Introduction
Osteosarcoma is the most common primary malignant
tumor of the bone. Remarkable advances in the treatment of
osteosarcoma have been made in the past 2–3 decades. These include
the introduction of adjuvant chemotherapy and appropriate surgical
excision (1,2). However, there have also been advances
in the field of immunotherapy for osteosarcoma that have received
less attention (3,4). We developed a method using dendritic
cells (DCs) to enhance tumor-specific immunoreactions based on the
premise that DCs are the main antigen-presenting cells initiating
cell-mediated immune responses in vivo (5). Our current strategy involves
eliminating immunosuppressive factors such as regulatory T cells
(Tregs) and enhancing cell-mediated immunity.
The glucocorticoid-induced tumor necrosis factor
receptor (GITR) family-related protein is constitutively expressed
at high levels on Tregs and presented ubiquitously at lower levels
on various immune subsets including cytotoxic T lymphocytes (CTLs)
(6,7). GITR ligation provides a costimulatory
signal that enhances CD4+ and CD8+ T cell
proliferation and effector functions, particularly in the context
of suboptimal T cell receptor stimulation (8,9).
Signaling through GITR, using agonist anti-GITR antibodies or GITR
ligands abrogates the suppressive effects of Tregs (7,10) and
enhances T cell responses (6,8,9,11).
Administration of agonist anti-GITR antibodies promotes the
activation of CTLs, and interferon (IFN)-γ is reportedly required
for the antitumor response induced by anti-GITR antibodies
(12,13). Recently, several studies showed that
in vivo GITR ligation by using anti-GITR antibodies can
augment antitumor T cell responses and induce tumor rejection
(14–16). However, the efficacy of the
combination of tumor lysate-pulsed DCs and agonist anti-GITR
antibodies in an osteosarcoma model has not been evaluated.
Therefore, we hypothesized that an antitumor effect may be
triggered if Tregs are controlled, resulting in the activation of
CTLs and inhibition of tumor growth.
We investigated how immunotherapies that target the
inhibitory pathways of Tregs using anti-GITR-mAbs can potentially
synergize the effects of cryotreated tumor lysate-pulsed DCs to
generate systemic antitumor immunity. We verify that, in contrast
to tumor lysate-pulsed DC or anti-GITR-Ab treatment alone, the
combination therapy enhanced antitumor immunity and slowed the
growth.
Materials and methods
Cell line
LM8 cells, derived from Dunn osteosarcoma, were
provided by the Riken BioResource Center (Saitama, Japan). The
cells were maintained in complete medium consisting of RPMI-1640
supplemented with 10% heat-inactivated fetal bovine serum, 100
μg/ml streptomycin and 100 U/ml penicillin. Cells were
cultured at 37°C in 5% CO2.
A total of 1×106 LM8 cells (a murine
osteosarcoma cell line) was hypodermically implanted into the
subcutaneous gluteal region of 20 female C3H mice 6–8 weeks old. We
purchased the C3H mice from Sankyo Labo Inc. (Toyama, Japan) and
housed them in a specific pathogen-free animal facility in our
laboratory.
DC generation
Bone marrow-derived DCs were generated as described
by Lutz and Rössner (17) with
minor modifications (5). Two weeks
after tumor inoculation, we resected the primary tumor lesion and
soaked the entire tumor in liquid nitrogen to kill the tumor cells.
The freeze-thawed tumor lysate was added to the DC cultures on day
6 at a ratio of five DC equivalents to one tumor cell (i.e., 5:1)
and incubated at 37°C in an atmosphere containing 50 ml
CO2 per liter. The homogenate was passed through a
0.2-μm filter to remove bacteria and tissues and mixed with
the DCs for 24 h. After 24 h of incubation, non-adherent cells
including DCs were harvested by gentle pipetting.
Antibody administration
Mice received 0.5 mg/mouse of agonistic
affinity-purified anti-GITR monoclonal antibody (rat anti-mouse
IgG; BioExpress). The control antibody is monoclonal antibody IgG
(rat anti-mouse IgG, isotype control antibodies, 0.5 mg/mouse).
Study design
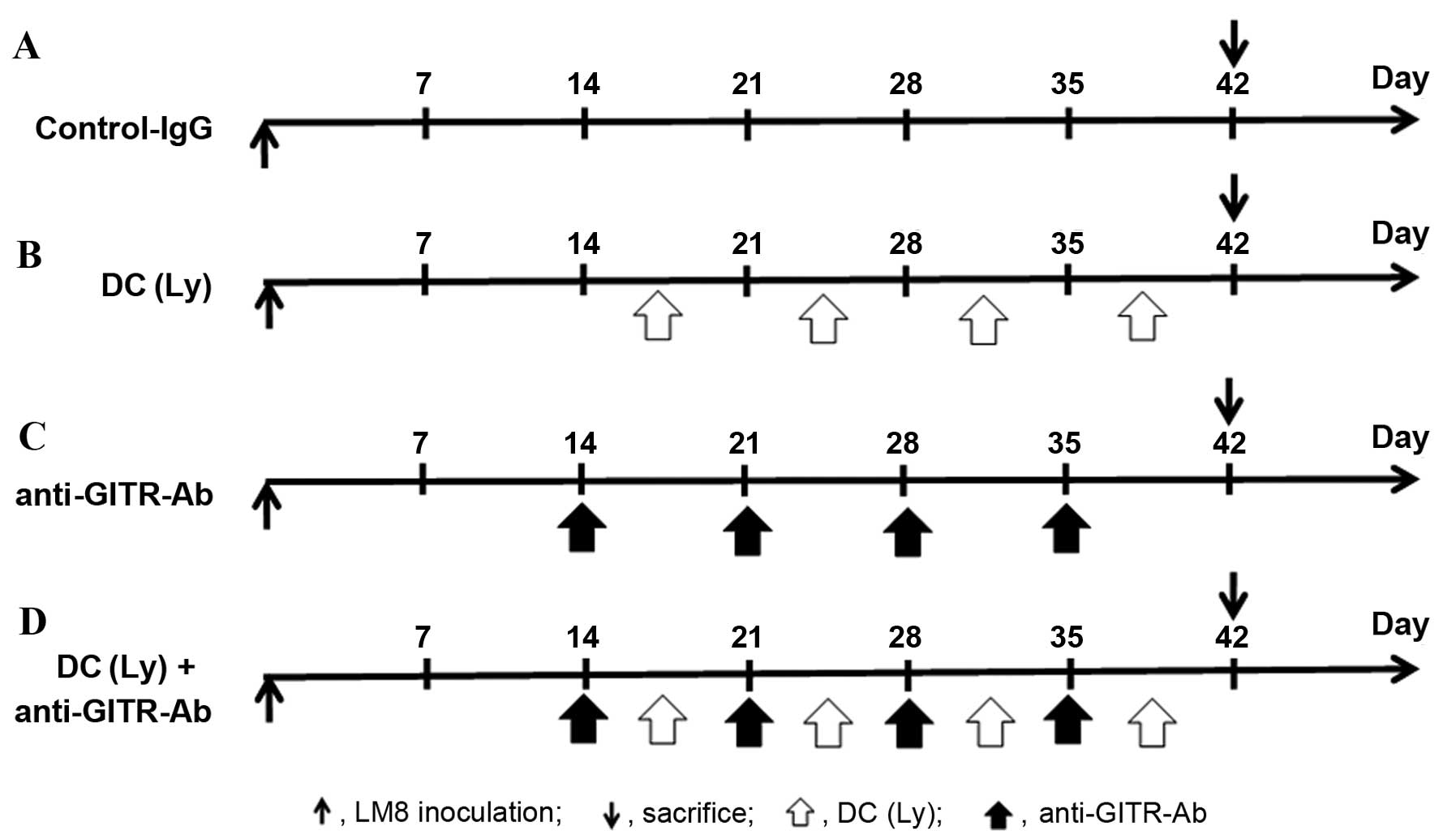
All the animals developed tumors. The following 4
groups were established (Fig. 1):
i), control IgG (control, n=5); ii), DCs exposed to cryotreated
tumor lysates were injected twice a week into the subcutaneous
contralateral gluteal region [DC(Ly), n=5]; iii), intraperitoneal
injection of agonist anti-GITR antibody was performed twice per
week (anti-GITR-Ab, n=5); and iv), DCs exposed to cryotreated tumor
lysates and injected twice a week into the subcutaneous
contra-lateral gluteal region and intraperitoneal injection of
agonist anti-GITR antibody was performed twice per week [DC(Ly) +
anti-GITR-Ab, n=5]. All experiments were performed under the
guidelines for animal experiments as stipulated by the Oita
University Graduate School of Medical Science. Tumor size was
measured in 2 perpendicular dimensions parallel with the surface
and the depth of the tumor in mice using a caliper.
