Introduction
Chronic myeloid leukemia (CML) is a major
hematopoietic malignancy characterized by expansion of myeloid
cells. In Western countries, CML accounts for 15–20% of all adult
leukemias, and its prevalence in the US is predicted to increase
from 70,000 in 2010 to 112,000 in 2020 to 181,000 cases in 2050
(1,2). The BCR-ABL tyrosine kinase provides
the ideal molecular target for the therapy of CML (3,4). The
use of imatinib or STI571, a tyrosine kinase inhibitor (TKI),
drastically improved the prognosis of patients with CML. However,
some portion of CML cells do not 'addict' to BCR-ABL and show very
low sensitivity to TKIs widely used for CML treatment (5,6). These
studies indicate the importance of developing a new therapeutic
strategy for CML.
Green tea (Camellia sinensis) is widely
consumed worldwide. Recent epidemiological studies have indicated a
possible protective effect of green tea intake against the risk of
hematopoietic malignancy (7–9). For
example, based on a cohort study, in 41,761 Japanese adults aged
40–79 years, the risk of hematologic malignancy was negatively
correlated with green tea consumption (9). The multivariate-adjusted hazard ratio
of hematologic malignancies for five cups or more compared with
<1 cup/day of green tea was 0.58 with a 95% confidence interval
of 0.37–0.89 (9).
In a phase II clinical trial, green tea extract had
an anticancer effect in patients with chronic lymphocytic leukemia
(CLL) (10). Of 42 patients, 29
(69%) fulfilled the criteria for a biological response with a
sustained ≥20% decline in ALC and/or a ≥30% reduction in the sum of
the products of all nodal areas at some point during 6 months of
treatment without severe adverse effects (10). Importantly, a green tea extract,
polyphenon E, has been approved by the US Food and Drug
Administration as the first botanical drug for the treatment of
external genital and perianal warts (11).
Epigallocatechin-O-gallate (EGCG) is the
predominant polyphenol catechin in green tea extract, and plays a
central role in the anticancer effects of green tea polyphenols
(12). Recent studies have shown
that EGCG has anticancer effects in hematopoietic malignancy
(13–16). However, several mechanisms have been
suggested for EGCG-induced cell death (17–19)
including the inhibition of anti-apoptosis protein, B-cell lymphoma
(17), radical oxygen species (ROS)
production (18) and VEGF receptor
inhibition (19).
A previous model of plasma membranes assumed a
homogeneous lipid bilayer randomly studded with membrane proteins
(20). However, it has become clear
that plasma membranes are heterogeneous and that clusters of lipids
in a more ordered state are present within the generally disordered
lipid environment of the membrane (20). These clusters are known as lipid
rafts. Recent studies have shown that changes in membrane structure
were induced in cancer cells treated with anticancer agents,
including cisplatin (21). Notably,
cisplatin increased in lipid raft cluster through acid
sphingomyelinase (ASM) activation and the cluster increase plays a
central role in its anticancer effect. In the present study, we
show the impact of EGCG on lipid raft clustering. We also show that
EGCG-induced ASM activation plays the crucial role in the
anticancer effect of EGCG.
Materials and methods
Materials
Penicillin and streptomycin were purchased from
Meiji Seika Pharma (Tokyo, Japan), fetal calf serum (FCS) was
obtained from Biowest (Nuaillé, France). RPMI-1640 was obtained
from Nissui Pharmaceutical Co. Ltd. (Tokyo, Japan). Catalase, EGCG,
NS2028, superoxide dismutase (SOD), BODIPY-C12-sphingomyelin (SM)
and desipramine were obtained from Sigma-Aldrich. Anti-PKCδ
antibody was provided by Santa Cruz Biotechnology (Santa Cruz, CA,
USA). Anti-phospho-PKCδ antibody at Ser664 antibodies was purchased
from Abcam. Vardenafil was provided by TRC (Toronto, Canada). Bay
41-2272 was obtained from Enzo Life Sciences (Villeurbanne,
France).
