Introduction
Globally, stomach cancer is the fifth most common
type of cancer and the third leading cause of cancer deaths, making
up 7% of cases and 9% of deaths (1). In 2012, 950,000 people contracted
stomach cancer worldwide, leading to 723,000 deaths (1). In 2014, the United States recorded an
estimated 22,220 new cases and 10,990 deaths from stomach cancer
(2). Outcomes are often poor with a
<10% 5-year survival rate globally, and a 28% 5-year survival
rate in the United States (3).
Gastric cancer is a multifactorial disease. The most common cause
is infection by the bacteria Helicobacter pylori, which is
responsible for 65–80% of gastric cancers (4), though other factors such as genetics,
smoking, and diet (especially eating pickled vegetables) have also
been shown to play an important role in the development of gastric
cancer. Surgery, chemotherapy, radiation therapy, and targeted
therapy are commonly used to treat this disease (5), and if treated late, palliative care
may also be advised (4).
Chemotherapy aims to initiate apoptosis in gastric
cancer cells (6,7); however, these drugs can be toxic.
Therefore, recent studies have made an effort to investigate
alternative treatments that have fewer and less potent side
effects. Lactic acid bacteria (LAB) may represent a useful approach
for the treatment of cancer. LAB are present in many foods such as
yogurt and have been shown to elicit antitumor effects. Probiotics
have been shown to act preventatively in in vitro studies
and during carcinogenesis in studies on animals bearing tumors. For
example, multiple studies show various LAB strains exert inhibitory
effects on the growth of different types of tumors in rodents
(8–11).
Earlier studies also reveal that different strains
of LAB have anti-proliferative effects against human cancer cell
lines. For example, Lactococcus lactis ssp. lactis (L.lac
CF) induces apoptosis on the human colon cancer cell line SNUC2A
(12), L. casei rhamnosus
induces apoptosis in the human monocytic leukemia cell line THP-1
(13), and L. reuteri
enhances tumor necrosis factor (TNF)-induced apoptosis in human
chronic myeloid leukemia-derived cells (14). Furthermore, probiotic consumption
might be associated with reducing the incidence of colon tumors, as
shown in epidemiological studies (15). This data suggests that fermented
milk products and/or the fermentative bacteria themselves may have
chemoprotective effects without the toxic side effects of
conventional therapeutic drugs.
Probiotics Fermentation Technology (PFT), a kefir
grain product, is a natural mixture composed primarily of
Lactobacillus kefiri P-IF, a specific strain of L.
kefiri with unique growth characteristics. Our recent studies
have demonstrated the ability of PFT to induce apoptosis on human
MDR myeloid leukemia (HL60/AR) cells in vitro (16). The present study was designed to
examine the possible apoptotic effect of PFT against other types of
cancer, specifically the human gastric cancer AGS cells, and murine
breast cancer 4T1 cells, in vitro. It was of interest to
note that PFT selectively exerts apoptotic effects on AGS cells,
but not on 4T1 cells. Furthermore, PFT showed no apoptotic effect
on human PBMCs. The mechanism underlying the effect of PFT was
examined.
Materials and methods
Tumor cell lines
Two cancer cell lines were used in the present
study, namely: AGS, a human gastric adenocarcinoma cell line, and
4T1, a murine breast cancer cell line. The cells were purchased
from American Tissue and Culture Collection (ATCC) (Manassas, VA,
USA). AGS tumor cells were maintained in Dulbecco's modified
Eagle's medium (DMEM) (Invitrogen Corp., Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS) and 2 mM glutamine,
and 100 µg/ml streptomycin and penicillin. 4T1 tumor cells
were maintained in RPMI-1614 (Invitrogen Corp.) supplemented with
10% FBS and 2 mM glutamine, and 100 µg/ml streptomycin and
penicillin. Cells were routinely maintained in log phase in a
humidified incubator at 37°C with 5% CO2.
Probiotics Fermentation Technology (PFT)
kefir grain product
PFT is a mixture that consists mainly (~90%) of a
freeze-dried form of heat-killed L. kefiri P-IF. In
addition, PFT contains ~2–3% each, of one bacterial strain L.
kefiri P-B1, as well as the yeast strains Kazachstania
turicensis, Kazachstania unispora and Kluyveromyces
marxianus. P-IF is a specific strain of LAB that has a unique
DNA sequence, and PET scans show a 99.6% homology with regular
kefiries. Its characteristics were recently reported (16). PFT was provided by Paitos Co., Ltd.,
yokohama, Kanagawa, Japan.
PFT induces apoptosis on cancer cells -
flow cytometry study
7-Aminoactinomycin D (7-AAD staining) was used to
detect cancer cell viability. AGS cells were cultured in the
presence or absence of PFT at different concentrations (0.0, 0.3,
0.6, 1.2, 2.5 and 5 mg/ml) for 3 days and the percentage of dead
cancer cells was examined by 7-AAD (BD Biosciences, San Diego, CA,
USA) technique using a FACSCalibur (Becton-Dickinson, San Jose, CA,
USA). Briefly, the cells were stained for 30 min at room
temperature in the dark with 5 µl of 7-AAD and analyzed by
FACSCalibur.