Flow cytometry
The markers Foxp3 and CD4, which are expressed on
the surface of Tregs, were counted with a FACSVerse™ flow cytometer
(Becton-Dickinson, San Jose, CA, USA) and stained them with
fluorochrome-conjugated antibody (BD Pharmingen, Tokyo, Japan) for
the following markers: phycoerythrin (PE)-conjugated anti-mouse
Foxp3 staining kit (eBioscience, San Diego, CA, USA) and
fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD4
(clone, RM4-5; BD Pharmingen). Data analysis was performed with
FACSuite™ software (Becton-Dickinson).
Immunohistofluorescence
Immunohistochemistry was used to measure the levels
of Foxp3, a marker of Tregs, and CD8, a marker of CTLs, inside
primary tumor lesions. Lung specimens were fixed in frozen section.
Five samples per mouse were cut into 15-μm-thick slices.
Rehydrated tissue sections were incubated with primary Abs against
CD8+ (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and
Foxp3 (Abcam, Cambridge, MA, USA) diluted at 1:200 in Ab Diluent
(Dako ChemMate, Dako, Japan) overnight at room temperature. For
CD8+ staining with FITC donkey anti-rabbit IgG and
Foxp3+ staining with Texas red goat anti-rat IgG
(Invitrogen, Carlsbad, CA, USA), secondary antibodies were diluted
at 1:300 in Ab Diluent and added for 60 min at room temperature in
the dark. Digital images were taken on a BIOREVO microscope
equipped with a confocal microscopy system (BZ-9000; Keyence,
Japan).
ELISA
We measured murine IFN-γ and IL-10 release by
enzyme-linked immunosorbent assay using Quantikine®
(R&D Systems, Minneapolis, MN, USA) according to the
manufacturer's instructions using a Skanlt for Multiskan FC
microplate reader (Thermo Fisher Scientific, Tokyo, Japan).
Western blot analysis
Tumor tissue was dissected and washed briefly with
chilled PBS, then cut into smaller pieces whilst keeping on ice.
The tissue was placed in a homogenizer adding RIPA buffer (500
μl per 10 mg of tissue) with protease inhibitor. Tissue was
homogenized thoroughly and kept on ice for 30 min. Total cellular
protein (15 μg) was resolved on a precast 10% Tris-HCl
Criterion 10-well gel (Bio-Rad) at 200 V (300 mAmp) for 30 min. The
gel was wet-transferred to a PVDF membrane for 1 h, and blocked
with PBST containing 5% instant dry non-fat milk for 30 min at room
temperature. Antibodies against transforming growth factor (TGF)-β
(#3711) was obtained from Cell Signaling Technology (Tokyo, Japan);
IL-10 (sc-7888) was obtained from Santa Cruz Biotechnology (Dallas,
TX, USA); and IL-6 (ab6672) and β-actin (ab16039) were from Abcam
(Cambridge, UK). Immunocomplexes were visualized with horseradish
peroxidase-conjugated anti-rabbit immunoglobulin G antibodies, and
developed using ECL Plus system with a ChemiDoc camera (ImageQuant
LAS 4000 mini) (all from GE Healthcare, Tokyo, Japan). The
quantification of western blot signals was performed by the
densitometry with ImageQuant TL software (GE Healthcare). All
western blot experiments were repeated at least 3 times.
Cell isolation from fresh tumor tissues
and spleen
LM8 cells were inoculated into C3H mice, and 28 days
after the injection, spleen cells were prepared. The tumor tissues
were dissected from the mice and minced.
Fresh tumor specimens were gently minced over a wire
mesh screen to obtain a cell suspension. The cell suspension was
layered over Ficoll-Hypaque (GE Healthcare) and centrifuged at 500
x g for 30 min. After density gradient centrifugation, mononuclear
cells were collected and washed with RPMI-1640 medium (Gibco,
Carlsbad, CA, USA) containing 5% fetal bovine serum and 1%
penicillin/streptomycin. Peripheral blood mononuclear cells (PBMCs)
were also isolated by Ficoll-Hypaque density gradient
centrifugation. PBMCs were collected, washed, and analyzed
immediately. Viable cell counts were obtained using trypan blue
dye. For isolation of
CD3+CD4+CD25high
CD127low, the cells prepared from tumor tissues were
stained with APC/Cy7-conjugated anti-CD3 mAb (M1/70),
FITC-conjugated anti-CD4 mAb (M1/70), Pacific Blue-conjugated
anti-CD25 mAb (M1/70) and Alexa Fluor 647-conjugated anti-CD127 mAb
(RB6-8C5) (all from BD Biosciences, San Jose, CA, USA). The
percentages of CD25high CD127low cells were
determined using BD LSRFortessa™ X-20 cell analyzer and analyzed
with BD FACSDiva (BD Biosciences). The population of
CD25high CD127low cells was isolated by
FACSAria II (BD Biosciences). The purity of the isolated cells was
consistently >95%.
