Introduction
Cancer cells utilize glucose at a high rate to
support their rapid proliferation and biosynthesis, and this occurs
via glycolysis rather than metabolizing pyruvate through oxidative
phosphorylation in the TCA cycle (1). Pyruvate kinase (PK) is a key
rate-limiting enzyme in the glycolytic pathway and has a
well-defined role in the Warburg effect (2,3).
Pyruvate kinase catalyzes the transfer of a phosphate group from
phosphoenolpyruvate (PEP) to ADP producing ATP and pyruvate
(1). Two genes encode for four
isoforms of pyruvate kinase that are specifically expressed in
different tissues. PKL and PKR isoforms are encoded by the
PKLR gene, while PKM1 and pyruvate kinase M2 (PKM2) are
encoded by PKM gene. By alternative splicing, PKM2 carries
exon 10, but not exon 9 as found in PKM1 (4). PKM2 exists in three forms including an
inactive monomer, a low activity dimer, and a high activity
tetramer. In cancer cells, a dominant form of PKM2 is the dimer
conformation, which paradoxically leads to a high lactate
production as in the Warburg effect (5). Highly proliferating cancer cells
require building blocks for the synthesis of lipids, nucleotides
and amino acids. Decreased PKM2 activity will slow glycolysis rate
enabling cancer cells to produce macromolecule precursors from
glycolytic intermediates, such as glucose-6-phosphate (G6P) through
the pentose phosphate pathway (PPP) and the hexosamine biosynthesis
pathway (HBP) (2,3,6).
O-GlcNAcylation is a discrete glycosylation
in which target proteins are modified at the hydroxyl group of
serine and/or threonine by one molecule of
N-acetyl-D-glucosamine (O-GlcNAc) (7). This glycosylation may take place in
the cytoplasm, nucleus, and mitochondria. Two key enzymes,
O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA)
regulate O-GlcNAcylation by catalyzing the addition and
removal of an O-GlcNAc group from proteins, respectively.
Enzymatic activity of OGT is sensitive to the concentration of its
donor substrate; uridine diphospho-N-acetylglucosamine
(uDP-GlcNAc) (8). UDP-GlcNAc is the
end product of the HBP and is built up from a variety of nutrients
including glucose, glutamine, fatty acids (acetyl) and uridine
(7,9). Cancer cells can take up both glucose
and glutamine more than normal cells (10). Increasing flux of glucose and
glutamine through the HBP leads to increased
O-GlcNAcylation. Enhanced level of O-GlcNAcylation is
observed in most cancers including colorectal cancer (11,12).
Growing evidence reveals that O-GlcNAcylation
has extensive crosstalk with phosphorylation either on the same or
adjacent sites of various proteins (13). At first glance, interplay between
O-GlcNAcylation and O-phosphorylation is proposed as
a yin-yang model with a shared modification site in which
O-GlcNAc on/off cycle is rapidly regulated in similar time
scale and cellular state as phosphorylation (13). Effects in several proteins are
reciprocal, while others are not (14). The interplay of these two
post-translational protein modifications can work simultaneously
and regulate protein function, stabilization, translocation,
complex formation and enzyme activity, subsequently affecting
cellular signaling pathways.
Previously, we showed that the levels of
O-GlcNAcylation and OGT were increased in primary breast and
colorectal cancer tissues and many proteins were selectively
modified by O-GlcNAc in both cancers (12,15).
In this study, we explored the interplay of O-GlcNAcylation
and phosphorylation by knocking down OGT gene in colorectal
cancer cell lines. Using a combination of proteomic approaches,
two-dimensional gel electrophoresis (2DE), O-GlcNAc and
serine phosphorylation immunoblotting, followed with LC-MS/MS
analysis, we identified a number of proteins associated with
aberrant O-GlcNAcylation and phosphorylation. In addition,
by using siOGT and OGA inhibitor approaches, we demonstrated that
reduction of O-GlcNAc led to decreased phosphorylation of
PKM2 whereas increasing O-GlcNAc gave the opposite results.
Furthermore, pyruvate kinase activity as well as expression levels
of PKM2 were modulated in response to alterations of global
O-GlcNAcylation levels in colorectal cancer cell lines.
Materials and methods
Cell culture, Thiamet-G, and siRNA
transfection
Cell culture medium and supplementary products were
purchased from Invitrogen. Normal colon epithelial cell, CCD841 CoN
(ATCC; American Type Culture Collection) was a gift from Dr
Jutamaad Satayavivad, Chulabhorn Research Institute, Thailand.
CCD841 CoN cells were cultured in Dulbecco's modified Eagle's
medium (DMEM) with high glucose and pyruvate supplemented with 10%
FBS and 1% L-glutamine. The human colorectal cancer cell lines,
HT29, SW480 and SW620 were purchased from ATCC. HT29 was cultured
in DMEM supplemented with 10% FBS. SW480 and SW620 were maintained
in RPMI-1640 medium supplemented with 10% FBS. All the cells were
supplemented with 1% penicillin/streptomycin and maintained at 37°C
in humidified 5% CO2 incubator. HT29 cells were treated
with Thiamet-G (TMG; Sigma), an inhibitor of OGA at 10 μM
for 48 h. Stealth siRNA targeting OGT gene was as follows:
sense, 5′-UAAUCAUUUCAAUAA CUGCUUCUGC-3′ and anti-sense,
5′-GCAGAAGCAGUUA UUGAAAUGAUUA-3′ (Invitrogen). Stealth scrambled
siRNA medium GC duplex (Invitrogen) was used as a negative control.
To transiently knockdown OGT, stealth siOGT or scrambled siRNA
(control) was transfected to HT29 cells for 72 h using
Lipofectamine™ 2000 (Invitrogen) according to manufacturer's
protocol.
Antibodies
Antibodies for O-GlcNAc, OGA, polyclonal PKM2
antibody were purchased from Abcam. Antibody for OGT was purchased
from Sigma. Monoclonal antibody for PKM2 was purchased from Santa
Cruz Biotechnology. Antibodies for phosphoserine and
phosphothreonine were purchased from Qiagen.
Preparation of cell lysates and western
blot analysis
Cell lysate extraction was prepared in RIPA buffer
as previously described (15).