PFT induces apoptosis on AGS cancer cells
- morphological analysis
Cytospin preparations stained with Giemsa allowed us
to examine PFT-induced apoptotic cancer cells. Several
morphological characteristics have been used to identify apoptotic
cells including cell swelling, membrane blebbing and chromatin
condensation (17).
Growth of monolayer cancer cells in
6-well plates
We followed our earlier model assay system (18) to examine apoptosis of monolayer AGS
cells post-culturing with PFT. Apoptosis in adherent and
non-adherent AGS cells was monitored. Cancer cells were allowed to
grow in 6-well plates (25×36 mm each, CellStar, Greiner Bio-One,
Monroe, NC, USA). A cover glass was placed at the bottom of each
well. AGS cells/ml (1×105/2 ml) were pipetted into each
well, allowed to adhere and reach 50–60% confluency. PFT (5.0
mg/ml) was added to each well and incubated at 37°C and 5%
CO2. At 0.5 and 24 h, both non-adherent and adherent
cells were examined as follows:
a) Non-adherent cells
The supernatant containing non-adherent tumor cells
(1 ml) was mixed with 100 µl of trypan blue to make cytospin
preparations (Shandon Southern Instruments, Sewickly, PA, USA).
Preparations were fixed in 100% methanol, air-dried, stained with
4% Giemsa for 20 min (Sigma-Aldrich Corp., St. Louis, MO, USA) and
examined using oil immersion and a light microscope fitted with a
100x objective (Leica DMLB microscope and Leica DFC310FX digital
color camera, Germany). The supernatant was also used to count the
number of apoptotic non-adherent cancer cells using a
hemocytometer.
b) Adherent cells
Cover glasses containing adherent cells were
carefully removed, air-dried, mounted on slides, fixed in methanol
and stained with Giemsa as above. The cells were analyzed
morphologically using oil immersion and a light microscope fitted
with a 100x objective (Leica microscope). In addition, the
preparations were used to calculate the percentage of apoptotic
adherent cancer cells. The number of adherent cells was calculated
from observations at 5 different sites on the cover slip, each
containing ~80 cells. The percentage of apoptotic cells was
determined by dividing the number of apoptotic cells by the total
number of cells calculated.
Mechanism underlying PFT effect
a) Expression of Bcl-2
For detection of Bcl-2, cells were first fixed and
permeabilized with ice-cold 70% methanol. Cells were then stained
with FITC-labeled anti-Bcl-2 or isotype control (Dako Corp.,
Carpinteria, CA, USA), washed and analyzed by FACSCalibur. The
percentage of cells expressing Bcl-2 and mean fluorescent intensity
(an indicator of density of the molecules/cell) was determined.
b) Detection of mitochondrial membrane
potential (MMP)
Variations of the mitochondrial transmembrane
potential ∆ψm during apoptosis were studied using
tetramethylrho-damine ethylester (TMRE, Molecular Probes, Eugene,
OR, USA). After treatment with PFT for 3 days, cancer cells
(5×105 cells/ml) were incubated with 50 nM TMRE for 30
min at 37°C, washed with PBS, and analyzed with FACSCalibur. The
side scatters were used to gate and exclude cellular debris using a
FACSCalibur. The cells were excited at 488 nm and the emission was
collected on the FL2 channel. Five thousand cells were analyzed.
The data were acquired and analyzed using CellQuest software
(Becton-Dickinson). A decrease in red fluorescence indicates loss
of membrane potential ∆ψm.
Effect of PFT with human peripheral blood
mononuclear cells (PBMCs)
PBMCs from three normal healthy donors [approved by
the Institutional Review Board (IRB), Charles Drew University, Los
Angeles, CA, USA] were separated over Ficoll-hypaque density
gradient centrifugation. Cells (1×106/ml) were cultured
with or without PFT (5.0 mg/ml) for 3 days. Cells were examined for
the percentage of apoptosis.
Statistical analysis
Using the Student's t-test, we tested the
significance of difference in the percent changes of apoptotic
cancer cells and PBMCs post-culture with PFT as compared to control
untreated cells alone. The level of significance was set at
p<0.05.
Results
Percent apoptotic cancer cells by flow
cytometry
AGS and 4T1 cells were cultured with PFT at
concentrations 0–5 mg/ml for 3 days, and the percent apoptotic
cancer cells was determined by flow cytometry using 7-AAD dye.
Fig. 1 shows that PFT induced
apoptosis in AGS cancer cells in a dose-dependent manner. An
increase in the percentage of apoptotic AGS cells was detected at
lower concentrations of PFT (0.3 and 0.6 mg/ml). The percent
apoptotic cells became significant at a concentration of 1.2 mg/ml
(37.0%, p<0.05), with a further increase at 2.5 mg/ml (53.1%,
p<0.001) and maximized at 5 mg/ml (66.3%, p<0.0001). Notably,
PFT does not induce apoptosis in 4T1 cancer cells ≤5 mg/ml.
