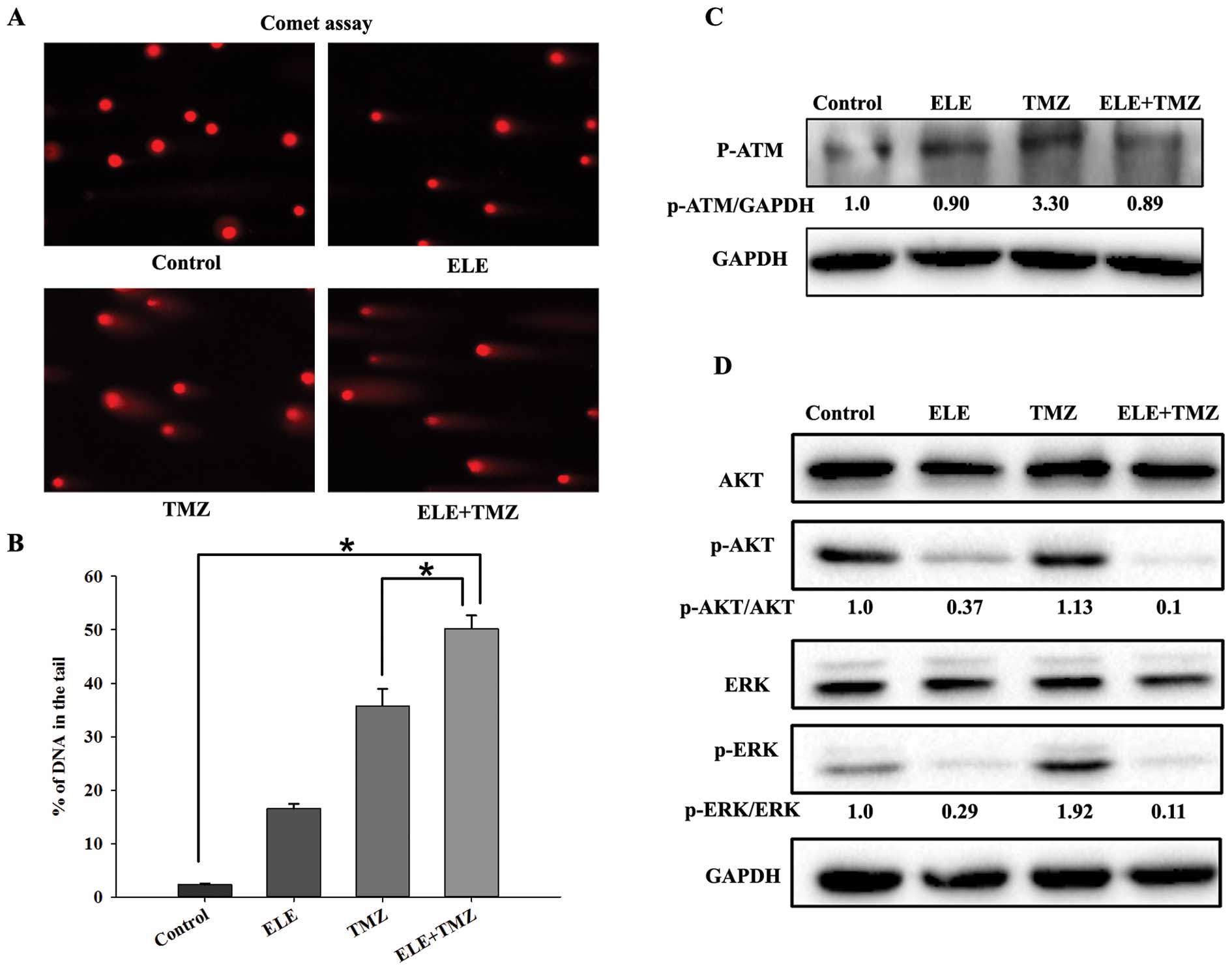
We used comet assay to examine the effects of
β-elemene combined with radiation on the DNA damage in GBM cells.