Protein concentration was determined using Bradford assay.
Extracted proteins (10–30 μg) were separated in 10% SDS-PAGE
and transferred to PVDF membranes (Millipore). Blots were probed
with indicated antibodies. Bands on immunoblots were detected using
WesternBright ECL (Advansta) and chemiluminescent signals images
were captured using ImageQuant™ LAS 4000 digital imaging system (GE
Healthcare).
Two-dimensional gel electrophoresis and
western blot analysis
For two-dimensional isoelectric focusing (IEF) and
polyacrylamide gel electrophoresis (2D-IEF/PAGE), cells were lysed
in 2D lysis buffer and extracted proteins (100–150 μg) were
separated by IEF using Immobiline Drystrips (7 cm, pH 3–10,
non-linear; GE Healthcare). The 2DE was performed as previously
described (15). After
electrophoresis was finished, proteins in gels were transferred to
PVDF membranes. The membranes were stained by SYPRO Ruby (Thermo
Scientific) using the manufacturer's recommendations and the
signals were scanned using Ettan DIGE Imager (GE Healthcare) prior
to probing with O-GlcNAc and phosphoserine antibodies. In
order to normalize the O-GlcNAc and phosphoserine-modified
protein spots with SYPRO Ruby protein staining on 2DE, proteins
extracted from siOGT and siScramble cells were loaded equally. The
exposure times on Ettan DIGE Imager and chemiluminescence were
exactly the same.
In-gel digestion
Protein spots, in which phosphoserine levels were
altered in siOGT-knockdown HT29 cells and consistently observed in
three independent 2DE experiments, were excised and subjected to
in-gel trypsin digestion as previously described (16). Briefly, excised gels were destained
with 50% ACN in 0.1 M NH4HCO3, reduced with
10 mM DTT, alkylated in 100 mM iodoacetamide and digested with
trypsin (Promega) at 37°C overnight. The digested peptides were
dried and collected for protein identification by LC-MS/MS.
Protein identification by LC-MS/MS
The digested peptides were identified using Nanoflow
liquid chromatography coupled with the amaZon speed ion trap mass
spectrometry (Bruker, USA) as previously described (17). Protein identification was performed
using Mascot search engine with NCBInr version 20130630 sequence
databases (http://www.matrix-science.com). Search parameters were
set as follows: peptide mass tolerance was 1.2 Da, MS/MS ion mass
tolerance was 0.6 Da, allowance was set to 1 missed cleavage,
enzyme set as trypsin, the limit of peptide charges was
1+, 2+, and 3+. Proteins with
molecular weight and pI consistent to spot on 2DE gel were
considered positively identified.
Pyruvate kinase activity assay
The pyruvate kinase activity of cell lysates (5
μg) of HT29, SW480 and SW620 was determined according to the
manufacturer's protocol (BioVision). One unit of pyruvate kinase is
the amount of enzyme that transfers a phosphate group from PEP to
ADP, yielding 1.0 μmol of pyruvate/min at 25°C. The specific
activity of PKM2 was calculated using the pyruvate kinase activity
divided by the protein amount of PKM2 (immunoblot intensity).
Immunoprecipitation
Whole cell lysate (500 μg) was incubated with
3 μg of polyclonal PKM2 antibody with gentle end-over-end
mixing overnight at 4°C. The antibody-sample complex was incubated
with 20 μl of protein A/G agarose resin (Thermo Scientific)
with gentle end-over-end mixing for 2 h at 4°C. Unbound fraction
was discarded by centrifugation at 1,000 × g for 1 min. The agarose
resin was washed with TBS buffer three times and boiled in 2X SDS
sample buffer for 10 min. Eluent was collected by centrifugation
for 5 min at 1,000 × g.
Real-time RT-PCR
RNA was extracted from HT29 cells using RNeasy Mini
kit (Qiagen) following the manufacturer's instructions. To
synthesize first-strand cDNA, reverse transcription of 2 μg
of total RNA was catalyzed by SuperScript™ III Reverse
Transcriptase (Invitrogen). The primers used are PKM2 forward, GAG
GCC TCC TTC AAG TGC T and reverse, CCA GAC TTG GTG AGG ACG AT; and
PKM1 forward, ACC GCA AGC TGT TTG AAG AA and reverse, TCC ATG AGG
TCT GTG GAG TG. Real-time PCR was performed in StepOnePlus™
Real-Time PCR system (Applied Biosystems) using KAPA
SYBR®FAST qPCR kits (Kapa Biosystems). To compare the
relative mRNA expression of each treatment, 18S rRNA was used as an
internal control. The thermal profile was set as follows: an
initial incubation step at 95°C for 15 min, followed by 40 cycles
of denaturation at 95°C for 15 sec, annealing at 65°C for 30 sec,
and extension at 72°C for 30 sec, respectively. Mean threshold
cycle (CT) was calculated from triplicates. Data were analyzed
using 2−ΔCt method as previously described (18).
Statistical analysis
The statistical analysis was analyzed using unpaired
Student's t-test to test for the difference between two groups. The
statistical significance was defined at p≤0.05.
Results
Alteration of O-GlcNAcylation, OGT and
OGA levels in colorectal cancer cells
Immunoblotting with antibodies specific to
O-GlcNAc (RL2), OGT and OGA were used to determine the
levels of global O-GlcNAcylation, and O-GlcNAc
cycling enzymes in colorectal cancer cells, respectively.
O-GlcNAcylation, OGT and OGA levels were obviously increased
in HT29 cells in comparison to normal colon epithelia cells, CCD841
CoN (Fig. 1A). As OGT catalyzes an
addition of O-GlcNAc on target proteins observed in various
cancers, OGT transient-knockdown HT29 cells were established.
Transfection of siOGT remarkably reduced OGT and
O-GlcNAcylation levels when compared to siScramble control
(Fig. 1B). Furthermore, when a
potent OGA inhibitor, TMG, was used to decrease removal of the
O-GlcNAc molecule, global O-GlcNAcylation was
enhanced. O-GlcNAc level was clearly elevated in HT29 cells
treated with TMG (Fig. 1B).