Morphological analysis of apoptotic
cancer cells by Giemsa staining
Cancer cells were cultured with PFT (5.0 mg/ml) and
the percentage/number of apoptotic cells among the non-adherent and
monolayer adherent cancer cells was examined.
a) Non-adherent apoptotic AGS cancer
cells
AGS cancer cells were cultured with PFT for 0.5 and
24 h and the supernatant containing non-adherent cells was
collected and the number of apoptotic cancer cells was examined by
trypan blue staining and hemocytometer. Data depicted in Fig. 2 show a significant increased level
of apoptotic non-adherent cells at 0.5 h. The number of apoptotic
cancer cells was further increased at 24 h, showing a 2-fold
increase in comparison to the 0.5 h.
b) Monolayer adherent apoptotic AGS
cells
i) Morphological characteristics
We were able to identify the apoptotic AGS cancer
cells in Giemsa-stained monolayer AGS cells grown on cover glass
post-culture with PFT (5.0 mg/ml) for 24 h. PFT induces the common
morphological characteristics of apoptosis including cell swelling,
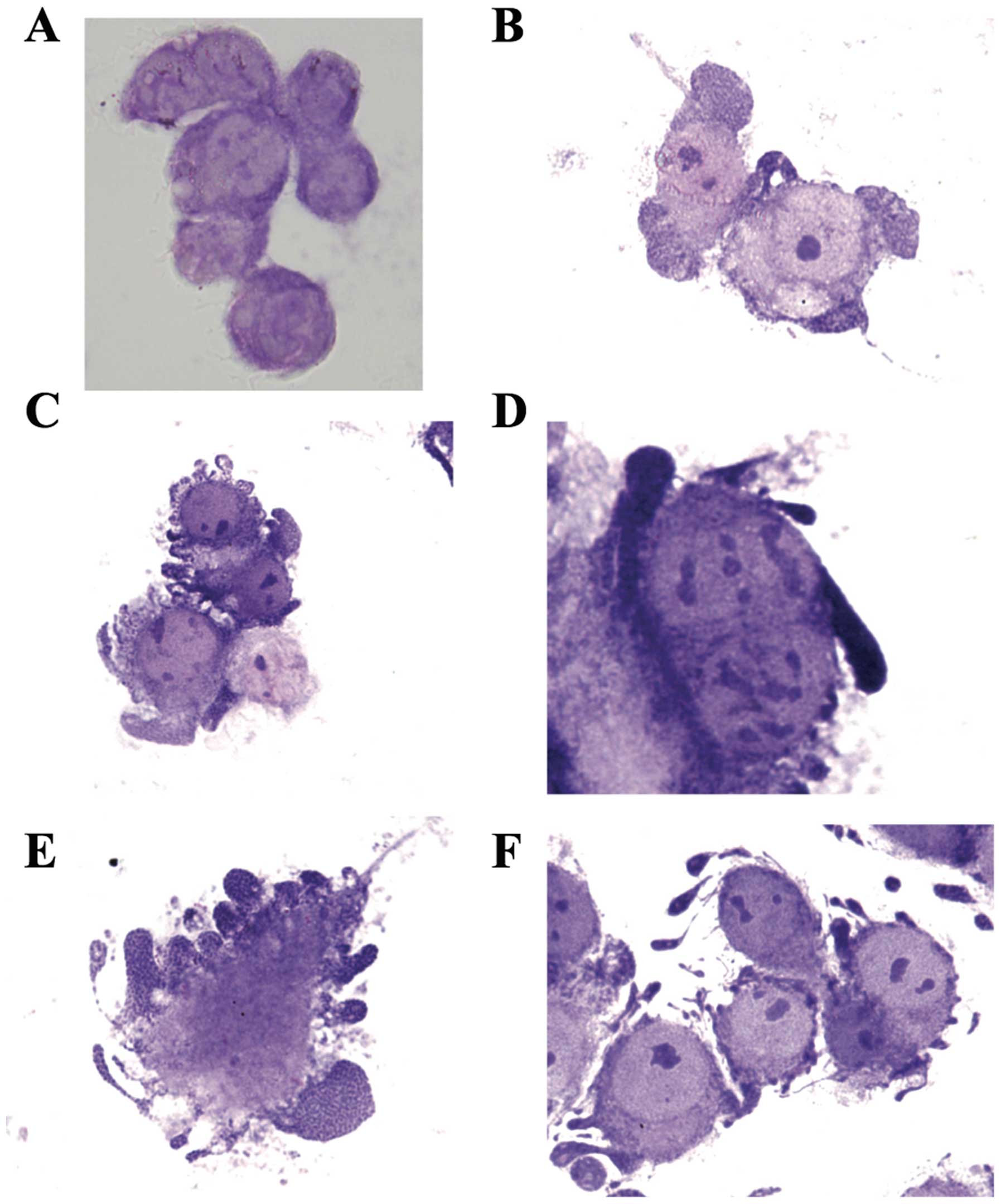
membrane blebbing, and chromatin condensation. Fig. 3A shows non-apoptotic adherent AGS
cells. Note the absence of chromatin condensation and membrane
blebbing. Cancer cells undergoing apoptosis begin with chromatin
condensation, in which the nucleus shrinks to about half the size
of the cell (Fig. 3B). This is
followed by membrane blebbing (Fig.