Cell cultures and cell-based assay
The KU812 human CML cell line was provided by the
Japanese Cancer Research Resources Bank (Tokyo, Japan) and
maintained in RPMI-1640 supplemented with 10% (v/v) FCS, 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C in 5%
CO2 at 100% humidity. To evaluate the anticancer effect
of EGCG, KU812 cells were seeded in 24-well plates at
5×104 cells/ml and treated with indicated concentrations
of EGCG for 96 h in RPMI-1640 medium supplemented with 1% FCS,
catalase (200 U/ml) and SOD (5 U/ml). A lipid-raft clustering assay
was performed using two different fluorescent probe-tagged cholera
toxin B subunits (Alexa Fluor® 488 and Alexa
Fluor® 594 labeled cholera toxin B subunits) obtained
from Life Technologies (Carlsbad, CA, USA). KU812 cells were
stained with Alexa Fluor® 488 and Alexa
Fluor® 594 labeled cholera toxin B subunits for 1 h on
ice, and cells were treated at 37°C with EGCG for indicated times.
All fluorescence images were captured with a fluorescence
microscope (BZ-8100; Keyence). Measurement of ASM activity was
performed as previously described (22). Briefly, KU812 cells were lysed in
lysis buffer containing 50 mM Tris-HCl (pH 4.5), 150 mM NaCl, 1%
Triton X-100, 1 mM EDTA, 50 mM NaF, 30 mM
Na4P2O7, 1 mM
phenylmethanesulfonyl fluoride, 2 mg/ml aprotinin and 1 mM
pervanadate and incubated for 1 h at 4°C, followed by
centrifugation at 15,000 × g for 15 min. The supernatant was
incubated for 18 h at 37°C with substrate buffer (400 pmol
BODIPY-C12-SM, 1% Triton X-100 and 200 mM sodium acetate in
dH2O). The enzyme reaction was stopped by addition of
chloroform:methanol [2:1 (v/v)].
Western blot analysis
Cells were lysed in lysis buffer, and ~50 µg
of protein was suspended in Laemmli sample buffer (0.1 M Tris-HCl
buffer, pH 6.8; 1% SDS; 0.05% mercaptoethanol; 10% glycerol; and
0.001% bromophenol blue), boiled and electrophoresed on
SDS-polyacrylamide gels. Gels were electroblotted onto Trans-Blot
nitrocellulose membranes (Bio-Rad) and incubated with the indicated
antibodies in Tween-20 PBS (TPBS) containing 2.5% BSA. Blots were
washed with TPBS and incubated in HRP-conjugated anti-rabbit or
anti-mouse antibody.
Statistical analyses
All data are presented as means ± SEM. Significance
of differences between experimental variables was determined by
Tukey's test. Statistical analyses were performed with KyPlot. A
P-value of <0.05 was considered to indicate a statistically
significant result. Isobologram analysis of growth inhibition was
performed with CalcuSyn 2.0 software (Biosoft) as previously
described (23).
Results
EGCG induces the reduction of viable cell
numbers in the human CML cell accompanied with lipid raft
clustering
We evaluated the anticancer effect of EGCG in the
presence of SOD and catalase. Our results showed that EGCG
treatment reduced the viable number of human CML KU812 cells in a
dose-dependent manner. The 50% inhibitory concentration
(IC50) of EGCG was ~18.3 µM (Fig. 1A).
Since the resolution limit of fluorescence
microscopy is ~200 nm, it is impossible to evaluate the size of
lipid raft clusters by conventional method. Förster resonance
energy transfer or fluorescence-detected resonance energy transfer
(FRET), describing the energy transfer between two fluorescent
probe molecules, has been applied as an important tool in
structural biology (23). The
efficiency of this energy transfer is inversely proportional to the
sixth power of the distance between donor and acceptor, making FRET
extremely sensitive to small changes in distance. The distance
between the donor and the acceptor is typically in the range of
1–10 nm when FRET occurs. In the present study, we evaluated the
lipid raft using both Alexa Fluor® 488 and Alexa
Fluor® 594 labeled cholera toxin B subunits, well-known
probes detecting ceramide-rich lipid rafts (21). In this system, when lipid rafts
aggregate and form larger lipid rafts, FRET signaling is increased.