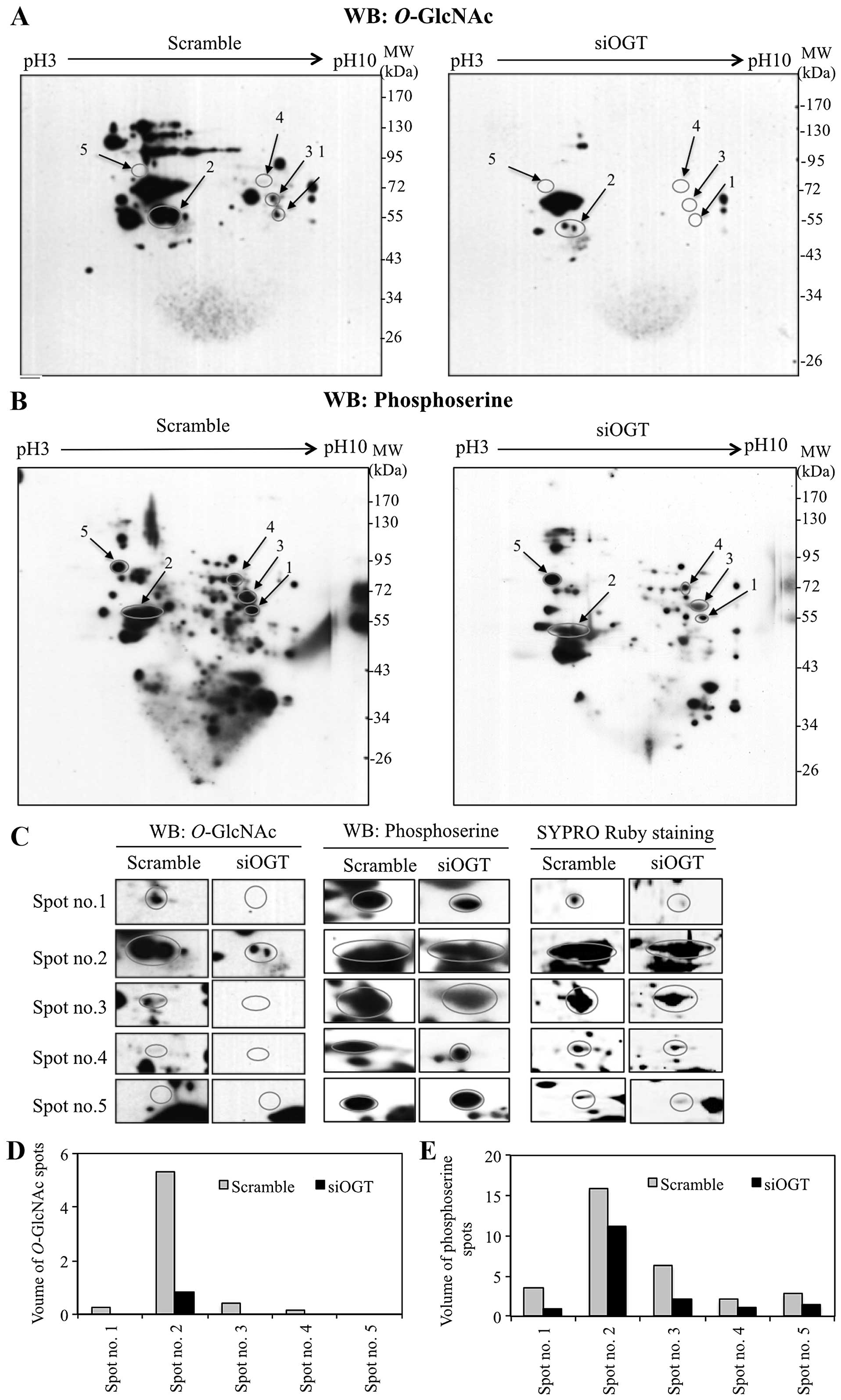
Interplay of O-GlcNAcylation and
phosphorylation in response to OGT transient-knockdown
Since O-GlcNAcylation is selective for serine
and/or threonine residues, changes in global O-GlcNAcylation
may influence the phosphorylation of various proteins. To
demonstrate an association between O-GlcNAcylation and
O-phosphorylation in colorectal cancer cells, 2DE was
performed on proteins isolated from OGT-knockdown and scrambled
siRNA-transfected HT29 cells, followed by immunoblotting with
antibodies specific to O-GlcNAc and phosphoserine. 2DE
immunoblots of siOGT-knockdown showed decreased
O-GlcNAcylation of various proteins compared to
siScramble-transfected cells (Fig.
2A). Interestingly, siOGT-knockdown resulted in reduction of
serine phosphorylation compared to scramble control cells (Fig. 2B). O-GlcNAc and phosphoserine
immunoblots were aligned to SYPRO Ruby staining blots and five
protein spots were clearly matched to the spots on immunoblots
(Fig. 2C). O-GlcNAc and
serine phosphorylation levels of five protein spots (spot no. 1–5)
were decreased or undetectable in siOGT-treated cells, when
compared to scramble control cells (Fig. 2D and E). These phosphorylated
protein spots were cut, digested with trypsin and subjected to
LC-MS/MS. Five proteins were identified and observed in three
independent experiments, as shown in Table I. Serine phosphorylation levels of
serine hydroxymethyltransferase (SHMT) (spot no. 1), cytokeratin-8
(spot no. 2), heterogeneous nuclear ribonucleo-protein L (HNRNPL)
(spot no. 4), and lamin-B1 (spot no. 5) were decreased in
siOGT-knockdown cells (Fig. 2B and
E). Four spots (spot no. 1–4) showed O-GlcNAc
modification on O-GlcNAc-2DE blots whereas O-GlcNAc
modification of lamin-B1 (spot no. 5) was not observed in either
scrambled or siOGT-knockdown HT29 cells (Fig. 2A and D). Notably, PKM2 (spot no. 3)
showed a remarkable reduction of O-GlcNAc as well as serine
phosphorylation in siOGT-knockdown HT29 cells but not in
threonine-phosphorylation (data not shown).
 | Table IThe identified
serine-phosphorylated-modified proteins in HT29 cells. |
Table I
The identified
serine-phosphorylated-modified proteins in HT29 cells.