RNA extraction, cDNA synthesis and
quantitative real-time PCR
Total RNA was extracted from prepared isolated Tregs
with the TRIzol reagent (Invitrogen) and cDNA was synthesized
according to the manufacturer's instructions (Roche). Quantitative
real-time PCR (qRT-PCR) was performed using a LightCycler 480 Probe
Master system (Roche), and PCR-specific amplification was conducted
in the LightCycler® Nano (Roche). The relative
expression of genes, TGF-β, IL-6, IL-10 and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), were calculated
with the 2−ΔΔCt method. The primer and probe kit of
TGF-β, IL-6, IL-10 and GAPDH were obtained from Applied Biosystems
(Nagoya, Japan).
Statistical analysis
We determined differences among the 4 groups using a
non-repeated measures analysis of variance (ANOVA) and the
Scheffe's test. All analyses were conducted using SPSS®
18.0 software (SPSS Japan Inc., Tokyo, Japan). Results were
expressed as the mean ± standard deviation, and P<0.01 was
considered statistically significant. For survival analysis, the
differences in survival rates were analyzed by log-rank test.
Results
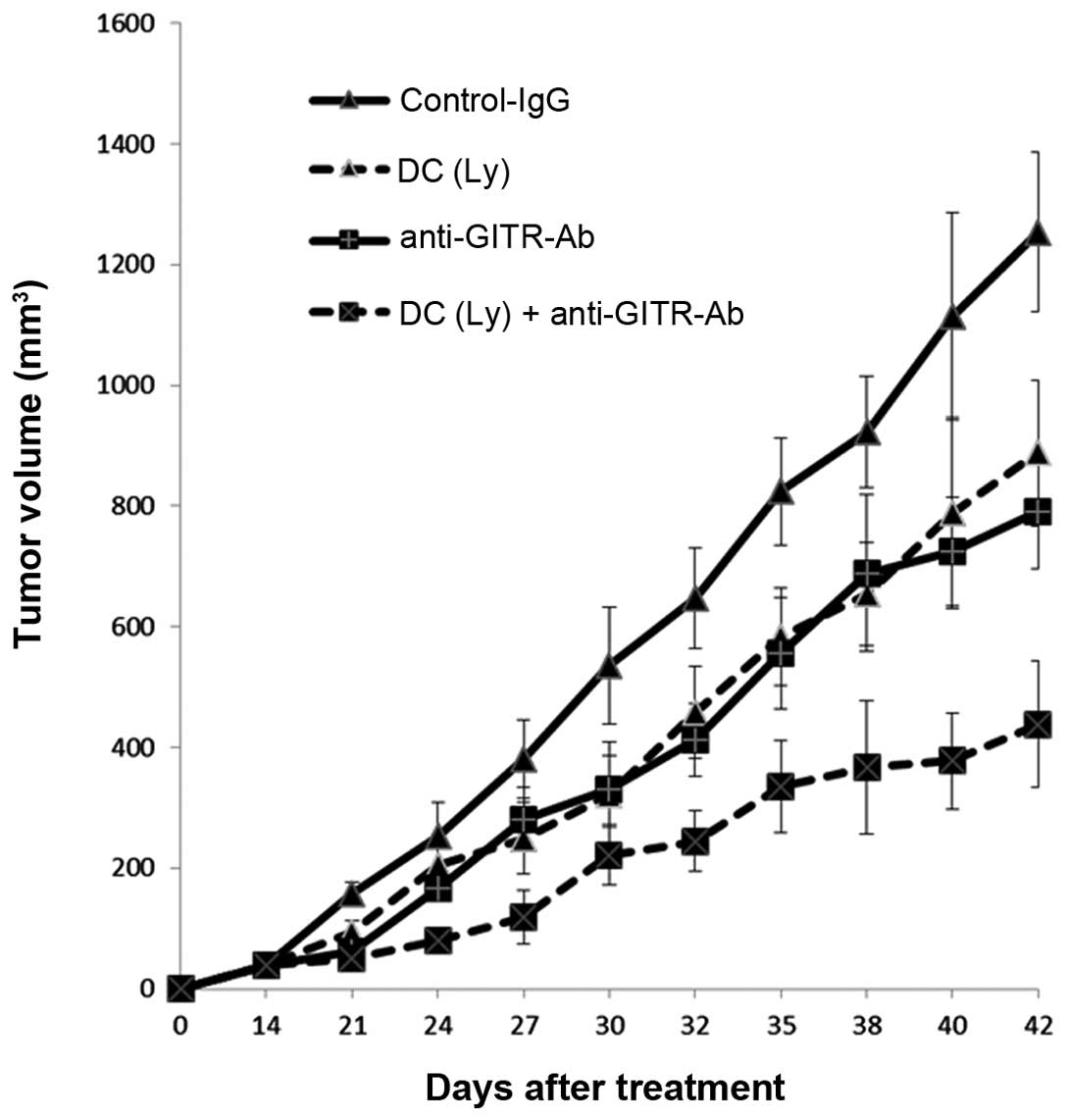
Tumor volume of the primary tumor
Forty-two days after inoculation, the volume of the
primary lesion in mice that received tumor lysate-pulsed DCs and
the agonist anti-GITR antibody (271.86±139.11 mm3) was
lower (P<0.01) than in the mice that received tumor
lysate-pulsed DCs (627.06±119.13 mm3) or the agonist
anti-GITR antibody alone (571.08±149.47 mm3). The volume
of the primary lesion in mice that received tumor lysate-pulsed DCs
and the agonist anti-GITR antibody (438.45±103.97 mm3)
was lower (P<0.01) than that in the mice that received tumor
lysate-pulsed DCs (887.87±121.19 mm3) or the agonist
anti-GITR antibody alone (701.47±95.97 mm3) (Fig. 2).