The tail intensity (percent DNA in the tail) in the comet assay is
a marker for the degree of DNA damage. The human GBM cell lines
U87-MG and T98G were used in the examination. Cells were pretreated
with β-elemene (40 μg/ml) for 24 h, exposed to radiation at 4 Gy
and were collected immediately or after 4 and 24 h to study the
repair of DNA damage. We found that the DNA damage of the GBM cells
was almost repaired at 24 h after radiation, while the cells
pretreated with β-elemene still had obvious DNA damage at the same
time-point (Fig. 3). The results
showed that β-elemene inhibited the repair of the DNA damage caused
by radiation.
β-elemene inhibits the phosphorylation of
ATM, H2AX, AKT and ERK signaling pathways to reduce the repair of
DNA damage after radiation in GBM cells
To understand the mechanism of the radiosensitivity
caused by β-elemene in GBM, human GBM cell lines U87-MG, T98G and
U251 were pretreated with β-elemene at 20 and 40 μg/ml for 24 h,
exposed to radiation at 10 Gy and collected for immunoblotting
after 5 min. Phosphorylated ATM and γH2AX levels are shown in
Fig. 4A–C. Furthermore, we used
U87-MG cells to examine the activation of AKT and ERK following the
indicated treatments. The levels of total and phosphorylated AKT
and ERK were analyzed and are shown in Fig. 4D. The results showed that β-elemene
significantly inhibited the radiation-induced phosphorylation of
ATM, AKT and ERK.
β-elemene increases the inhibitory
effects of TMZ on the proliferation and survival of GBM cells
In order to determine the combinatorial effects of
β-elemene and TMZ in GBM, we used MTT and colony formation assays
to examine the effects of β-elemene plus TMZ in GBM cell lines
U87-MG, T98G, U251 and C6 cells. T98G has been reported to be
resistant to TMZ (11). We examined
the different effects of TMZ at 0, 100, 250, 500 and 1,000
μM on GBM cells for 48 h by MTT assay and selected 500
μM for the following combination experiments. We treated the
cells with β-elemene alone (40 μg/ml), TMZ alone (500
μM) or both for 48 h, and then examined the effects using
the MTT assay (Fig. 5A–D). We found
that β-elemene markedly increased the TMZ-induced inhibition of
cell proliferation in the four cell lines and the CI was
calculated: 0.87 for U87-MG, 0.672 for T98G, 0.857 for U251, 0.699
for C6 cells. For the colony formation assay (Fig. 5E), the cells were seeded in 6-well
plate for 24 h at 500/well, and treated with the indicated
concentrations of β-elemene alone, TMZ alone or the combination for
48 h, and then cultured for 14-20 days. The numbers of the colonies
were fewer and the sizes were smaller in the combination treatment
group when compared to the group treated with TMZ alone or the
control. The data demonstrated that β-elemene strongly enhanced the
inhibition of cell proliferation and survival induced by TMZ in the
human and rat GBM cell lines and it was more effective in the
TMZ-resistant cell line T98G.
β-elemene inhibits TMZ-induced activation
of ATM, AKT and ERK signaling and enhances DNA damage of TMZ
We used the comet assay to examine the DNA damage of
GBM cells treated with β-elemene and TMZ, and assessed the change
in the p-ATM and total/phosphorylated levels of AKT and ERK
proteins by immunoblotting at the same time. The human GBM cell
line T98G was treated with β-elemene alone (40 μg/
ml), TMZ alone (500 μM) or both for 48 h and collected for
comet assay and immunoblotting. The percentage of DNA in the tail
was almost 50% in the combined group and was higher than that in
the group treated with TMZ alone. We also found that TMZ enhanced
the phosphorylation of ATM, AKT and ERK, whereas β-elemene reduced
their phosphorylated counterparts compared to the total protein
levels which were not affected in T98G cells (Fig. 6). Thus, β-elemene decreased the
phosphorylation of ATM, AKT and ERK and inhibited the DNA damage
repair caused by TMZ.
Discussion
The resistance to radiotherapy and chemotherapy of
glioblastoma multiforme is a critical issue associated with its
poor prognosis and reduced patient survival. New treatments to
reverse the resistance are urgently needed. β-elemene, a Chinese
medicine, has demonstrated antitumor effects in many types of
tumors (13–17) with low toxicity, and has been widely
used in China. Some reports have shown that β-elemene has a
sensitization effect on radiotherapy and cisplatin in lung cancer
cells (19,20). We carried out clinical experiments
of β-elemene on patients with GBM. We found that injection of
β-elemene through the internal carotid artery had significant
effects on reducing the size of tumors. The antitumor effect of
β-elemene on GBM patients is definite, and β-elemene has few
side-effects with some local vasculitis (21). It has been reported that β-elemene
increased the ratio of Bax:Bcl-2, and activated caspase-9, -3 and
-7 to induce the apoptosis of GBM cells (22,23).