Our results showed that in cells treated with 10 µM of EGCG,
the FRET signal increased in a time-dependent manner (Fig. 1B and C). In contrast, control cells
showed no change (Fig. 1C). These
results show that EGCG induced ceramide-rich lipid raft clustering
in CML cells.
EGCG, but not other EGCG-related
compounds induces ASM activation in human CML cells
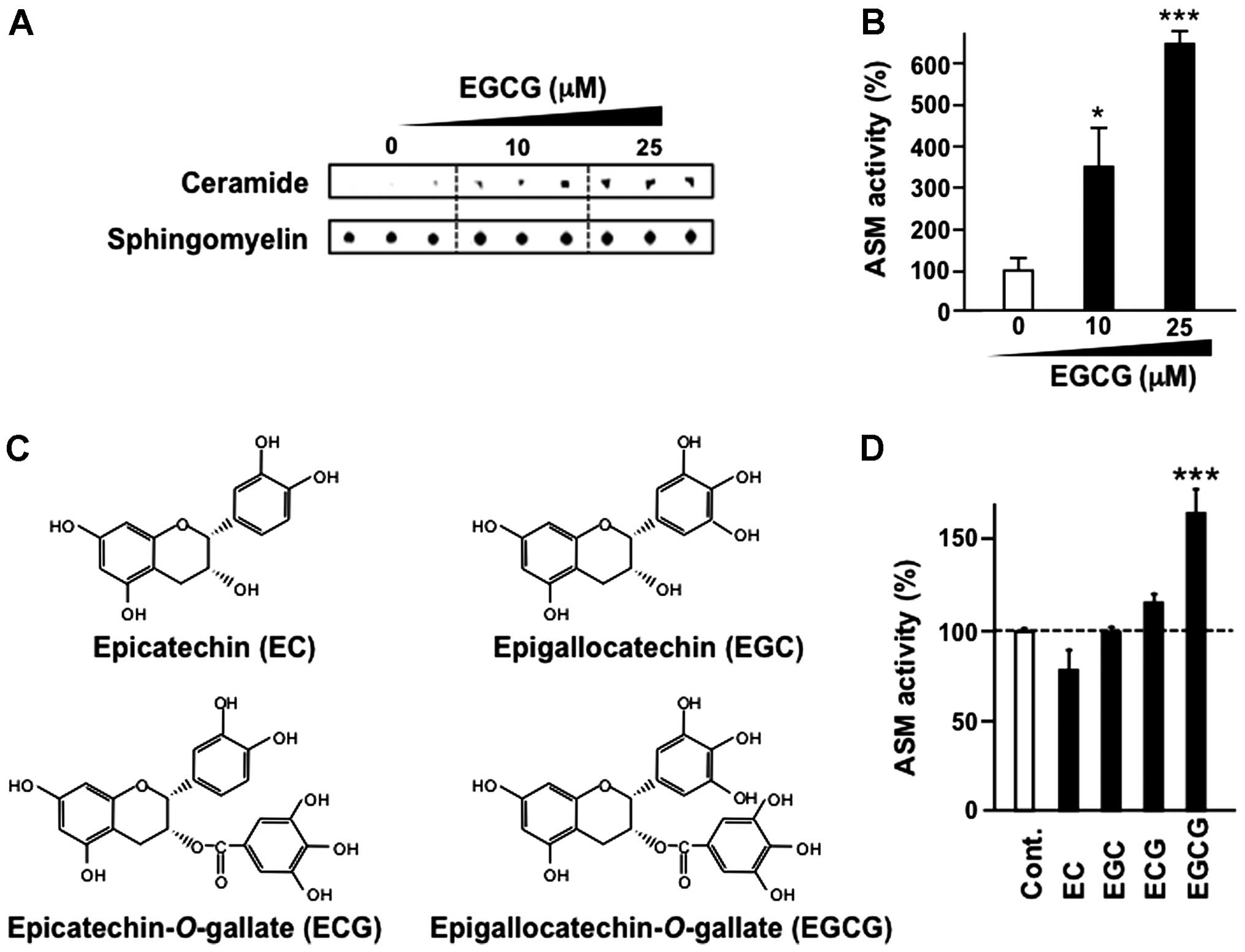
KU812 cells were treated with EGCG for 3 h and ASM
activity was evaluated by thin-layer chromatography. Our results
suggested that EGCG treatment increased ASM activity in a
dose-dependent manner (Fig. 2A and
B).