| Volume of
phosphoserinea
| |
|---|
| Spot no. | Accession nos. | Protein names | MW/PI
(kDa/pI) | Peptide
matches | Coverage (%) | Scores | SC | siOGT |
Fold-changesb | Reports for
O-GlcNAcylation |
|---|
| 1 | gi|19923315 | Serine
hydroxymethyl-transferase, mitochondrial | 56414/8.76 | 38 | 45 | 489 | 3.49 | 0.903 | −3.86 | N/A |
| 2 | gi|181573 | Cytokeratin-8 | 53529/5.52 | 19 | 33 | 623 | 15.9 | 11.2 | −1.42 | Chou et al
(28) |
| 3 | gi|189998 | M2-type pyruvate
kinase | 58447/7.95 | 19 | 37 | 440 | 6.29 | 2.16 | −2.91 | Champattanachai
et al (15) |
| 4 | gi|119577231 | Heterogeneous
nuclear ribonucleoprotein L | 64720/8.46 | 16 | 21 | 160 | 2.09 | 1.11 | −1.88 | N/A |
| 5 | gi|5031877 | Lamin-B1 isoform
1 | 66653/5.11 | 36 | 38 | 537 | 2.82 | 1.54 | −1.83 | N/A |
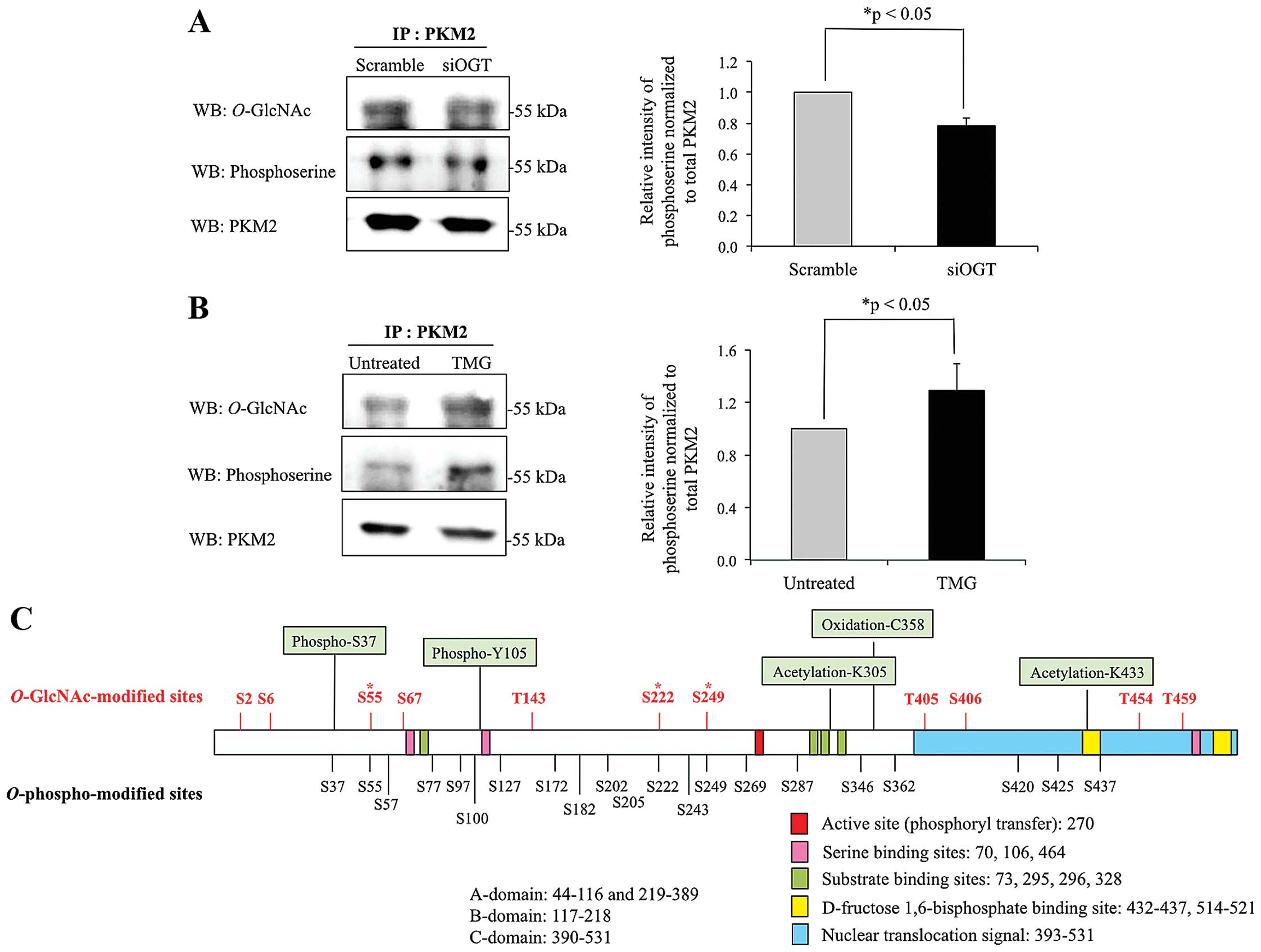
Association between O-GlcNAcylation and
serine phosphor-ylation of PKM2
According to the results from 2DE, PKM2 was
O-GlcNAcylated in HT29 cells, but the level of
O-GlcNAc was reduced in cells with siOGT knocked down. To
confirm whether PKM2 is O-GlcNAcylated, PKM2 from controls,
siOGT-knockdown and TMG-treated HT29 cell lysates were
immunoprecipitated, separated on SDS-PAGE and probed with antibody
specific to O-GlcNAc. The results showed that
O-GlcNAcylation of PKM2 was reduced in siOGT-knockdown cells
but increased in TMG-treated cells (Fig. 3A and B). We further verified an
association between O-GlcNAcylation and phosphorylation of
pulled down PKM2. After siOGT knockdown, decreased
O-GlcNAcylation resulted in lower O-GlcNAcylated and
serine phosphorylated PKM2, while upon treatment with TMG,
increased O-GlcNAcylation significantly enhanced serine
phosphorylation of PKM2 (Fig. 3A and
B). O-GlcNAc sites of PKM2 were computationally
predicted using two available websites including YinOyang and
O-GlcNAcScan (http://www.cbs.dtu.dk/services/YinOyang/ and
http://cbsb.lombardi.georgetown.edu/hulab/OGAP.html).