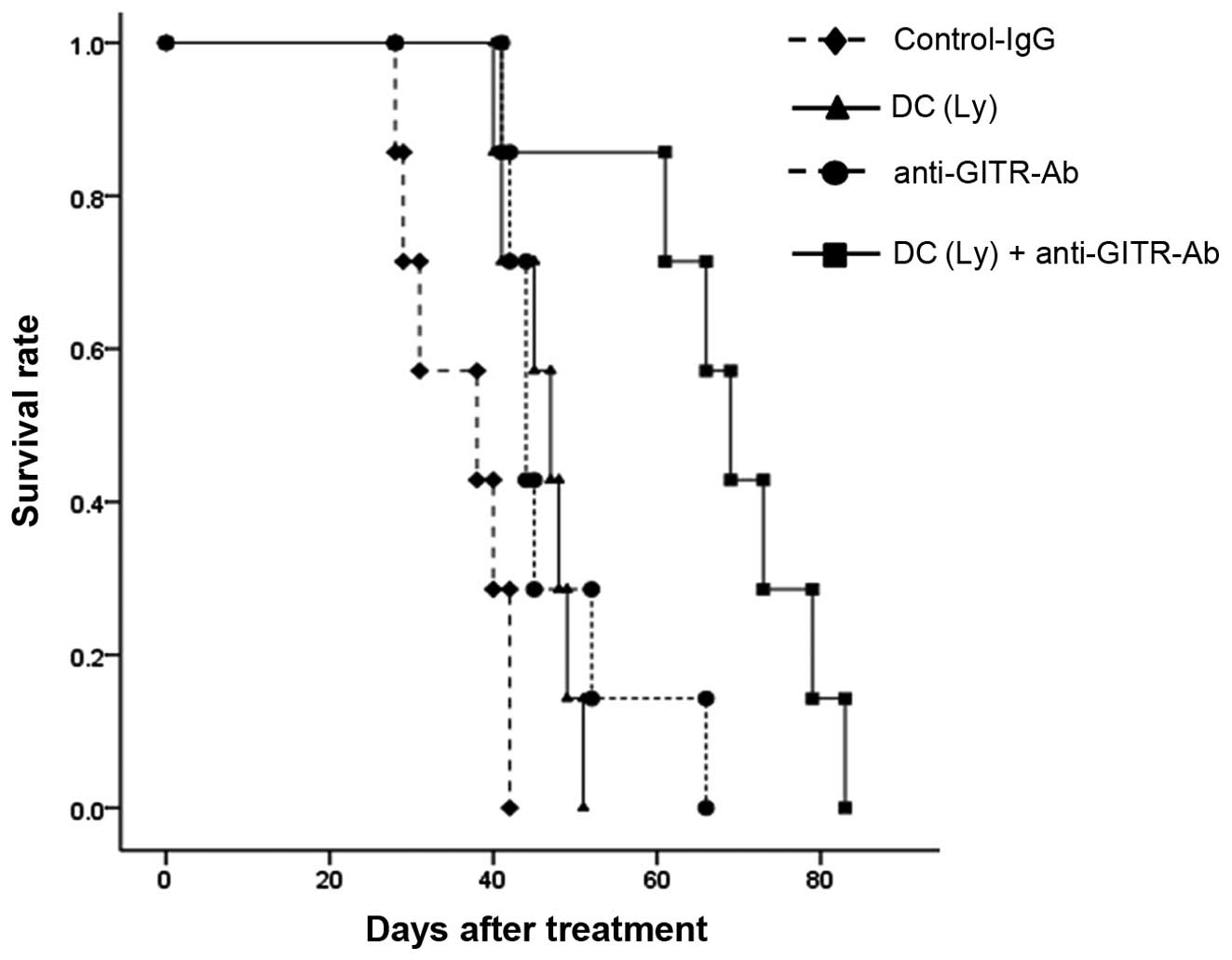
Survival rate
The median survival time was in IgG control, 35.7
days (range, 28–42); tumor lysate-pulsed DCs, 45.9 days (range,
40–51); anti-GITR antibody, 47.7 days (range, 41–66); and the tumor
lysate-pulsed DCs and the anti-GITR-4 antibody group, 67.4 days
(range, 41–83). Survival was significantly prolonged but
differences in tumor lysate-pulsed DCs alone and anti-GITR antibody
alone group were small compared with control IgG group (P<0.01).
There was no significant difference between the tumor lysate-pulsed
DCs alone and anti-GITR antibody alone groups. Further lifetime
prolongation was observed in tumor lysate-pulsed DCs and the
anti-GITR antibody group compared with the tumor lysate-pulsed DCs
alone and anti-GITR antibody alone groups (P<0.01, Fig. 3).
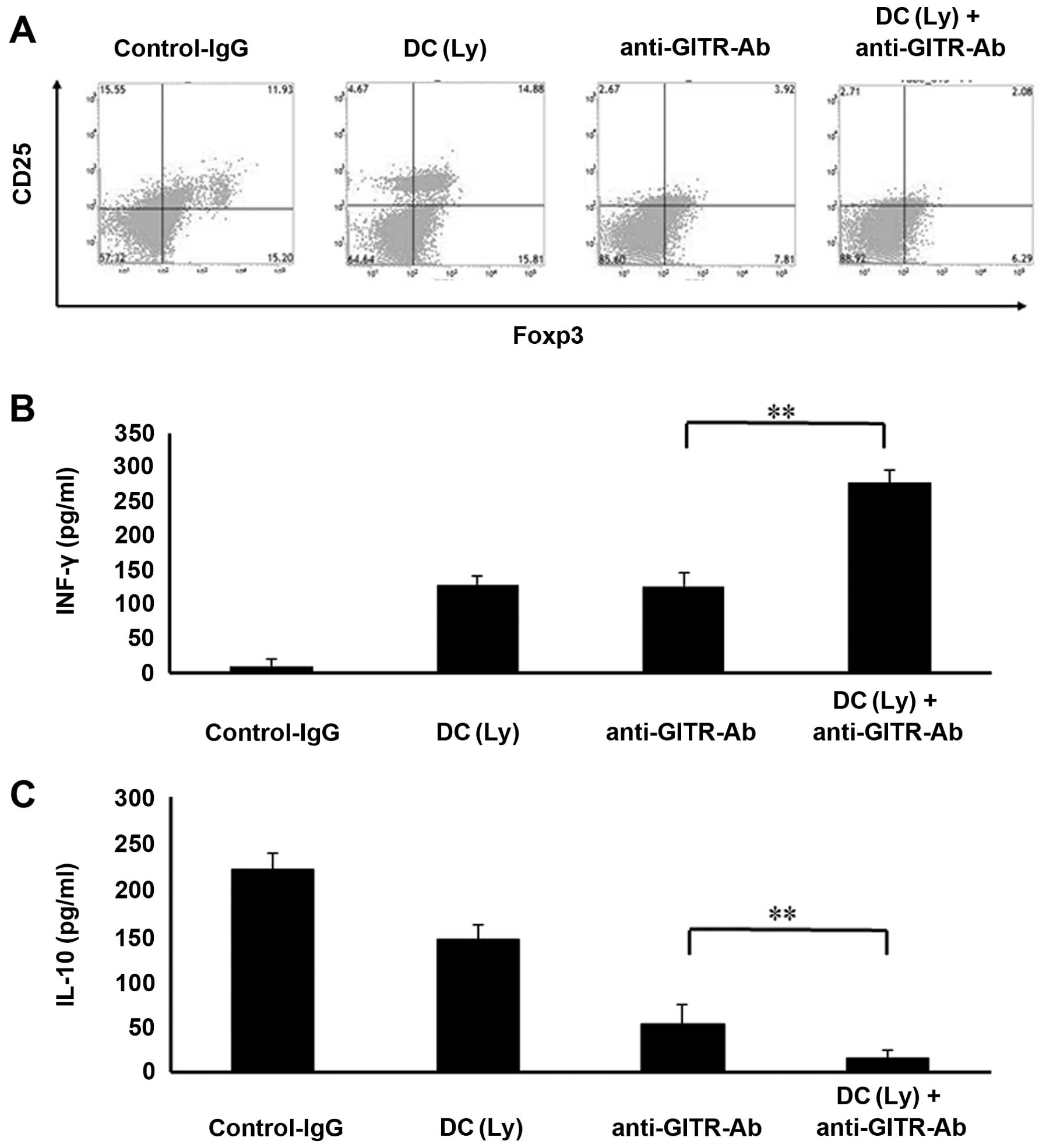
Characterization of distinct subsets of
CD25+Foxp3+ T cells in the spleen
Agonist anti-GITR antibodies markedly reduced the
CD4+Foxp3+ Treg population in the spleen. The
groups that received agonist anti-GITR antibodies alone or in
combination with tumor lysate-pulsed DCs displayed marked decreases
in the proportion of CD4+Foxp3+ cells
compared to the control IgG or tumor lysate-pulsed DC-treated
groups (Fig. 4A).