The major green tea catechins are EGCG, epicatechin
(EC), epigallocatechin (EGC) and epicatechin-3-gallate (ECG) as
shown in Fig. 2C. These major tea
catechins are characterized by dihydroxyl or trihydroxyl
substitutions on the B ring and the m-5,7-dihydroxyl
substitutions on the A ring. The B ring appears to be the principal
site of antioxidant reactions, and the antioxidant activity is
further increased by the trihydroxyl structure on the D ring
(gallate) in EGCG and ECG (24).
Notably, other tea catechins, including EC, EGC and ECG,
structurally related compounds, did not affect ASM activity,
whereas EGCG activated ASM in KU812 cells (Fig. 2D).
ASM plays the crucial role in the
anti-CML effect of EGCG
We found that EGCG induced the reduction of viable
cell number in CML accompanied with lipid raft clustering (Fig. 1A and B). ASM activation is a
well-known mechanism in cisplatin-induced lipid raft clustering
(21,25). We also found that EGCG, yet not
other structurally related compounds, activated ASM (Fig. 2D). To determine the role of ASM in
EGCG-induced viable cell reduction, we evaluated the effect of the
ASM inhibitor desipramine, a tricyclic antidepressant on the
anti-CML effect of EGCG (Fig. 3A).
Our results showed that desipramine significantly reduced anti-CML
effect of EGCG, suggesting that ASM plays the central role in the
anti-CML effect of EGCG.
The 67-kDa laminin receptor (67LR) is the molecular
target of EGCG (26). We previously
reported that EGCG induced cell death in multiple myeloma cells
through activation of the cell surficial protein 67-LR (14). Several studies have shown that 67LR
also mediates the effects of EGCG, including an anti-acute myeloid
leukemia (13), an anticervical
cancer (27) and antimelanoma
effects (28,29).
Cyclic guanosine monophosphate (cGMP) is one of the
secondary messengers that plays the central role in the regulation
of vascular homeostasis and sexual arousal-induced penile erection.
Regulation of cGMP is a well-established strategy for vasodilation
and increased blood flow. Soluble guanylyl cyclase (sGC) is an
enzyme involved in EGCG-induced cGMP upregulation (14). We previously reported the role of
cGMP as the secondary messenger that transmits EGCG-induced
67LR-dependent apoptosis (14).
Indeed, EC, EGC and ECG that have little affinity for 67LR
(26) did not affect the
intracellular cGMP level (14).
Since EC, EGC and ECG, which have low affinity to 67LR, did not
affect ASM activity, whereas EGCG activated ASM in KU812 cells
(Fig. 2D), we hypothesized that
EGCG increased ASM activity via an sGC-dependent mechanism. To
assess the role of sGC in EGCG-induced ASM activation, we
pretreated CML cells with the sGC inhibitor NS2028 before treatment
with EGCG (Fig. 3B and C). NS2028
pretreatment significantly reduced EGCG-induced ASM activation.
These findings suggested that EGCG-induced ASM activation via
sGC-dependent mechanisms.