3C) and nuclear fragmentation (Fig.
3D). Finally, nuclear fragments become encased in membrane
vesicles (Fig. 3E), and
subsequently these vesicles become detached from the cell. Note
that spherical and ovular vesicles are completely detached and
autonomous from the apoptotic cell (Fig. 3F). On the other hand, we observed
absence of chromatin condensation and membrane blebbing in 4T1
cancer cells post-treatment with PFT (Fig. 4).
ii) Percentage of monolayer apoptotic AGS
cells
The percentage of adherent AGS cells having
morphological characteristics of apoptosis was examined at 0.5 and
24 h post-culture of cancer cells with PFT. Fig. 5 shows that a significant percentage
of apoptosis was detected as early as 0.5 h post-culture of PFT
with AGS cells (p<0.01). The apoptotic effect of PFT was further
increased and became highly significant at 24 h (p<0.001). The
percent of apoptotic cancer cells post-treatment with PFT for 24 h
was 2.4-fold of those treated at 0.5 h.
Mechanisms underlying the PFT effect
a) Mitochondrial membrane potential
(MMP)
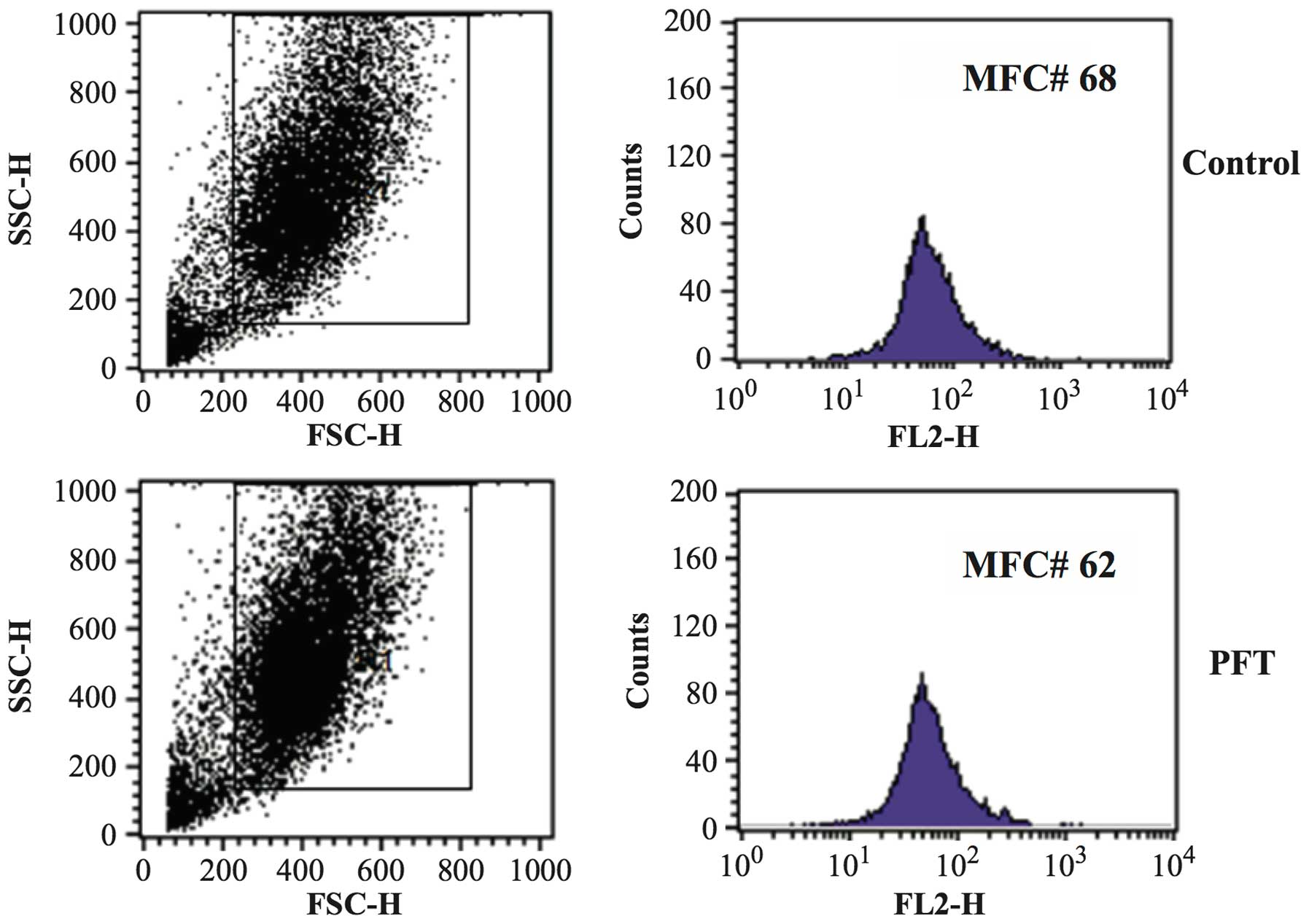
The effect of PFT (2.5 mg/ml) on the MMP of AGS and
4T1 cells was examined by flow cytometry. Data in Fig. 6 show that treatment of AGS cancer
cells with PFT for 3 days resulted in a significant decrease in
mitochondrial potential as compared with control untreated cells
(p= 0.007). Fig. 6A shows a
representative flow histogram and Fig.