Cytokine release
Mice treated with tumor lysate-pulsed DCs and the
agonist anti-GITR antibody displayed higher serum IFN-γ levels
(278.33±18.64 pg/ml, P<0.01) than those that received tumor
lysate-pulsed DCs (129.6±13.28 pg/ml) or the anti-GITR antibody
alone (144.98±20.37 pg/ml, Fig.
4B). Serum IL-10 levels were lower (P<0.01) in mice that
received the anti-GITR antibody alone (53.24±21.29 pg/ml) than in
those that received tumor lysate-pulsed DCs alone (145.43±16.38
pg/ml). Serum IL-10 levels were lower (P<0.01) in mice that
received tumor lysate-pulsed DCs and the anti-GITR antibody
(15.38±9.26 pg/ml) than in those that received the anti-GITR
antibody alone (53.24±21.29 pg/ml, Fig.
4C).
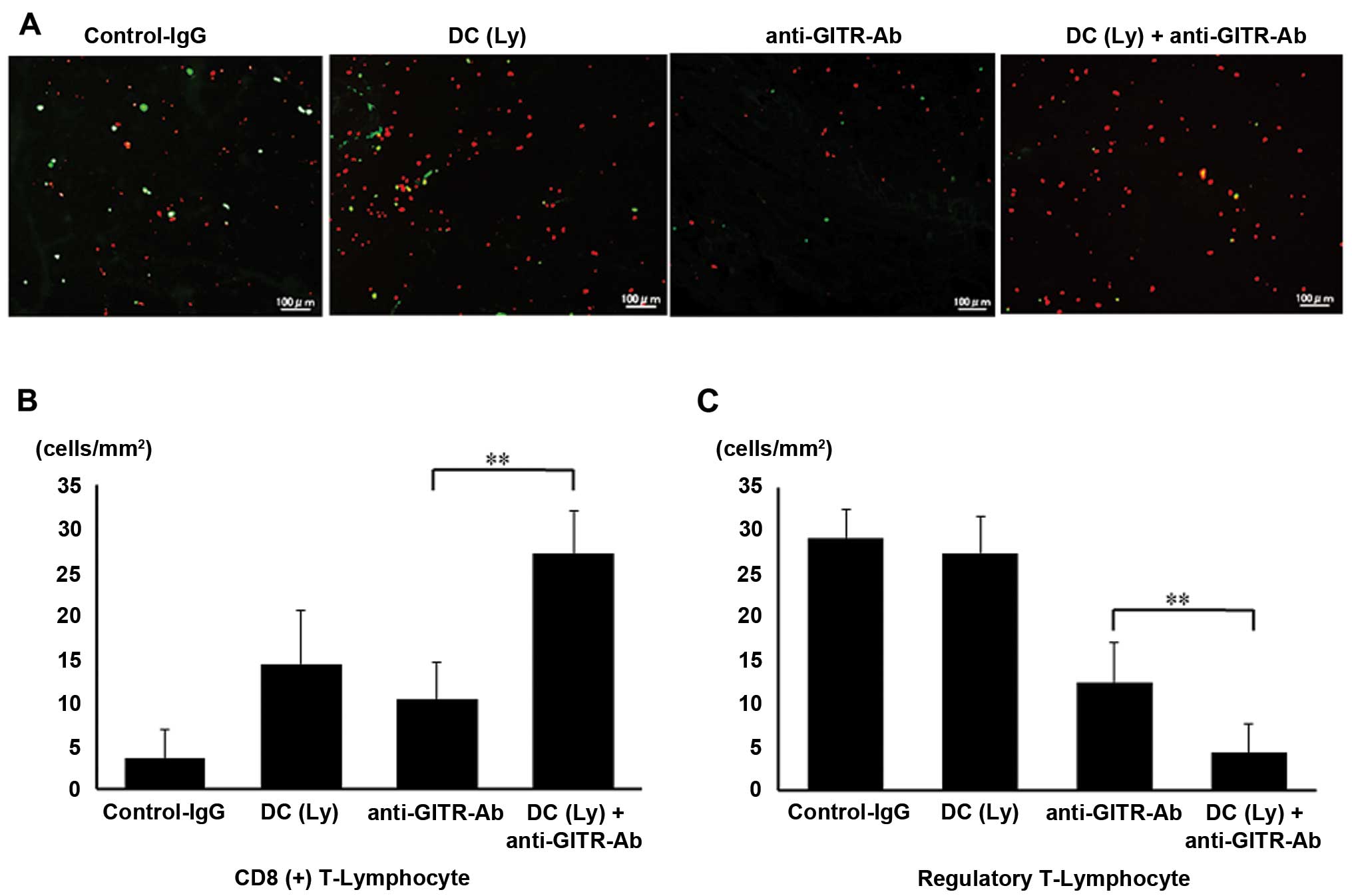
Infiltration of CD8+ T
lymphocyte and Tregs in the tumor
Foxp3 levels were significantly decreased, whereas
CD8+ T cell numbers were significantly increased in the
primary tumor lesions in the agonist anti-GITR antibody-treated
group. Foxp3+ cells were not recruited to the primary
area in the agonist anti-GITR antibody-treated group, but were
recruited in the control IgG-treated group (Fig. 5A). The number of CD8+ T
lymphocytes per unit area was higher (P<0.01) in mice that
received tumor lysate-pulsed DCs and the agonist anti-GITR antibody
(26.99±5.03 cells/mm2) than in those that received tumor
lysate-pulsed DCs (12.34±6.22 cells/mm2) or agonist
anti-GITR antibody alone (11.18±4.32 cells/mm2, Fig. 5B). The number of Foxp3+ T
lymphocytes per unit area was lower (P<0.01) in mice that
received the agonist anti-GITR antibody (12.49±4.59
cells/mm2) than in those that received tumor
lysate-pulsed DCs (27.38±4.31 cells/mm2). The number of
Foxp3+ T lymphocytes per unit area was lower (P<0.01)
in mice that received tumor lysate-pulsed DCs and the agonist
anti-GITR antibody (4.31±3.29 cells/mm2) than in those
that received the agonist anti-GITR antibody alone (Fig. 5C).