The combination of O-GlcNAc sites from the two programs are
shown in Fig. 3C. YinOYang
predicted seven O-GlcNAc sites, which are Ser2, Ser6,
Ser222, Thr405, Ser406, Thr454 and Thr459, while
O-GlcNAcScan also predicted seven sites including Ser6,
Ser55, Ser67, Thr143, Ser249, Ser406 and Thr454. Shared possible
O-GlcNAc sites from the two programs are Ser6, Ser222,
Ser406 and Thr454. Together, eleven O-GlcNAc-modified sites
were predicted and found in various parts throughout the PKM2
structure. Serine phosphorylated sites of PKM2 (data from
PhosphoSitePlus database) are also shown in Fig. 3C, with only three sites, Ser55,
Ser222 and Ser249 having potential to be competitively occupied by
O-GlcNAc or O-phospho modification.
O-GlcNAcylation regulates expression
levels and pyruvate kinase activity of PKM2
The mRNA expression levels of PKM1 and PKM2 were
determined using real-time RT-PCR, with results showing that PKM2
mRNA expression level was >10-fold higher in comparison to PKM1
mRNA level in HT29 cells (Fig. 4A).
This indicated that the expression of PKM2 predominates in cancer
cells. To elucidate role of O-GlcNAcylation on PKM2 gene
expression level, the expression levels of PKM2 gene were
determined in siOGT-knockdown HT29 cells, compared to
scramble-treated cells, and in TMG-treated cells compared to
untreated cells. PKM2 expression level was significantly
downregulated in siOGT-knockdown cells but upregulated in
TMG-treated cells (Fig. 4A).
Consistent with the real-time RT-PCR results, immunoblots of PKM2
showed alteration in expression levels of PKM2, in harmony with
levels of O-GlcNAcylation of PKM2 (Fig. 4B). In addition, pyruvate kinase (PK)
activity of whole cell lysates of siOGT-knockdown and TMG-treated
HT29 cells were measured as shown in Fig. 4C. Lower PK activity was observed in
both siOGT-knockdown and TMG-treated HT29 cells compared to their
controls. However, since global O-GlcNAcylation levels
regulated expression of PKM2 enzyme, the ratio of PK activity to
PKM2 expression levels detected by immunoblotting was calculated as
PKM2-specific activity shown in Fig.
4D. PKM2-specific activity was significantly higher in
siOGT-knockdown than scramble-treated HT29 cells. However, the
opposite result was observed in TMG-treated HT29 cells compared to
untreated controls.
Differential level and activity of
O-GlcNAc-modified PKM2 in colorectal cancer cell lines originated
from primary and metastatic sites
Further study was performed on O-GlcNAc
modification of PKM2 in primary (non-metastatic) SW480 and
metastatic SW620 colorectal cancer cells. Western blotting of whole
cell lysates of SW480 and SW620 cells did not show major
differences in global O-GlcNAcylation, OGT and OGA enzyme
levels (Fig. 5A). However,
immunoblotting for PKM2 showed that the expression level of PKM2
was significantly enhanced in SW620 cells compared to SW480 cells
(Fig. 5B). Assay of PKM2 enzyme
activity demonstrated that the SW480 and SW620 cells did not differ
significantly (Fig. 5C). However,
due to the higher level of PKM2 protein in SW620 cells compared to
SW480 cells (Fig. 5B), the specific
activity of PKM2 in SW620 appears to be much lower than in SW480
cells (Fig. 5D). To estimate the
extent of O-GlcNAc modification, PKM2 was immunoprecipitated
from whole cell lysates, and interestingly, the level of
O-GlcNAc modification observed in pulled down PKM2 tended to
increase in SW620 cells compared to SW480 cells (Fig. 5E).
Discussion
The increase in O-GlcNAcylation in various
cancers is well known, and correlates with the increase in glucose
and glutamine uptake in cancer cells, which allows cancer cells to
utilize available nutrients and accumulate cellular
O-GlcNAc. Previous study has shown that
O-GlcNAcylation level was globally increased in colon cancer
in comparison to adjacent benign tissues (11,12).
Here, we demonstrate that levels of O-GlcNAcylation, OGT and
OGA were notably increased in the colorectal cancer cell line,
HT29, while less change was found in a normal colorectal epithelium
cell, CCD841 Con (Fig. 1A).
However, basal levels of O-GlcNAcylation and its cycling
enzymes, OGT and OGA, did not differ between the colorectal cancer
cell lines originating from primary (SW480) and metastatic sites
(SW620) of the same patient (Fig.
5A). Thus, the role of O-GlcNAcylation in modulating
complex signaling pathways in cancer still needs to be
understood.
Apart from the potential roles of
O-GlcNAcylation in regulating myriad signaling pathways,
crosstalk between O-GlcNAcylation and phosphorylation has
also emerged as a significant fine-tuning regulatory mechanism in
various diseases including diabetes, Alzheimer's and cancer
(13). We therefore interfered with
the basal level of O-GlcNAcylation by knocking down the
OGT gene or inhibiting OGA activity using the potent
inhibitor TMG, and observed the subsequent phosphorylation
profiles. The results from 2DE immunoblots indicated that the
intensity of serine phosphorylation was significantly decreased in
at least 5 protein spots including SHMT, cytokeratin-8, PKM2,
HNRNPL and lamin-B1 upon decrease of global O-GlcNAcylation
(Fig. 2). This observation suggests
that the 'yin-yang' model, in which the two modifications compete
for the same or proximal sites (19), is inadequate. Consistent with our
findings, globally increased O-GlcNAcylation in NIH-3T3
cells by inhibition of OGA induced an unexpected elevation of
phosphorylation at 149 sites (~18% of phosphosites), caused no
alteration in ~48% of phosphosites and decreased phosphorylation at
280 sites (~33% of phosphosites) from 1,564 identified proteins
(20). Thus, it appears that
crosstalk between O-GlcNAcylation and phosphorylation is not
exclusively antagonistic, but can lead to a synergistic outcome. A
possible explanation is that each modification may regulate the
cycling enzymes of the other modifications. Indeed, OGT itself is
phosphorylated at Tyr979, although a well-defined function of
phosphorylation on OGT activity is still awaited (13). Furthermore, O-GlcNAcylation
has been reported to regulate the activities of some kinases
including Ca2+/calmodulin-dependent protein kinase Iv
(CaMKIV) (21), Akt (22) and PKC (23). Dias et al also studied a wide
range of kinases and revealed 42 kinases which acted as substrates
for OGT (14).