EGCG activates PKCδ attributed to ASM
activation in CML
Previous studies suggested that PKCδ is one of the
novel PKCs activated by diacylglycerol or 12-otetradecanoylphorbol
13-acetate. PKCδ is well known as a kinase that acts as a trigger
of ASM activation. In fact, the PKCδ knockout mouse shows a
phenotype of dysfunction in UV-induced ASM-dependent apoptosis
(30). To assess the effect of EGCG
on the phos-phorylation of PKCδ at Ser664 that involved apoptotic
cell death (31) was measured. EGCG
induced phosphorylation of PKCδ at Ser664 in a dose-dependent
manner (Fig. 4A and B).
Discussion
A previous study showed that EGCG killed
hematopoietic malignancy cells by the production of ROS in an in
vitro model (18). Although the
vicinal trihydroxy structure of EGCG contributes to these
antioxidative activities of tea polyphenols, it also renders these
compounds susceptible to air oxidation at alkaline or even neutral
pH (24,25). Autooxidation leads to the generation
of superoxide anions and H2O2 (12). Several studies have also shown that
high concentrations of EGCG induce ROS-dependent cell death
(12,32). The oxygen partial pressure in the
internal organs is normally much lower than that under cell culture
conditions (<40 vs. 160 mm Hg), and cells contain antioxidative
enzymes such as SOD and glutathione peroxidase (12), recent studies recommend the use of
SOD and catalase to halt EGCG-induced ROS production to avoid
artifacts (12,32). In the presence of SOD and catalase,
EGCG also induced significant anticancer activities (14,16,22,27–29,33),
suggesting that the effects of EGCG on cancer cells are independent
of ROS. Our data showed that by co-treatment with SOD and catalase,
EGCG exerted its anti-CML effect in a sGC-dependent ASM activation
pathway.
A lipid raft consists of mostly saturated
hydrocarbon chains with several kinds of tightly intercalated
sphingolipids and cholesterol organized the liquid-ordered state in
plasma membranes (34). Lipid rafts
play an essential role in the regulation of various signaling, cell
growth, and apoptosis. Proteins located in lipid rafts include
glycosylphosphatidylinositol–anchored proteins, doubly acylated
proteins such as Src-family kinases or α-subunits of heterotrimeric
G proteins, cholesterol-linked and palmitoylated proteins such as
Hedgehog, epidermal growth factor receptor (EGFR) and transmembrane
proteins, particularly palmitoylated ones (34). Several studies indicate that EGCG
affects lipid raft function (35,36) in
its anticancer effect. Adachi et al reported that EGCG has
an inhibitory effect on activation of EGFR via reduction of the
lipid (35). In that study, EGCG
reduced cholesterol-rich lipid rafts in a dose-dependent manner
(35). As a result, EGCG
drastically reduced epidermal growth factor-induced EGFR
phosphorylation, which plays the crucial role in tumor cell growth
and survival. However, little is known about the effect of EGCG on
lipid raft clustering in CML cells. In the present study, we showed
that EGCG induces lipid raft clustering in CML.
Ceramide and its metabolites influence cellular
processes that include apoptosis, autophagy and inflammation
(37). Enzymes of sphingolipid
metabolism determine cellular levels of ceramide, so that knowledge
of the regulation of these enzymes provides insight into the
cellular mechanisms underlying ceramide generation, accumulation
and action. Ceramide can be generated by hydrolysis of complex
sphingolipids or by the recently characterized ceramide salvage
pathway. ASM, also known as sphingomyelin phosphodiesterase 1
(SMPD1), is a member of the SMPD family and occupies a prominent
position in sphingolipid catabolism, catalyzing the hydrolysis of
sphingomyelin to ceramide and phosphorylcholine. In a recent study,
ASM-null mice were protected against a variety of stress stimuli,
including Fas ligand, lipopolysaccharide, ionizing radiation and
photocytotoxicity, ischemia/reperfusion injury, cisplatin and tumor
necrosis factor-α, as a result of impaired ceramide generation.