β-elemene can also activate the p38 MAPK (17) and inactivate RAF/MEK/ERK signaling
pathways to induce cell cycle arrest of GBM cells in the G0/G1
phase (23).
We found that β-elemene inhibited the proliferation
of GMB cell lines and had a sensitization effect on radiotherapy.
Our data showed that β-elemene co-treated with radiation increased
the inhibition of DNA damage repair.
The ataxia telangiectasia mutated (ATM) kinase is
triggered by DSB formation and initiates a series of signaling
events by phosphorylating substrate proteins (8). ATM is a 350-kDa protein that exists as
an inactive dimer and undergoes monomerization following DSB
induction. Phosphorylated ATM regulates cell cycle checkpoints
through p53 phosphorylation at Ser15 and activates checkpoint
kinase 2 (CHK2) through phosphorylation at Thr68 (24,25).
As ATM kinase is a potential therapeutic target for the therapy of
GBM, the ATM inhibitor increases the death of GBM cells combined
with radiation (9,26,27).
Histone H2AX can be phosphorylated by p-ATM to γH2AX and takes
parts in the repair of DNA damage directly, thus, we not only
tested the level of p-ATM but also γH2AX to measure the DNA damage
repair after radiotherapy.
In the present study, we showed that β-elemene
inhibited the phosphorylation of ATM after radiation. The levels of
p-ATM decreased depending on the concentrations of β-elemene. The
downstream protein, γH2AX was also decreased by β-elemene. We also
found that untreated T98G cells had a higher level of p-ATM, and
this may be the reason why T98G is more resistant to radiotherapy.
Thus, β-elemene enhances the sensitivity of GBM cells to radiation
via inhibiting the activation of the ATM signaling pathway to
reduce DNA damage repair.
The constitutive activation of AKT and ERK in GBM
cells increases the ability of survival, proliferation, migration
and invasion (10). The
radiosensitivity of GBM cells can be affected by AKT and ERK
signaling pathways through promoting the activation of the
catalytic subunit of DNA-dependent protein kinase (DNA-PKcs)
(28). ERK and AKT signaling
pathways have positive effects on DSB repair in an ATM-dependent
manner (29). It has been
discovered that ATM indirectly induces AKT and ERK phosphorylation,
and inhibiting the ATM kinase with an ATM-specific inhibitor was
found to reduce the phosphorylation of AKT and ERK (30,31).
Meanwhile, the phosphorylated ERK promotes ATM-dependent foci
formation, making ATM and ERK signaling under the control of a
regulatory feedback loop (32). Our
findings showed that the total AKT and ERK did not change
significantly after treatment while the phosphorylation of AKT and
ERK was inhibited by β-elemene with increased concentrations.
As TMZ is the first-line alkylating agent in
clinical therapy and has an antitumor effect by inducing DNA
damage, we examined the effect of β-elemene combined with TMZ and
found that β-elemene had a chemosensitizing effect on TMZ. The
phosphorylation of ATM, AKT and ERK induced by TMZ were reduced by
β-elemene to inhibit the repair of DNA damage. In addition, the
effect on the TMZ-resistant cell line T98G was more pronounced
following the combination of β-elemene and TMZ which indicated that
β-elemene could reverse the resistance of GBM cells to TMZ.
The present study demonstrated that β-elemene not
only inhibited the proliferation but also enhanced the
radiosensitivity and chemosensitivity of GBM cells. We found that
β-elemene inhibited the DNA damage repair induced by both radiation
and TMZ by decreasing the levels of phosphorylated ATM, AKT and
ERK. Thus, β-elemene may be used as a potential novel drug in
combination with the radiotherapy and chemotherapy of GBM.
Acknowledgments
The present study was supported by funds from the
National Science Foundation of China (no. 81202964), the Liaoning
Province Natural Science Foundation of China (no. 2013023043). Y.Z.
and Y.Y. received the funding.