Immunosuppressive activity of
tumor-infiltrating Tregs and tumor tissue
To confirm the immunosuppressive activity of Tregs
which gathered in the tumor, we analyzed the expression levels of
TGF-β, IL-6 and IL-10 on Tregs sorted from tumor tissues. The
expression of TGF-β (0.59-fold), IL-6 (0.61-fold), and IL-10
(0.59-fold) were significantly lower in DC with anti-GITR-Ab group
compared with the control-IgG, DC alone and anti-GITR-Ab alone
group as determined by real-time quantitative RT-PCR (Fig. 6A). We also performed immunoblot
analysis to evaluate the protein levels of those immunosuppressive
molecules using tumor tissue lysate. Western blot analysis showed
that the expression levels of TGF-β, IL-10 and IL-6 of tumor tissue
dramatically decreased in DC and anti-GITR-Ab compared with
control-IgG, DC alone, and anti-GITR-Ab alone group (Fig. 6B).
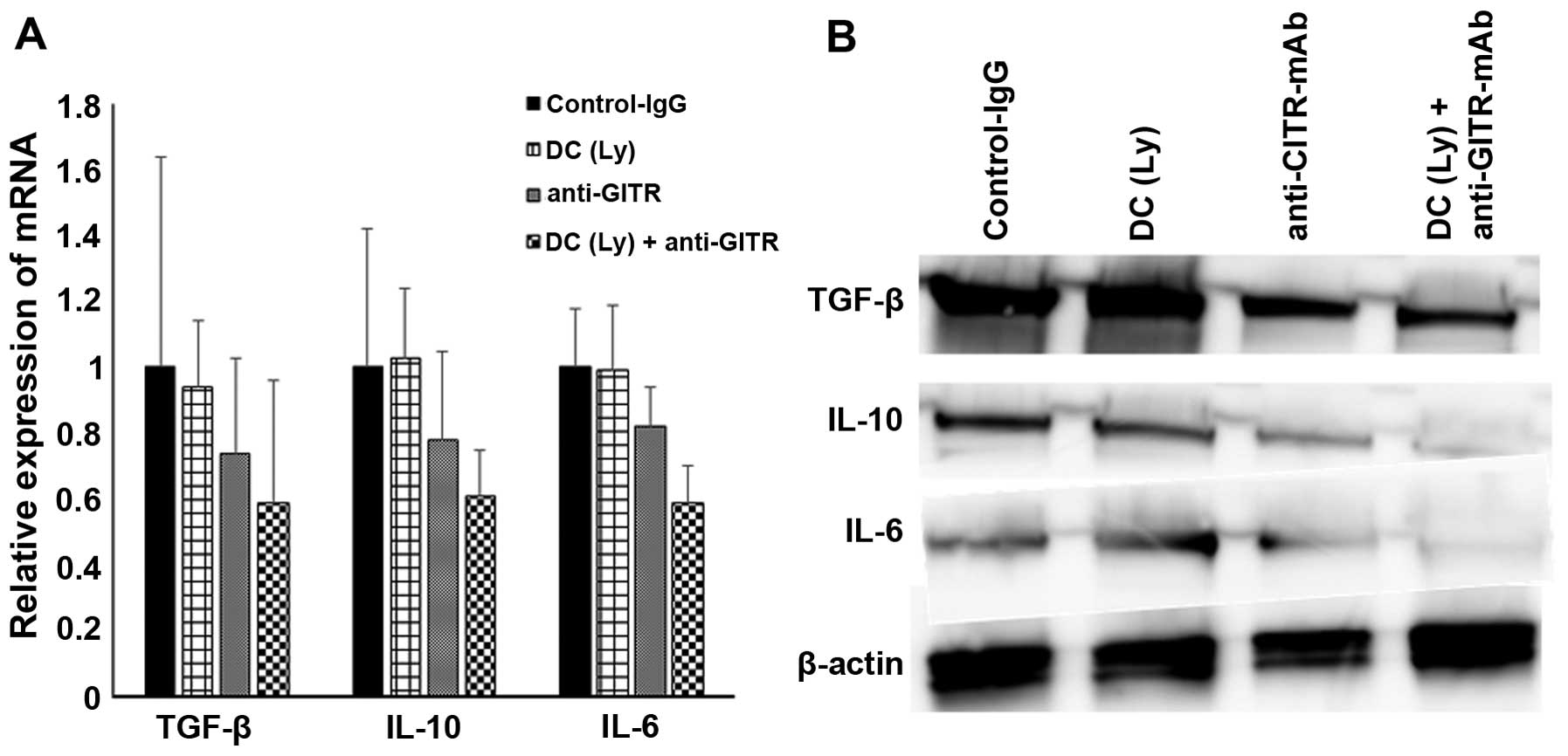 | Figure 6The TIL mRNA expression level of
TGF-β, IL-10 and IL-6 in control, DC(Ly), anti-GITR antibody, and
DC(Ly) + anti-GITR antibody groups was measured by qRT-PCR (A). A
western blot analysis of tumor tissue for TGF-β, IL-10 and IL-6 in
control, DC(Ly), anti-GITR antibody, and DC(Ly) + anti-GITR
antibody groups (B). GITR, glucocorticoid-induced tumor necrosis
factor receptor; Ly-DC, lysate-pulsed dendritic cell. |
Discussion
Most osteosarcoma patients are treated with some
combination of surgery, radiation and chemotherapy. Despite recent
advances in local therapies with curative intent, chemotherapeutic
treatments for primary disease are unsatisfactory owing to severe
adverse effects and incomplete long-term remission. Therefore, the
development of novel therapeutic options is of great interest.
Several immunotherapies have been investigated as new methods to
overcome progressive cancers (18-21).
We focused on the inability to control immunosuppressive factors
such as Tregs, which inhibit attacker cells, such as DCs and
CD8+ T lymphocytes; as a result, Tregs are a major cause
of insufficient antitumor effects. Since the initial discovery that
GITR stimulation drives T cell immunity (10), agonistic anti-GITR has been used
extensively for tumor immunotherapy, and GITR stimulation has been
shown to drive potent CD8+ T cell-mediated tumor
protection (13,22). The proportion of Tregs in tumor
tissues is dramatically reduced, which appears to be a direct
consequence of depletion (23–25).