Notably, previous studies (21,25)
showed that cisplatin, the first member of a class of
platinum-containing anticancer drugs, induced apoptosis through ASM
activation and thereby caused ceramide-dependent lipid raft
clustering. These findings initiated our interest to investigate
the effect of EGCG on ASM activity. Importantly, ASM activation was
induced by EGCG, whereas its analog could not induce ASM
activation, showing that this pathway is specifically activated by
EGCG.
67LR is highly upregulated in hematopoietic
malignancies, including multiple myeloma (14), acute myeloid leukemia (13) and CLL (16), compared with normal peripheral blood
mono-nuclear cells (PBMCs). Indeed, EGCG selectively kills those
cancer cells without affecting normal PBMCs (13,14,33).
Thus, EGCG selectively suppresses CML cells without affecting
normal cells. In the last 3 years, severe adverse effects of the
second- and third-generation TKIs have been reported (1). These findings suggest EGCG as a choice
for the CML treatment.
Furthermore, we have reported that cGMP transmits an
anticancer effect and that the presence of a negative regulator of
cGMP protects against EGCG-induced cell death (14,23).
Indeed, the present study based on multiple myeloma cells showed
that cGMP production is the 'choke point' of the anticancer effect
of EGCG (14). Moreover, we
reported that phosphodiesterase 5 (PDE5) inhibition synergistically
enhanced the anticancer effect of EGCG in multiple myeloma
(14) and acute myeloid leukemia
cells (33). These data suggested
that pharmacological inhibition of a sGC negative regulator could
be an ideal approach to enhance the anti-CML effect of EGCG.
In conclusion, the present study demonstrated that
EGCG-induced lipid raft clustering in human CML cells. Indeed, the
present study further reveals that EGCG induced the cell death via
the sGC/ASM pathway. The present study clarifies the molecular
mechanism of EGCG in CML, and suggests that EGCG as a choice for
the CML treatment and pharmacological inhibition of a sGC negative
regulator could be an ideal approach to enhance the anti-CML effect
of EGCG.
Acknowledgments
This study was supported in part by a Grant-in-Aid
for the Scientific Research (S) (grant no. 22228002; to H.T.), and
the Research Fellowships from the Japan Society for the Promotion
of Science (PD to M.K. 14J30004; DC1 to Y.H. 13J03437).
References
|
1
|
Apperley JF: Chronic myeloid leukaemia.
Lancet. 385:1447–1459. 2015. View Article : Google Scholar
|
|
2
|
Hehlmann R, Hochhaus A and Baccarani M;
European LeukemiaNet: Chronic myeloid leukaemia. Lancet.
370:342–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Druker BJ, Tamura S, Buchdunger E, Ohno S,
Segal GM, Fanning S, Zimmermann J and Lydon NB: Effects of a
selective inhibitor of the Abl tyrosine kinase on the growth of
Bcr-Abl positive cells. Nat Med. 2:561–566. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Druker BJ, Talpaz M, Resta DJ, Peng B,
Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R,
Ohno-Jones S, et al: Efficacy and safety of a specific inhibitor of
the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J
Med. 344:1031–1037. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao C, Chen A, Jamieson CH, Fereshteh M,
Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, et al:
Hedgehog signalling is essential for maintenance of cancer stem
cells in myeloid leukaemia. Nature. 458:776–779. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Perl A and Carroll M: BCR-ABL kinase is
dead; long live the CML stem cell. J Clin Invest. 121:22–25. 2011.
View Article : Google Scholar :
|
|
7
|
Zhang M, Zhao X, Zhang X and Holman CD:
Possible protective effect of green tea intake on risk of adult
leukaemia. Br J Cancer. 98:168–170. 2008. View Article : Google Scholar
|
|
8
|
Kuo YC, Yu CL, Liu CY, Wang SF, Pan PC, Wu
MT, Ho CK, Lo YS, Li Y and Christiani DC; Kaohsiung Leukemia
Research Group: A population-based, case-control study of green tea
consumption and leukemia risk in southwestern Taiwan. Cancer Causes
Control. 20:57–65. 2009. View Article : Google Scholar
|
|
9
|
Naganuma T, Kuriyama S, Kakizaki M, Sone
T, Nakaya N, Ohmori-Matsuda K, Hozawa A, Nishino Y and Tsuji I:
Green tea consumption and hematologic malignancies in Japan: The
Ohsaki study. Am J Epidemiol. 170:730–738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shanafelt TD, Call TG, Zent CS, Leis JF,
LaPlant B, Bowen DA, Roos M, Laumann K, Ghosh AK, Lesnick C, et al:
Phase 2 trial of daily, oral polyphenon E in patients with
asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia.