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Preusser M, de Ribaupierre S, Wöhrer A,
Erridge SC, Hegi M, Weller M and Stupp R: Current concepts and
management of glioblastoma. Ann Neurol. 70:9–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wilson TA, Karajannis MA and Harter DH:
Glioblastoma multiforme: State of the art and future therapeutics.
Surg Neurol Int. 5:642014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ostrom QT, Gittleman H, Farah P, Ondracek
A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2006–2010. Neuro Oncol.
15(Suppl 2): ii1–ii56. 2013. View Article : Google Scholar :
|
|
5
|
Bai RY, Staedtke V and Riggins GJ:
Molecular targeting of glioblastoma: Drug discovery and therapies.
Trends Mol Med. 17:301–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang G, Li LT, Xin Y, Zhang L, Liu YQ and
Zheng JN: Strategies to improve the killing of tumors using
temozolomide: Targeting the DNA repair protein MGMT. Curr Med Chem.
19:3886–3892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bei R, Marzocchella L and Turriziani M:
The use of temozolomide for the treatment of malignant tumors:
Clinical evidence and molecular mechanisms of action. Recent
Patents Anticancer Drug Discov. 5:172–187. 2010. View Article : Google Scholar
|
|
8
|
Begg AC, Stewart FA and Vens C: Strategies
to improve radiotherapy with targeted drugs. Nat Rev Cancer.
11:239–253. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eich M, Roos WP, Nikolova T and Kaina B:
Contribution of ATM and ATR to the resistance of glioblastoma and
malignant melanoma cells to the methylating anticancer drug
temozolo-mide. Mole Cancer Ther. 12:2529–2540. 2013. View Article : Google Scholar
|
|
10
|
Nathanson DA, Gini B, Mottahedeh J,
Visnyei K, Koga T, Gomez G, Eskin A, Hwang K, Wang J, Masui K, et
al: Targeted therapy resistance mediated by dynamic regulation of
extrachro-mosomal mutant EGFR DNA. Science. 343:72–76. 2014.
View Article : Google Scholar :
|
|
11
|
Vlachostergios PJ, Hatzidaki E, Befani CD,
Liakos P and Papandreou CN: Bortezomib overcomes MGMT-related
resistance of glioblastoma cell lines to temozolomide in a
schedule-dependent manner. Invest New Drugs. 31:1169–1181. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Williams TM, Flecha AR, Keller P, Ram A,
Karnak D, Galbán S, Galbán CJ, Ross BD, Lawrence TS, Rehemtulla A,
et al: Cotargeting MAPK and PI3K signaling with concurrent
radiotherapy as a strategy for the treatment of pancreatic cancer.
Mol Cancer Ther. 11:1193–1202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang G, Li X, Huang F, Zhao J, Ding H,
Cunningham C, Coad JE, Flynn DC, Reed E and Li QQ: Antitumor effect
of beta-elemene in non-small-cell lung cancer cells is mediated via
induction of cell cycle arrest and apoptotic cell death. Cell Mol
Life Sci. 62:881–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li QQ, Wang G, Liang H, Li JM, Huang F,
Agarwal PK, Zhong Y and Reed E: β-Elemene promotes
cisplatin-induced cell death in human bladder cancer and other
carcinomas. Anticancer Res. 33:1421–1428. 2013.PubMed/NCBI
|
|
15
|
Yu Z, Wang R, Xu L, Xie S, Dong J and Jing
Y: β-Elemene piperazine derivatives induce apoptosis in human
leukemia cells through downregulation of c-FLIP and generation of
ROS. PLoS One. 6:e158432011. View Article : Google Scholar
|
|
16
|
Li QQ, Lee RX, Liang H, Wang G, Li JM,
Zhong Y and Reed E: β-elemene enhances susceptibility to cisplatin
in resistant ovarian carcinoma cells via downregulation of ERCC-1
and XIAP and inactivation of JNK. Int J Oncol. 43:721–728.
2013.PubMed/NCBI
|
|
17
|
Yao YQ, Ding X, Jia YC, Huang CX, Wang YZ
and Xu YH: Anti-tumor effect of beta-elemene in glioblastoma cells
depends on p38 MAPK activation. Cancer Lett. 264:127–134. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fischel JL, Formento P and Milano G:
Epidermal growth factor receptor double targeting by a tyrosine
kinase inhibitor (Iressa) and a monoclonal antibody (Cetuximab).