SHMT is a key one-carbon donor required for de
novo nucleotide biosynthesis and DNA methylation (24,25).
Two isoforms of SHMT, SHMT1 and SHMT2, were found as cytosolic and
mitochondrial isoforms, respectively. However, only the
mitochondrial isoform SHMT2, and not the cytosolic isoform, has a
significant impact on cancer cell proliferation (26). Here, we found decreased
phosphorylation of SHMT2, when global O-GlcNAcylation was
decreased (Fig. 2 and Table I). However, there is still no
information on the role of serine phosphorylation of SHMT2.
According to 2DE immunoblots, SHMT2 is also modified by
O-GlcNAcylation in HT29 cells. Since SHMT2 is a crucial
enzyme in mitochondrial glycine synthesis pathway, further study on
regulation of SHMT2 by O-GlcNAcylation would be of
interest.
Cytokeratin-8 or keratin-8 (CK8) is an intermediate
filament protein in epithelial cells and classified as a
basic-to-neutral type II keratin (27). Chou et al first reported
O-GlcNAc-modification of CK8 in 1992 (28). Cellular CK8 exists in two forms,
which are the soluble and filamentous forms, respectively. Higher
O-GlcNAcylation in conjunction with enhanced serine
phosphorylation of CK8 was notably found in the soluble fraction of
immortalized Chang cells and heat stress-induced HepG2 cells
(29–31). Our finding of synergistic effect of
these two PTMs is therefore inconsistent with previous studies.
HNRNPL is an RNA-binding protein, which exhibits a
binding preference for CA-repeat and CA-rich RNA elements (32). HNRNPL has been reported as a
substrate for CaMKIV (33). The
Ser513 phosphorylated site of HNRNPL is crucial for an interaction
of HNRNPL with the CaMKIV-responsive RNA element 1 of stress
axis-regulated exon of the Slo1 mRNA that governs splicing
patterns and provides composition of potassium channels. Here, we
demonstrated that HNRNPL is potentially O-GlcNAc-modified in
HT29 cells (Fig. 2 and Table I). Moreover, O-GlcNAcylation
of many HNRNP family members was reported in cancer tissues,
suggesting the role of O-GlcNAcylation in tumorigenesis
(15).
Lamin-B1 (LB1) is a main nuclear structural protein
that has important roles in the regulation of various processes
taking place in nucleus including DNA, RNA processes and chromatin
remodeling (34). Nuclear PKC
catalyzes phosphorylation of LB1, which subsequently induces cell
cycle progression through mitosis (35,36).
However, we did not observe O-GlcNAcylation of LB1 on the
2DE blot from HT29 whole cell lysates. Subcellular enrichment of
nuclear fraction may enhance detection (Fig. 2 and Table I).
In HT29 cells, we showed that serine phosphorylation
level of PKM2 was modulated owing to changes of global
O-GlcNAcylation level (Fig. 3A
and B). We also examined the effect of O-GlcNAcylation
on pyruvate kinase activity. The results showed that PK activity of
PKM2 was decreased when O-GlcNAcylation was increased by
inhibiting OGA enzyme (Fig. 4).
This is consistent with a study from yi et al that PK
activity was modestly decreased in PUGNAc-treated and
OGT-overexpressing 293T cells (37). Furthermore, OGT knock- down in HT29
cells also caused a significantly higher PKM2-specific activity. In
SW620 cells, higher O-GlcNAcylation of PKM2 was observed, in
conjunction with lower PKM2-specific activity, when compared to
SW480 cells (Fig. 5). As shown in
Fig. 3C, the predicted
O-GlcNAc-modification sites are located in various regions
throughout the PKM2 structure, so regulation of PK activity by
interfering with the tetramer to dimer ratio or blocking allosteric
regulation may occur (38,39). PKM2 may be modified by various PTMs,
including phosphorylation, acetylation and oxidation as shown in
Fig. 3C, which may regulate its
catalytic activity.
Furthermore, one of the most striking observations
of this study is the role of O-GlcNAcylation in regulating
PKM2 expression, both at mRNA and protein levels (Fig. 4). The expression of PKM2 was
modulated in consonance with levels of O-GlcNAcylation.
Interestingly, it has been previously reported that overexpression
of hepatic forkhead box O1 (FoxO1) decreased an expression level of
liver pyruvate kinase (PKL) through suppression
O-GlcNAcylation of carbohydrate response element binding
protein (Chrebp) (40).
O-GlcNAcylation stabilized Chrebp by inhibiting
ubiquitination that enhanced the recruitment of Chrebp to PKL
promoter. PKM2 expression is also regulated by SP1 transcription
factor (41). In high glucose
environment, dephosphorylation of SP1 induces binding of SP1, thus
increasing PKM2 expression level (41). Interestingly, Jackson and Tjian
reported O-GlcNAcylation of SP1 (42). Moreover, reciprocal interplay
between O-GlcNAcylation and phosphorylation of SP1 was
observed in HT29 cells as PUGNAc-treated HT29 cells exhibited a
reduction of phosphorylation on SP1 (43). It is likely that elevated
O-GlcNAcylation enhances binding activity of SP1 to PKM2
promoter leading to upregulation of PKM2 expression. Therefore,
understanding the mechanism of O-GlcNAcylation in regulating
PK activity and the expression profile of PKM2 is still a
challenge.