6B shows a bar graph representing the mean ± SD of 3 different
experiments. In contrast, data in Fig.
7 show that PFT had no effect on MMP of 4T1 cells.
b) Bcl2 expression
Bcl2 expression of AGS cells post- culture with PFT
(2.5 mg/ml) for 3 days was examined. Flow cytometry studies show
that treatment with PFT resulted in a significant downregulation of
the expression of Bcl2 of AGS cells (p=0.004) as compared to
control untreated cancer cells. Fig.
8A shows a representative flow histogram and Fig. 8B shows a bar graph representing the
mean ± SD of 3 different experiments.
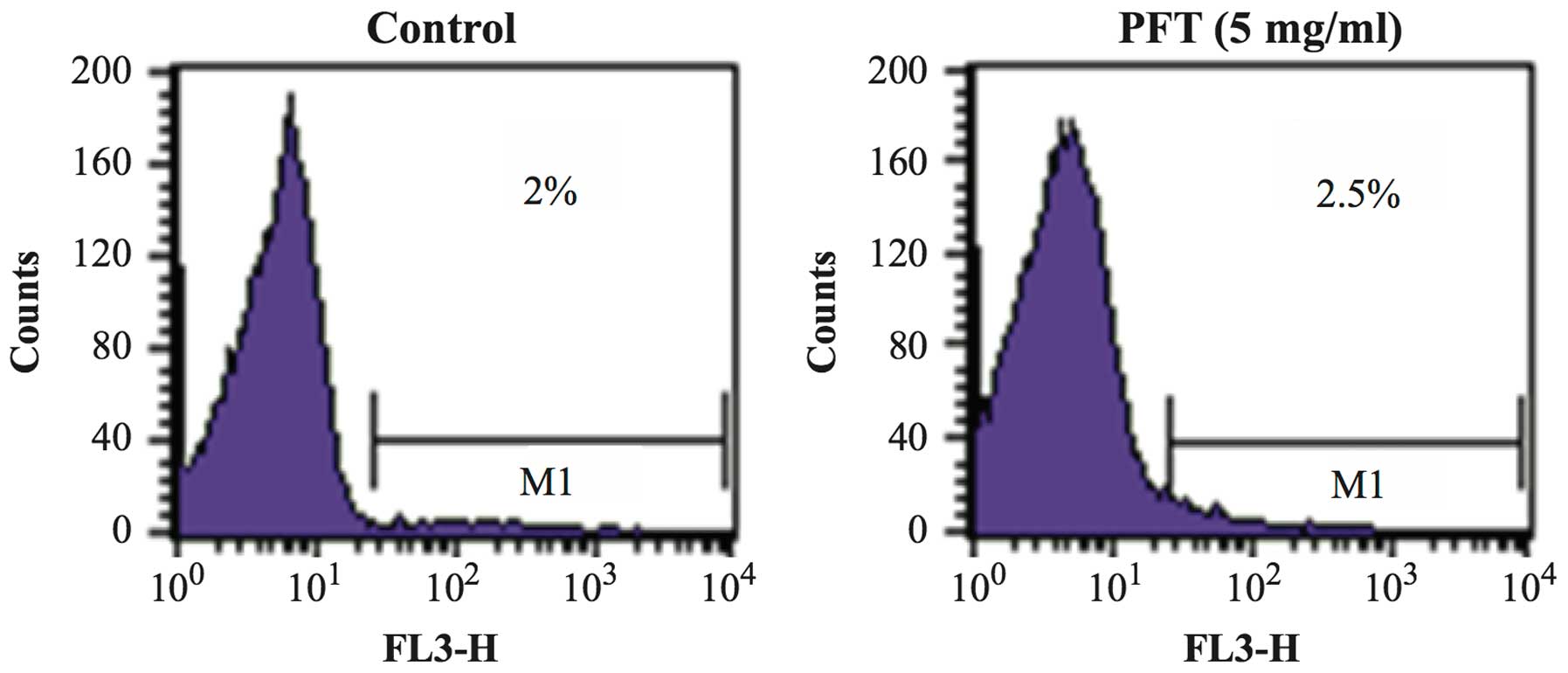
Effect of PFT on human PBMC
The effect of PFT treatment on PBMCs with respect to
changes on the percentage of apoptotic cells was examined. PBMCs
were co-cultured with PFT (5.0 mg/ml) for 3 days and the percentage
of apoptotic cells was examined by flow cytometry. Fig. 9 shows that treatment of PBMCs with
PFT caused no significant change in the percentages of apoptotic
PBMCs as compared with control untreated cells.
Discussion
Lactic acid bacteria (LAB) has been found in milk
products for thousands of years and is associated with inhibiting
the growth of spoilage agents. Recently, scientists have revealed
the additional potential of these bacteria as anticancer agents.