Impact on cell growth and molecular factors. Br J Cancer.
92:1063–1068. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li QQ, Wang G, Huang F, Li JM, Cuff CF and
Reed E: Sensitization of lung cancer cells to cisplatin by
β-elemene is mediated through blockade of cell cycle progression:
Antitumor efficacies of β-elemene and its synthetic analogs. Med
Oncol. 30:4882013. View Article : Google Scholar
|
|
20
|
Zou K, Tong E, Xu Y, Deng X and Zou L:
Down regulation of mammalian target of rapamycin decreases
HIF-1alpha and survivin expression in anoxic lung adenocarcinoma
A549 cell to elemene and/or irradiation. Tumour Biol. 35:9735–9741.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou HY, Xu YH and Luo QZ: The clinical
studies of β-elemene’s anti-tumor effect on gliomas. J Med J
Liaoning. 17:189–191. 2003.
|
|
22
|
Zhang H, Xu F, Xie T, Jin H and Shi L:
β-elemene induces glioma cell apoptosis by downregulating survivin
and its interaction with hepatitis B X-interacting protein. Oncol
Rep. 28:2083–2090. 2012.PubMed/NCBI
|
|
23
|
Zhao YS, Zhu TZ, Chen YW, Yao YQ, Wu CM,
Wei ZQ, Wang W and Xu YH: Β-elemene inhibits Hsp90/Raf-1 molecular
complex inducing apoptosis of glioblastoma cells. J Neurooncol.
107:307–314. 2012. View Article : Google Scholar
|
|
24
|
Barlow C, Brown KD, Deng CX, Tagle DA and
Wynshaw-Boris A: Atm selectively regulates distinct p53-dependent
cell-cycle checkpoint and apoptotic pathways. Nat Genet.
17:453–456. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsuoka S, Rotman G, Ogawa A, Shiloh Y,
Tamai K and Elledge SJ: Ataxia telangiectasia-mutated
phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA.
97:10389–10394. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biddlestone-Thorpe L, Sajjad M, Rosenberg
E, Beckta JM, Valerie NC, Tokarz M, Adams BR, Wagner AF, Khalil A,
Gilfor D, et al: ATM kinase inhibition preferentially sensitizes
p53-mutant glioma to ionizing radiation. Clin Cancer Res.
19:3189–3200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Golding SE, Rosenberg E, Adams BR,
Wignarajah S, Beckta JM, O’Connor MJ and Valerie K: Dynamic
inhibition of ATM kinase provides a strategy for glioblastoma
multiforme radiosensitiza-tion and growth control. Cell Cycle.
11:1167–1173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mukherjee B, McEllin B, Camacho CV,
Tomimatsu N, Sirasanagandala S, Nannepaga S, Hatanpaa KJ, Mickey B,
Madden C, Maher E, et al: EGFRvIII and DNA double-strand break
repair: A molecular mechanism for radioresistance in glioblastoma.
Cancer Res. 69:4252–4259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khalil A, Morgan RN, Adams BR, Golding SE,
Dever SM, Rosenberg E, Povirk LF and Valerie K: ATM-dependent ERK
signaling via AKT in response to DNA double-strand breaks. Cell
Cycle. 10:481–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Viniegra JG, Martínez N, Modirassari P,
Hernández Losa J, Parada Cobo C, Sánchez-Arévalo Lobo VJ, Aceves
Luquero CI, Alvarez-vallina L, Ramón y Cajal S, Rojas JM, et al:
Full activation of PKB/Akt in response to insulin or ionizing
radiation is mediated through ATM. J Biol Chem. 280:4029–4036.
2005. View Article : Google Scholar
|
|
31
|
Golding SE, Rosenberg E, Neill S, Dent P,
Povirk LF and Valerie K: Extracellular signal-related kinase
positively regulates ataxia telangiectasia mutated, homologous
recombination repair, and the DNA damage response. Cancer Res.
67:1046–1053. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Munshi A and Ramesh R: Mitogen-activated
protein kinases and their role in radiation response. Genes Cancer.
4:401–408. 2013. View Article : Google Scholar : PubMed/NCBI
|