In conclusion, we have explored the interplay of
O-GlcNAcylation and serine phosphorylation by knocking down
OGT gene in colorectal cancer cell lines. Decreasing
O-GlcNAc levels led to a reduction of serine phosphorylation
of many proteins. Using the combination of 2DE O-GlcNAc and
phospho-immunoblotting, followed by LC-MS/MS analysis, we found
that PKM2 was modified by O-GlcNAc and phospho-serine.
Importantly, an alteration of global O-GlcNAcylation
affected levels of serine-phosphorylation on PKM2 that may relate
to kinase activity of PKM2. Here, we demonstrated that lower global
O-GlcNAcylation led to decreased PKM2 expression, but
induced a higher PKM2-specific activity, while increasing
O-GlcNAcylation by TMG treatment had the opposite result. In
addition, the metastatic colorectal cancer SW620 cells were found
to have more O-GlcNAc-PKM2 and exhibited lower PKM2-specific
activity in comparison to the non-metastatic colorectal cancer
SW480 cells. Taken together, these data suggest that
O-GlcNAcylation of PKM2, at least in part, controls PKM2
activity and expression, thereby regulating the glycolytic pathway
to facilitate the Warburg effect that is beneficial for
bioenergetics and biosynthesis of cancer cells.
Acknowledgments
This study was supported by the Chulabhorn Research
Institute (CRI), the Chulabhorn Graduate Institute (CGI), and the
Center of Excellence on Environmental Health and Toxicology
(EHT).
Abbreviations:
|
2DE
|
two-dimensional gel
electrophoresis
|
|
CaMKIV
|
Ca2+/calmodulin-dependent
protein kinase IV
|
|
CK8
|
cytokeratin-8 or keratin-8
|
|
FBP
|
fructose 1,6-bisphosphate
|
|
G6P
|
glucose-6-phosphate
|
|
HBP
|
hexosamine biosynthesis pathway
|
|
HNRNPL
|
heterogeneous nuclear
ribonucleoprotein L
|
|
IEF
|
isoelectric focusing
|
|
LB1
|
lamin-B1
|
|
O-GlcNAc
|
O-linked
N-acetyl-D-glucosamine
|
|
OGA
|
O-GlcNAcase
|
|
OGT
|
O-GlcNAc transferase
|
|
PEP
|
phosphoenolpyruvate
|
|
PK
|
pyruvate kinase
|
|
PKCδ
|
protein kinase C δ
|
|
PKM2
|
pyruvate kinase M2
|
|
SHMT
|
serine hydroxymethyltransferase
|
|
TMG
|
Thiamet-G
|
|
UDP-GlcNAc
|
uridine
diphospho-N-acetylglucosamine
|
References
|
1
|
Wong N, Ojo D, Yan J and Tang D: PKM2
contributes to cancer metabolism. Cancer Lett. 356:184–191. 2015.
View Article : Google Scholar
|
|
2
|
Tamada M, Suematsu M and Saya H: Pyruvate
kinase M2: Multiple faces for conferring benefits on cancer cells.
Clin Cancer Res. 18:5554–5561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Christofk HR, Vander Heiden MG, Harris MH,
Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL and
Cantley LC: The M2 splice isoform of pyruvate kinase is important
for cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang W and Lu Z: Regulation and function
of pyruvate kinase M2 in cancer. Cancer Lett. 339:153–158. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mazurek S, Boschek CB, Hugo F and
Eigenbrodt E: Pyruvate kinase type M2 and its role in tumor growth
and spreading. Semin Cancer Biol. 15:300–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanover JA, Krause MW and Love DC: The
hexosamine signaling pathway: O-GlcNAc cycling in feast or famine.
Biochim Biophys Acta. 1800:80–95. 2010. View Article : Google Scholar :
|
|
8
|
Lazarus MB, Nam Y, Jiang J, Sliz P and
Walker S: Structure of human O-GlcNAc transferase and its complex
with a peptide substrate. Nature. 469:564–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chaiyawat P, Netsirisawan P, Svasti J and
Champattanachai V: Aberrant O-GlcNAcylated proteins: New
perspectives in breast and colorectal cancer. Front Endocrinol
(Lausanne). 5:1932014.
|
|
10
|
Willems L, Jacque N, Jacquel A, Neveux N,
Maciel TT, Lambert M, Schmitt A, Poulain L, Green AS, Uzunov M, et
al: Inhibiting glutamine uptake represents an attractive new
strategy for treating acute myeloid leukemia. Blood. 122:3521–3532.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X,
Cong Q and Yu W: O-GlcNAcylation is a novel regulator of lung and
colon cancer malignancy. Biochim Biophys Acta. 1812:514–519. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Phueaouan T, Chaiyawat P, Netsirisawan P,
Chokchaichamnankit D, Punyarit P, Srisomsap C, Svasti J and
Champattanachai V: Aberrant O-GlcNAc-modified proteins expressed in
primary colorectal cancer. Oncol Rep. 30:2929–2936. 2013.PubMed/NCBI
|
|
13
|
Butkinaree C, Park K and Hart GW: O-linked
beta-N-acetylglu-cosamine (O-GlcNAc): Extensive crosstalk with
phosphorylation to regulate signaling and transcription in response
to nutrients and stress. Biochim Biophys Acta. 1800:96–106. 2010.
View Article : Google Scholar
|
|
14
|
Dias WB, Cheung WD and Hart GW:
O-GlcNAcylation of kinases. Biochem Biophys Res Commun.
422:224–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Champattanachai V, Netsirisawan P,
Chaiyawat P, Phueaouan T, Charoenwattanasatien R,
Chokchaichamnankit D, Punyarit P, Srisomsap C and Svasti J:
Proteomic analysis and abrogated expression of O-GlcNAcylated
proteins associated with primary breast cancer. Proteomics.
13:2088–2099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Srisomsap C, Sawangareetrakul P,
Subhasitanont P, Panichakul T, Keeratichamroen S, Lirdprapamongkol
K, Chokchaichamnankit D, Sirisinha S and Svasti J: Proteomic
analysis of cholangiocarcinoma cell line. Proteomics. 4:1135–1144.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tit-Oon P, Chokchaichamnankit D,
Khongmanee A, Sawangareetrakul P, Svasti J and Srisomsap C:
Comparative secretome analysis of cholangiocarcinoma cell line in
three-dimensional culture. Int J Oncol. 45:2108–2116.