Cancer. 119:363–370. 2013. View Article : Google Scholar
|
|
11
|
Hoy SM: Polyphenon E 10% ointment: In
immunocompetent adults with external genital and perianal warts. Am
J Clin Dermatol. 13:275–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang CS, Lambert JD, Ju J, Lu G and Sang
S: Tea and cancer prevention: Molecular mechanisms and human
relevance. Toxicol Appl Pharmacol. 224:265–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Britschgi A, Simon HU, Tobler A, Fey MF
and Tschan MP: Epigallocatechin-3-gallate induces cell death in
acute myeloid leukaemia cells and supports all-trans retinoic
acid-induced neutrophil differentiation via death-associated
protein kinase 2. Br J Haematol. 149:55–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumazoe M, Sugihara K, Tsukamoto S, Huang
Y, Tsurudome Y, Suzuki T, Suemasu Y, Ueda N, Yamashita S, Kim Y, et
al: 67-kDa laminin receptor increases cGMP to induce
cancer-selective apoptosis. J Clin Invest. 123:787–799.
2013.PubMed/NCBI
|
|
15
|
Shammas MA, Neri P, Koley H, Batchu RB,
Bertheau RC, Munshi V, Prabhala R, Fulciniti M, Tai YT, Treon SP,
et al: Specific killing of multiple myeloma cells by
(-)-epigallocat-echin-3-gallate extracted from green tea: Biologic
activity and therapeutic implications. Blood. 108:2804–2810. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumazoe M, Tsukamoto S, Lesnick C, Kay NE,
Yamada K, Shanafelt TD and Tachibana H: Vardenafil, a clinically
available phosphodiesterase inhibitor, potentiates the killing
effect of EGCG on CLL cells. Br J Haematol. 168:610–613. 2015.
View Article : Google Scholar
|
|
17
|
Leone M, Zhai D, Sareth S, Kitada S, Reed
JC and Pellecchia M: Cancer prevention by tea polyphenols is linked
to their direct inhibition of antiapoptotic Bcl-2-family proteins.
Cancer Res. 63:8118–8121. 2003.PubMed/NCBI
|
|
18
|
Nakazato T, Ito K, Ikeda Y and Kizaki M:
Green tea component, catechin, induces apoptosis of human malignant
B cells via production of reactive oxygen species. Clin Cancer Res.
11:6040–6049. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee YK, Bone ND, Strege AK, Shanafelt TD,
Jelinek DF and Kay NE: VEGF receptor phosphorylation status and
apoptosis is modulated by a green tea component,
epigallocatechin-3-gallate (EGCG), in B-cell chronic lymphocytic
leukemia. Blood. 104:788–794. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pike LJ: The challenge of lipid rafts. J
Lipid Res. 50(Suppl): S323–S328. 2009. View Article : Google Scholar :
|
|
21
|
Rebillard A, Tekpli X, Meurette O, Sergent
O, LeMoigne-Muller G, Vernhet L, Gorria M, Chevanne M, Christmann
M, Kaina B, et al: Cisplatin-induced apoptosis involves membrane
fluidification via inhibition of NHE1 in human colon cancer cells.