Several reports suggest that fermented milk products and/or the
fermentative bacteria themselves may have chemoprotective effects
against cancer without the toxic side effects of conventional
therapeutic drugs. In the present study we used a novel kefir
product, Probiotics Fermentation Technology PFT, in which
Lactobacillus kefiri P-IF is the main constituent. There are
several characteristics that may allow P-IF to act as a potent
anticancer agent. These include the ability of P-IF to grow
three-dimensionally due to carbohydrate chains found on its
surface, while other L. kefiri strains grow in a
lengthwise-dimensional pattern. Moreover, P-IF can utilize
galactose as a carbon source and produce carbonic acid (19). Our recent study demonstrated that
PFT induces apoptosis on human multidrug-resistant (MDR) myeloid
leukemia (HL60/AR) cells (16).
These results prompted us to examine the apoptotic effect of PFT on
other types of cancer. Data revealed that PFT exerts a selective
apoptotic effect on human AGS cancer cells and did not exhibit
apoptotic effects on 4T1 cells.
LAB can induce cancer cell death through a mechanism
that involves apoptosis. There are two major pathways of apoptosis
that have been extensively described in the literature; these are
the extrinsic and intrinsic pathways. The former is mediated by
activation of death receptors and caspase 8, while the latter
involves mitochondria and caspase 9 (20). The Bcl-2 family of proteins have
been shown to play an important role in the mitochondrial pathway
and in the maintenance of the MMP. In this study, treatment with
PFT caused significant downregulation in the level of Bcl-2 of AGS
cancer cells, this was associated with a decrease in the
mitochondrial polarization of AGS cells. This may result in the
release of pro-apoptotic molecules that cause the activation of
caspases and eventually lead to apoptosis. Similar effects were
noted on HL60/AR cells post-treatment with PFT (16).
Chemotherapy such as 5-fluorouracil (5-FU),
cisplatin, and doxorubicin, which are often used for the treatment
of gastric cancer, aim to initiate apoptosis in gastric cancer
cells (6,7). The apoptotic effect of PFT was shown
to be both dose- and time-dependent on AGS cells. Flow cytometry
studies showed that PFT induces apoptosis which was detected at a
concentration of 0.3 mg/ml and peaked at 66.3% at 5.0 mg/ml.
Morphological examination of Giemsa stained cytospin preparation
confirmed the apoptotic effect of PFT against AGS cells, where the
characteristics of apoptotic cells such as cell swelling, membrane
blebbing and chromatin condensation were clearly identified.
Induction of apoptosis by PFT was detected at 0.5 h post-treatment
and the percentage of apoptotic cells showed a 2-fold increase at
24 h. Earlier reports showed that other LAB agents such as L.lac CF
induced DNA fragmentation and chromatin condensation against human
stomach adenocarcinoma, SNU-1 (21).
The mitochondrial pathway appears to be the main
route for the induction of apoptosis against gastric cancer cells
by different types of probiotics such as P. freudenreichii
(22), L. paracasei IMPC2.1,
and L. rhamnosus GG (L.GG) (23). Similarly, induction of apoptosis via
the mitochondrial pathway was also noted in colon cancer cells
treated with probiotics including propionibacteria (24,25),
L. rhamnosus, Bifidobacterium latis (26), and L. delbrueckii (27). Furthermore, LAB induces the
mitochondrial pathway of apoptosis in myeloid leukemia as well. For
example, PFT induces apoptosis on human MDR myeloid leukemia
(HL60/AR) (16), as well as L.
reuteri (14) and L. casei
rhamnosus (13) on human
monocytic leukemia-derived cells.
Two alternative mechanisms can be proposed to
account for the induction of apoptosis by LAB. First of all it is
possible that LAB induces apoptosis by binding to Toll like
receptors (TLR) on cancer cells and triggers apoptosis. This
hypothesis is based on the reports that show some but not all TLRs
trigger apoptosis in cancer cells (28–30).
Second, it is possible that phagocytosed LAB may induce apoptosis.
This hypothesis is based on studies by us and others that show
cancer cells phagocytose microoraganism (31–33)
and subsequently cancer cells undergo apoptosis (17,34,35).
The above hypotheses are not mutually exclusive. The reason for the
inability of PFT to induce apoptosis in 4T1 may be due to the lack
of appropriate TLRs that bind to PFT or failure of 4T1 cells to
induce apoptosis post-phagocytosis of PFT.
Several studies have shown PFT to be a non-toxic
agent. In this study, we noted no significant change in the
percentage of apoptotic human PBMCs that were treated with PFT (5.0
mg/ml) for 3 days. Additionally, in vivo studies have shown
that PFT-treated mice had no change in body weight, and showed no
macroscopic or histopathological abnormalities in different organs
(36). These results suggest that
PFT is a selective apoptotic inducer for gastric cancer, and is
also a safe, non-toxic, potential therapy for the treatment of
gastric cancer.