2014.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Hu P, Shimoji S and Hart GW: Site-specific
interplay between O-GlcNAcylation and phosphorylation in cellular
regulation. FEBS Lett. 584:2526–2538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Gucek M and Hart GW: Cross-talk
between GlcNAcylation and phosphorylation: Site-specific
phosphorylation dynamics in response to globally elevated O-GlcNAc.
Proc Natl Acad Sci USA. 105:13793–13798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dias WB, Cheung WD, Wang Z and Hart GW:
Regulation of calcium/calmodulin-dependent kinase IV by O-GlcNAc
modification. J Biol Chem. 284:21327–21337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo B, Soesanto Y and McClain DA: Protein
modification by O-linked GlcNAc reduces angiogenesis by inhibiting
Akt activity in endothelial cells. Arterioscler Thromb Vasc Biol.
28:651–657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robles-Flores M, Meléndez L, García W,
Mendoza-Hernández G, Lam TT, Castañeda-Patlán C and
González-Aguilar H: Post-translational modifications on protein
kinase c isozymes. Effects of epinephrine and phorbol esters.
Biochim Biophys Acta. 1783:695–712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Renwick SB, Snell K and Baumann U: The
crystal structure of human cytosolic serine
hydroxymethyltransferase: A target for cancer chemotherapy.
Structure. 6:1105–1116. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Amelio I, Cutruzzolá F, Antonov A,
Agostini M and Melino G: Serine and glycine metabolism in cancer.
Trends Biochem Sci. 39:191–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jain M, Nilsson R, Sharma S, Madhusudhan
N, Kitami T, Souza AL, Kafri R, Kirschner MW, Clish CB and Mootha
VK: Metabolite profiling identifies a key role for glycine in rapid
cancer cell proliferation. Science. 336:1040–1044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schweizer J, Bowden PE, Coulombe PA,
Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DA,
Rogers MA, et al: New consensus nomenclature for mammalian
keratins. J Cell Biol. 174:169–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chou CF, Smith AJ and Omary MB:
Characterization and dynamics of O-linked glycosylation of human
cytokeratin 8 and 18. J Biol Chem. 267:3901–3906. 1992.PubMed/NCBI
|
|
29
|
Owens DW and Lane EB: The quest for the
function of simple epithelial keratins. Bioessays. 25:748–758.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Omary MB, Ku NO, Liao J and Price D:
Keratin modifications and solubility properties in epithelial cells
and in vitro. Subcell Biochem. 31:105–140. 1998.
|
|
31
|
Srikanth B, Vaidya MM and Kalraiya RD:
O-GlcNAcylation determines the solubility, filament organization,
and stability of keratins 8 and 18. J Biol Chem. 285:34062–34071.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rossbach O, Hung LH, Khrameeva E,
Schreiner S, König J, Curk T, Zupan B, Ule J, Gelfand MS and
Bindereif A: Cross-linking-immunoprecipitation (iCLIP) analysis
reveals global regulatory roles of hnRNP L. RNA Biol. 11:146–155.
2014. View Article : Google Scholar :
|
|
33
|
Liu G, Razanau A, Hai Y, Yu J, Sohail M,
Lobo VG, Chu J, Kung SK and Xie J: A conserved serine of
heterogeneous nuclear ribonucleoprotein L (hnRNP L) mediates
depolarization-regulated alternative splicing of potassium
channels. J Biol Chem. 287:22709–22716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shimi T, Butin-Israeli V, Adam SA,
Hamanaka RB, Goldman AE, Lucas CA, Shumaker DK, Kosak ST, Chandel
NS and Goldman RD: The role of nuclear lamin B1 in cell
proliferation and senescence. Genes Dev. 25:2579–2593. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hocevar BA, Burns DJ and Fields AP:
Identification of protein kinase C (PKC) phosphorylation sites on
human lamin B. Potential role of PKC in nuclear lamina structural
dynamics. J Biol Chem. 268:7545–7552. 1993.PubMed/NCBI
|
|
36
|
Fiume R, Ramazzotti G, Teti G, Chiarini F,
Faenza I, Mazzotti G, Billi AM and Cocco L: Involvement of nuclear
PLCbeta1 in lamin B1 phosphorylation and G2/M cell cycle
progression. FASEB J. 23:957–966. 2009. View Article : Google Scholar
|
|
37
|
Yi W, Clark PM, Mason DE, Keenan MC, Hill
C, Goddard WA III, Peters EC, Driggers EM and Hsieh-Wilson LC:
Phosphofructokinase 1 glycosylation regulates cell growth and
metabolism. Science. 337:975–980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dombrauckas JD, Santarsiero BD and Mesecar
AD: Structural basis for tumor pyruvate kinase M2 allosteric
regulation and catalysis. Biochemistry. 44:9417–9429. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mazurek S: Pyruvate kinase type M2: A key
regulator of the metabolic budget system in tumor cells. Int J
Biochem Cell Biol. 43:969–980. 2011. View Article : Google Scholar
|
|
40
|
Ido-Kitamura Y, Sasaki T, Kobayashi M, Kim
HJ, Lee YS, Kikuchi O, Yokota-Hashimoto H, Iizuka K, Accili D and
Kitamura T: Hepatic FoxO1 integrates glucose utilization and lipid
synthesis through regulation of Chrebp O-glycosylation. PLoS One.
7:e472312012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schäfer D, Hamm-Künzelmann B and Brand K:
Glucose regulates the promoter activity of aldolase A and pyruvate
kinase M2 via dephosphorylation of Sp1. FEBS Lett. 417:325–328.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jackson SP and Tjian R: O-glycosylation of
eukaryotic transcription factors: Implications for mechanisms of
transcriptional regulation. Cell. 55:125–133. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Haltiwanger RS, Grove K and Philipsberg
GA: Modulation of O-linked N-acetylglucosamine levels on nuclear
and cytoplasmic proteins in vivo using the peptide
O-GlcNAc-beta-N-acetylglucosaminidase inhibitor
O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-
phenylcarbamate. J Biol Chem. 273:3611–3617. 1998. View Article : Google Scholar : PubMed/NCBI
|