Cancer Res. 67:7865–7874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsukamoto S, Hirotsu K, Kumazoe M, Goto Y,
Sugihara K, Suda T, Tsurudome Y, Suzuki T, Yamashita S, Kim Y, et
al: Green tea polyphenol EGCG induces lipid-raft clustering and
apoptotic cell death by activating protein kinase Cδ and acid
sphingomyelinase through a 67 kDa laminin receptor in multiple
myeloma cells. Biochem J. 443:525–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
VanBeek DB, Zwier MC, Shorb JM and Krueger
BP: Fretting about FRET: Correlation between kappa and R. Biophys
J. 92:4168–4178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tachibana H: Green tea polyphenol sensing.
Proc Jpn Acad Ser B Phys Biol Sci. 87:66–80. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Micheau O, Solary E, Hammann A and
Dimanche-Boitrel MT: Fas ligand-independent, FADD-mediated
activation of the Fas death pathway by anticancer drugs. J Biol
Chem. 274:7987–7992. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tachibana H, Koga K, Fujimura Y and Yamada
K: A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol.
11:380–381. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Umeda D, Tachibana H and Yamada K:
Epigallocatechin-3-O- gallate disrupts stress fibers and the
contractile ring by reducing myosin regulatory light chain
phosphorylation mediated through the target molecule 67 kDa laminin
receptor. Biochem Biophys Res Commun. 333:628–635. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Umeda D, Yano S, Yamada K and Tachibana H:
Green tea poly-phenol epigallocatechin-3-gallate signaling pathway
through 67-kDa laminin receptor. J Biol Chem. 283:3050–3058. 2008.
View Article : Google Scholar
|
|
29
|
Tsukamoto S, Huang Y, Umeda D, Yamada S,
Yamashita S, Kumazoe M, Kim Y, Murata M, Yamada K and Tachibana H:
67-kDa laminin receptor-dependent protein phosphatase 2A (PP2A)
activation elicits melanoma-specific antitumor activity overcoming
drug resistance. J Biol Chem. 289:32671–32681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Humphries MJ, Limesand KH, Schneider JC,
Nakayama KI, Anderson SM and Reyland ME: Suppression of apoptosis
in the protein kinase Cdelta null mouse in vivo. J Biol Chem.
281:9728–9737. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hung JH, Lu YS, Wang YC, Ma YH, Wang DS,
Kulp SK, Muthusamy N, Byrd JC, Cheng AL and Chen CS: FTY720 induces
apoptosis in hepatocellular carcinoma cells through activation of
protein kinase C delta signaling. Cancer Res. 68:1204–1212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang CS, Wang X, Lu G and Picinich SC:
Cancer prevention by tea: Animal studies, molecular mechanisms and
human relevance. Nat Rev Cancer. 9:429–439. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kumazoe M, Kim Y, Bae J, Takai M, Murata
M, Suemasu Y, Sugihara K, Yamashita S, Tsukamoto S, Huang Y, et al:
Phosphodiesterase 5 inhibitor acts as a potent agent sensitizing
acute myeloid leukemia cells to 67-kDa laminin receptor-dependent
apoptosis. FEBS Lett. 587:3052–3057. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Simons K and Toomre D: Lipid rafts and
signal transduction. Nat Rev Mol Cell Biol. 1:31–39. 2000.
View Article : Google Scholar
|
|
35
|
Adachi S, Nagao T, Ingolfsson HI, Maxfield
FR, Andersen OS, Kopelovich L and Weinstein IB: The inhibitory
effect of (-)-epigallocatechin gallate on activation of the
epidermal growth factor receptor is associated with altered lipid
order in HT29 colon cancer cells. Cancer Res. 67:6493–6501. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Patra SK, Rizzi F, Silva A, Rugina DO and
Bettuzzi S: Molecular targets of (−)-epigallocatechin-3-gallate
(EGCG): Specificity and interaction with membrane lipid rafts. J
Physiol Pharmacol. 59(Suppl 9): S217–S235. 2008.
|
|
37
|
Jenkins RW, Canals D and Hannun YA: Roles
and regulation of secretory and lysosomal acid sphingomyelinase.
Cell Signal. 21:836–846. 2009. View Article : Google Scholar : PubMed/NCBI
|