Our aims were to evaluate Tregs by using the Foxp3 and CD4, in the
spleens of mice; measure the levels of Foxp3 and determine the
numbers of CD8+ T lymphocytes inside the primary and
primary tumor lesions; determine the changes in the primary and
tumor volumes; measure the levels of IFN-γ and IL-10; and measure
the expression levels of immune suppression factors, TGF-β, IL-10
and IL-6 from Tregs in the tumor tissue.
The group treated with the combination of tumor
lysate-pulsed DCs and the anti-GITR antibody displayed smaller
tumor lesions and the life time was prolonged. Importantly, the
result of tumor rejection in the combined therapy group correlated
with the intratumor ratio of CD8+ T cells to Tregs
(26). This suggests that
controlling immunosuppressive factors may facilitate the activity
of DCs and CTLs in the tumor. The Treg depletion using anti-GITR
antibody treatment combined with tumor lysate-pulsed DCs treatment
showed significantly improved survival in comparison to the tumor
lysate-pulsed DCs or anti-GITR antibody monotherapy groups.
The agonist anti-GITR antibody inhibited the
proliferation of Tregs in the spleen. Inhibition of Treg
accumulation in the spleen can enhance systemic cell-mediated
immunity through the activation of DCs or CTLs. We believe that
this result could reduce Treg accumulation and CD8+ T
lymphocyte proliferation inthe tumor tissues.
The agonist anti-GITR antibody inhibited the
accumulation of Tregs and induced the infiltration of
CD8+ T cells in the primary lesions. GITR signaling in
CD4+Foxp3+ Tregs is required for their
immunosuppressive capacity (6,8,27). We
demonstrated that stimulation of GITR led to the reduction of
Foxp3+ T cells in the tumor tissues.
The group treated with the combination of tumor
lysate-pulsed DCs and agonist anti-GITR antibodies also displayed
smaller primary lesions. Tregs comprise one of the major components
of the immunosuppressive microenvironment of tumor lesions
(28). This is consistent with our
results that tumor lesion volumes were significantly reduced in the
combined therapy group, suggesting that controlling
immunosuppressive factors may facilitate the activity of DCs and
CTLs in the tumor microenvironment.
Stimulation of GITR induced the activation of
cell-mediated immunity by increasing serum IFN-γ levels and
decreasing serum IL-10 levels. Tregs are among the major factors
that cause potent cytokine-mediated immunosuppression in tumor
cells, and GITR stimulation may be useful for enhancing the
efficacy of cancer therapy or vaccines (9). Our results revealed that stimulating
GITR using agonist anti-GITR antibody enhanced cell-mediated
immunity.
Tregs can inhibit immune cell functions either
directly through cell-cell contact or indirectly through the
secretion of immunosuppressive mediators, such as IL-10 and TGF-β
(29). Hence, it is possible that
by removing tumor-specific Tregs, antitumor immunity could be
enhanced. Many studies in mice have shown that removal or
inhibition of this subset of cells can enhance antitumor immune
responses (30,31). Decreasing immunosuppressive
cytokines by depleting tumor infiltrating Tregs by combining DCs
and anti-GITR Abs could represent an important adjunct to cancer
immunotherapy.
As a clinical application, GITR stimulatory therapy
should involve the induction of antitumor immunity without adverse
effects such as autoimmunity or cytokine storms. Continuous GITR
stimulation has been linked to the exacerbation of autoimmune
conditions (7,8). Several studies showed that
melanocyte-specific autoimmunity could be avoided entirely via
limited therapeutic administration of agonistic anti-GITR (14,32,33).
Taken together, our findings clearly support the
therapeutic potential of agonist anti-GITR antibodies in
osteosarcoma treatment. The effectiveness of GITR stimulation in
humans has yet to be demonstrated. The present studies of
concomitant and post-surgical immunity demonstrate that GITR
stimulation during primary tumor growth could be sufficient for
treating minimal residual disease or preventing tumor metastasis
and recurrence. The synergistic effect of this combined
chemoimmunotherapy using anticancer agents and immunotherapy with
agonist anti-GITR antibodies could enhance self-reactive CTL
responses, overcome self-tolerance, and induce long-lasting
antitumor immunity (24). Future
studies should be directed toward translating anti-GITR antibody
therapy into clinical trials for evaluation of osteosarcoma
treatments.
Acknowledgments
This study was performed at the Department of
Orthopaedic Surgery, Faculty of Medicine, Oita University, Oita,
Japan.
References
|
1
|
Ferrari S, Smeland S, Mercuri M, Bertoni
F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini
G, et al Italian and Scandinavian Sarcoma Groups: Neoadjuvant
chemotherapy with high-dose ifosfamide, high-dose methotrexate,
cisplatin, and doxorubicin for patients with localized osteosarcoma
of the extremity: A joint study by the Italian and Scandinavian
Sarcoma Groups. J Clin Oncol. 23:8845–852. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kager L, Zoubek A, Dominkus M, Lang S,
Bodmer N, Jundt G, Klingebiel T, Jürgens H, Gadner H and Bielack S;
COSS Study Group: Osteosarcoma in very young children: Experience
of the Cooperative Osteosarcoma Study Group. Cancer. 116:5316–324.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campbell CJ, Cohen J and Enneking WF:
Editorial: New therapies for osteogenic sarcoma. J Bone Joint Surg
Am. 57:143–44. 1975.PubMed/NCBI
|
|
4
|
Kawaguchi S, Wada T, Tsukahara T, Ida K,
Torigoe T, Sato N and Yamashita T: A quest for therapeutic antigens
in bone and soft tissue sarcoma. J Transl Med. 3:312005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawano M, Nishida H, Nakamoto Y, Tsumura H
and Tsuchiya H: Cryoimmunologic antitumor effects enhanced by
dendritic cells in osteosarcoma. Clin Orthop Relat Res.
468:1373–1383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanamaru F, Youngnak P, Hashiguchi M,
Nishioka T, Takahashi T, Sakaguchi S, Ishikawa I and Azuma M:
Costimulation via gluco-corticoid-induced TNF receptor in both
conventional and CD25+ regulatory CD4+ T
cells. J Immunol. 172:7306–7314. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McHugh RS, Whitters MJ, Piccirillo CA,
Young DA, Shevach EM, Collins M and Byrne MC: CD4(+)CD25(+)
immunoregulatory T cells: Gene expression analysis reveals a
functional role for the glucocorticoid-induced TNF receptor.
Immunity. 16:311–323. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kohm AP, Williams JS and Miller SD:
Cutting edge: Ligation of the glucocorticoid-induced TNF receptor
enhances autoreactive CD4+ T cell activation and
experimental autoimmune encephalomyelitis. J Immunol.