Acknowledgments
The authors would like to thank Paitos Co., Ltd.,
Yokohama, Kanagawa, Japan; grant no. T0099108. We would also like
to thank our colleague and our collaborator Dr S. Gollapudi, UC
Irvine, for his critical insight and guidance for this study. We
would also like to thank our collaborator D. Pan, who is partially
supported by NIH-NIMHD grant U54MD007598 (formerly U54RR026138) and
NIH/NIMHD grant S21 MD000103 (CDU Life Sciences Institute). Partial
support has also been provided by AXIS 5U54MD007598-06. Finally, we
greatly appreciate the help of Dr Ben Winjum for help in preparing
the figures and manuscript.
References
|
1
|
World Health Organization: The global and
regional burden of cancer. World Cancer Report 2014. Stewart BW and
Wild CP: (IARC Nonserial Publication). 2014
|
|
2
|
National Cancer Institute (NCI): Stomach
(gastric) cancer. http://www.cancer.gov/types/stomach.
Accessed July 1, 2014.
|
|
3
|
National Cancer Institute (NCI): SEER Stat
Fact Sheets: Stomach Cancer. http://seer.cancer.gov/statfacts/html/stomach.html.
Accessed June 18, 2014.
|
|
4
|
World Health Organization: Stomach cancer.
Gastric cancer prevention. World Cancer Report 2014. Stewart BW and
Wild CP: (IARC Nonserial Publication). 2014
|
|
5
|
National Cancer Institute (NCI): Gastric
Cancer Treatment (PDQ®). http://www.cancer.gov/types/stomach/patient/stomach-treatment-pdq#section/all/patient/stomach-treatment-pdq#section/all
NCI. Accessed July 1, 2014.
|
|
6
|
Sugamura K, Makino M, Shirai H, Kimura O,
Maeta M, Itoh H and Kaibara N: Enhanced induction of apoptosis of
human gastric carcinoma cells after preoperative treatment with
5-fluorouracil. Cancer. 79:12–17. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuhashi N, Saio M, Matsuo A, Sugiyama Y
and Saji S: The evaluation of gastric cancer sensitivity to
5-FU/CDDP in terms of induction of apoptosis: Time- and p53
expression-dependency of anticancer drugs. Oncol Rep. 14:609–615.
2005.PubMed/NCBI
|
|
8
|
Singh J, Rivenson A, Tomita M, Shimamura
S, Ishibashi N and Reddy BS: Bifidobacterium longum, a lactic
acid-producing intestinal bacterium inhibits colon cancer and
modulates the intermediate biomarkers of colon carcinogenesis.
Carcinogenesis. 18:833–841. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reddy BS and Rivenson A: Inhibitory effect
of Bifidobacterium longum on colon, mammary, and liver
carcinogenesis induced by 2-amino-3-methylimidazo[4,5-f]quinoline,
a food mutagen. Cancer Res. 53:3914–3918. 1993.PubMed/NCBI
|
|
10
|
Fukui M, Fujino T, Tsutsui K, Maruyama T,
Yoshimura H, Shinohara T, Fukui M and Nada O: The tumor-preventing
effect of a mixture of several lactic acid bacteria on
1,2-dimethylhy-drazine-induced colon carcinogenesis in mice. Oncol
Rep. 8:1073–1078. 2001.PubMed/NCBI
|
|
11
|
Abd el-Gawad IA, el-Sayed EM, Hafez SA,
el-Zeini HM and Saleh FA: Inhibitory effect of yoghurt and soya
yoghurt containing bifidobacteria on the proliferation of Ehrlich
ascites tumour cells in vitro and in vivo in a mouse tumour model.
Br J Nutr. 92:81–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim JY, Woo HJ, Kim YS, Kim KH and Lee HJ:
Cell cycle dysregulation induced by cytoplasm of Lactococcus lactis
ssp lactis in SNUC2A, a colon cancer cell line. Nutr Cancer.
46:197–201. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chiu YH, Hsieh YJ, Liao KW and Peng KC:
Preferential promotion of apoptosis of monocytes by Lactobacillus
casei rhamnosus soluble factors. Clin Nutr. 29:131–140. 2010.
View Article : Google Scholar
|
|
14
|
Iyer C, Kosters A, Sethi G, Kunnumakkara
AB, Aggarwal BB and Versalovic J: Probiotic Lactobacillus reuteri
promotes TNF-induced apoptosis in human myeloid leukemia-derived
cells by modulation of NF-kappaB and MAPK signalling. Cell
Microbiol. 10:1442–1452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McIntosh GH and Le Leu RK: The influence
of dietary proteins on colon cancer risk. Nutr Res. 21:1053–1066.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghoneum M and Gimzewski J: Apoptotic
effect of a novel kefir product, PFT, on multidrug-resistant
myeloid leukemia cells via a hole-piercing mechanism. Int J Oncol.
44:830–837. 2014.PubMed/NCBI
|
|
17
|
Ghoneum M and Gollapudi S: Induction of
apoptosis in breast cancer cells by Saccharomyces cerevisiae, the
baker's yeast, in vitro. Anticancer Res. 24:1455–1463.
2004.PubMed/NCBI
|
|
18
|
Ghoneum M and Gollapudi S: Modified
arabinoxylan rice bran (MGN-3/Biobran) enhances yeast-induced
apoptosis in human breast cancer cells in vitro. Anticancer Res.
25A:859–870. 2005.
|
|
19
|
Suzuki K, Tani H, Yabumoto T, Yabumoto Y
and Yoshida Y: Novel fermented milk product and use thereof. US
Patent No. US 20110123640. A1. Filed Jun 8, 2009; issued. May
26–2011
|
|
20
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim SY, Lee KW, Kim JY and Lee HJ:
Cytoplasmic fraction of Lactococcus lactis ssp. lactis induces
apoptosis in SNU-1 stomach adenocarcinoma cells. Biofactors.
22:119–122. 2004. View Article : Google Scholar
|
|
22
|
Cousin FJ, Jouan-Lanhouet S,
Dimanche-Boitrel MT, Corcos L and Jan G: Milk fermented by
Propionibacterium freudenreichii induces apoptosis of HGT-1 human
gastric cancer cells. PLoS One. 7:e318922012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Orlando A, Refolo MG, Messa C, Amati L,
Lavermicocca P, Guerra V and Russo F: Antiproliferative and
proapoptotic effects of viable or heat-killed Lactobacillus
paracasei IMPC2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric
and DLD-1 colon cell lines. Nutr Cancer. 64:1103–1111. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jan G, Belzacq AS, Haouzi D, Rouault A,
Métivier D, Kroemer G and Brenner C: Propionibacteria induce
apoptosis of colorectal carcinoma cells via short-chain fatty acids
acting on mitochondria. Cell Death Differ. 9:179–188. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lan A, Lagadic-Gossmann D, Lemaire C,
Brenner C and Jan G: Acidic extracellular pH shifts colorectal
cancer cell death from apoptosis to necrosis upon exposure to
propionate and acetate, major end-products of the human probiotic
propionibacteria. Apoptosis. 12:573–591. 2007. View Article : Google Scholar
|
|
26
|
Altonsy MO, Andrews SC and Tuohy KM:
Differential induction of apoptosis in human colonic carcinoma
cells (Caco-2) by Atopobium, and commensal, probiotic and
enteropathogenic bacteria: Mediation by the mitochondrial pathway.
Int J Food Microbiol. 137:190–203. 2010. View Article : Google Scholar
|
|
27
|
Wan Y, Xin Y, Zhang C, Wu D, Ding D, Tang
L, Owusu L, Bai J and Li W: Fermentation supernatants of
Lactobacillus delbrueckii inhibit growth of human colon cancer
cells and induce apoptosis through a caspase 3-dependent pathway.
Oncol Lett. 7:1738–1742. 2014.PubMed/NCBI
|
|
28
|
Fukata M, Shang L, Santaolalla R,
Sotolongo J, Pastorini C, España C, Ungaro R, Harpaz N, Cooper HS,
Elson G, et al: Constitutive activation of epithelial TLR4 augments
inflammatory responses to mucosal injury and drives
colitis-associated tumorigenesis. Inflamm Bowel Dis. 17:1464–1473.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fűri I, Sipos F, Germann TM, Kalmár A,
Tulassay Z, Molnár B and Műzes G: Epithelial toll-like receptor 9
signaling in colorectal inflammation and cancer: Clinico-pathogenic
aspects. World J Gastroenterol. 19:4119–4126. 2013. View Article : Google Scholar :
|
|
30
|
Li TT, Ogino S and Qian ZR: Toll-like
receptor signaling in colorectal cancer: Carcinogenesis to cancer
therapy. World J Gastroenterol. 20:17699–17708. 2014.PubMed/NCBI
|
|
31
|
Vandenberghe J, Verheyen A, Lauwers S and
Geboes K: Spontaneous adenocarcinoma of the ascending colon in
Wistar rats: The intracytoplasmic presence of a Campylobacter-like
bacterium. J Comp Pathol. 95:45–55. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghoneum M and Gollapudi S: Apoptosis of
breast cancer MCF-7 cells in vitro is induced specifically by yeast
and not by fungal mycelia. Anticancer Res. 26:2013–2022.
2006.PubMed/NCBI
|
|
33
|
Ghoneum M, Grewal I, Brown J, Osborne R,
Elembabi H and Gill G: Phagocytosis of candida albicans by
lymphatic tumour cells in vitro. Acta Histochem. 105:127–133. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ghoneum M, Hamilton J, Brown J and
Gollapudi S: Human squamous cell carcinoma of the tongue and colon
undergoes apoptosis upon phagocytosis of Saccharomyces cerevisiae,
the baker's yeast, in vitro. Anticancer Res. 25A:981–989. 2005.
|
|
35
|
Ghoneum M, Matsuura M, Braga M, Gollapudi
S, et al: S. cerevisiae induces apoptosis in human metastatic
breast cancer cells by altering intracellular Ca2+ and
the ratio of Bax and Bcl-2. Int J Oncol. 33:533–539.
2008.PubMed/NCBI
|
|
36
|
Paitos Co: Ltd. Yokohama, Kanagawa, Japan:
Increase the good bacteria held by nature, ideal AH21 is a
functional food, consider preventive medicine and food. http://www.bio-j.net/ken00.html.
Accessed June 14, 2013.
|