172:4686–4690. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tone M, Tone Y, Adams E, Yates SF, Frewin
MR, Cobbold SP and Waldmann H: Mouse glucocorticoid-induced tumor
necrosis factor receptor ligand is costimulatory for T cells. Proc
Natl Acad Sci USA. 100:15059–15064. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimizu J, Yamazaki S, Takahashi T, Ishida
Y and Sakaguchi S: Stimulation of CD25(+)CD4(+) regulatory T cells
through GITR breaks immunological self-tolerance. Nat Immunol.
3:135–142. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stephens GL, McHugh RS, Whitters MJ, Young
DA, Luxenberg D, Carreno BM, Collins M and Shevach EM: Engagement
of glucocorticoid-induced TNFR family-related receptor on effector
T cells by its ligand mediates resistance to suppression by
CD4+CD25+ T cells. J Immunol. 173:5008–5020.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ko K, Yamazaki S, Nakamura K, Nishioka T,
Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T and Sakaguchi
S: Treatment of advanced tumors with agonistic anti-GITR mAb and
its effects on tumor-infiltrating
Foxp3+CD25+CD4+ regulatory T
cells. J Exp Med. 202:885–891. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramirez-Montagut T, Chow A,
Hirschhorn-Cymerman D, Terwey TH, Kochman AA, Lu S, Miles RC,
Sakaguchi S, Houghton AN and van den Brink MR:
Glucocorticoid-induced TNF receptor family related gene activation
overcomes tolerance/ignorance to melanoma differentiation antigens
and enhances antitumor immunity. J Immunol. 176:6434–6442. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cohen AD, Diab A, Perales MA, Wolchok JD,
Rizzuto G, Merghoub T, Huggins D, Liu C, Turk MJ, Restifo NP, et
al: Agonist anti-GITR antibody enhances vaccine-induced CD8(+)
T-cell responses and tumor immunity. Cancer Res. 66:4904–4912.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishikawa H, Kato T, Hirayama M, Orito Y,
Sato E, Harada N, Gnjatic S, Old LJ and Shiku H: Regulatory T
cell-resistant CD8+ T cells induced by
glucocorticoid-induced tumor necrosis factor receptor signaling.
Cancer Res. 68:5948–5954. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Turk MJ, Guevara-Patiño JA, Rizzuto GA,
Engelhorn ME, Sakaguchi S and Houghton AN: Concomitant tumor
immunity to a poorly immunogenic melanoma is prevented by
regulatory T cells. J Exp Med. 200:771–782. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lutz MB and Rössner S: Factors influencing
the generation of murine dendritic cells from bone marrow: The
special role of fetal calf serum. Immunobiology. 212:855–862. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chauvin C, Philippeau JM, Hémont C, Hubert
FX, Wittrant Y, Lamoureux F, Trinité B, Heymann D, Rédini F and
Josien R: Killer dendritic cells link innate and adaptive immunity
against established osteosarcoma in rats. Cancer Res. 68:9433–9440.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawano M, Itonaga I, Iwasaki T, Tsuchiya H
and Tsumura H: Anti-TGF-β antibody combined with dendritic cells
produce antitumor effects in osteosarcoma. Clin Orthop Relat Res.
470:2288–2294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Southam CM, Marcove RC, Levin AG,
Buchsbaum HJ and Miké V: Proceedings: Clinical trial of autogenous
tumor vaccine for treatment of osteogenic sarcoma. Proc Natl Cancer
Conf. 7:91–100. 1972.PubMed/NCBI
|
|
21
|
Yu Z, Ma B, Zhou Y, Zhang M, Qiu X and Fan
Q: Activation of antitumor cytotoxic T lymphocytes by fusion of
patient-derived dendritic cells with autologous osteosarcoma. Exp
Oncol. 27:273–278. 2005.
|
|
22
|
Piao J, Kamimura Y, Iwai H, Cao Y, Kikuchi
K, Hashiguchi M, Masunaga T, Jiang H, Tamura K, Sakaguchi S, et al:
Enhancement of T-cell-mediated anti-tumour immunity via the
ectopically expressed glucocorticoid-induced tumour necrosis factor
receptor-related receptor ligand (GITRL) on tumours. Immunology.
127:489–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Coe D, Begom S, Addey C, White M, Dyson J
and Chai JG: Depletion of regulatory T cells by anti-GITR mAb as a
novel mechanism for cancer immunotherapy. Cancer Immunol
Immunother. 59:1367–1377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY,
Chang SY, Sakaguchi S and Kang CY: A combination of
chemoimmuno-therapies can efficiently break self-tolerance and
induce antitumor immunity in a tolerogenic murine tumor model.
Cancer Res. 67:7477–7486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishida H, Tsuchiya H and Tomita K:
Re-implantation of tumour tissue treated by cryotreatment with
liquid nitrogen induces anti-tumour activity against murine
osteosarcoma. J Bone Joint Surg Br. 90:1249–1255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawano M, Itonaga I, Iwasaki T and Tsumura
H: Enhancement of antitumor immunity by combining anti-cytotoxic T
lymphocyte antigen-4 antibodies and cryotreated tumor lysate-pulsed
dendritic cells in murine osteosarcoma. Oncol Rep. 29:1001–1006.
2013.PubMed/NCBI
|
|
27
|
Ronchetti S, Zollo O, Bruscoli S, Agostini
M, Bianchini R, Nocentini G, Ayroldi E and Riccardi C: GITR, a
member of the TNF receptor superfamily, is costimulatory to mouse T
lymphocyte subpopulations. Eur J Immunol. 34:613–622. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Viguier M, Lemaître F, Verola O, Cho MS,
Gorochov G, Dubertret L, Bachelez H, Kourilsky P and Ferradini L:
Foxp3 expressing CD4+CD25(high) regulatory T cells are
overrepresented in human metastatic melanoma lymph nodes and
inhibit the function of infiltrating T cells. J Immunol.
173:1444–1453. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nizar S, Meyer B, Galustian C, Kumar D and
Dalgleish A: T regulatory cells, the evolution of targeted
immunotherapy. Biochim Biophys Acta. 1806:7–17. 2010.PubMed/NCBI
|
|
30
|
Baba J, Watanabe S, Saida Y, Tanaka T,
Miyabayashi T, Koshio J, Ichikawa K, Nozaki K, Koya T, Deguchi K,
et al: Depletion of radio-resistant regulatory T cells enhances
antitumor immunity during recovery from lymphopenia. Blood.
120:2417–2427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Girardin A, McCall J, Black MA, Edwards F,
Phillips V, Taylor ES, Reeve AE and Kemp RA: Inflammatory and
regulatory T cells contribute to a unique immune microenvironment
in tumor tissue of colorectal cancer patients. Int J Cancer.
132:1842–1850. 2013. View Article : Google Scholar
|
|
32
|
Suvas S, Kim B, Sarangi PP, Tone M,
Waldmann H and Rouse BT: In vivo kinetics of GITR and GITR ligand
expression and their functional significance in regulating viral
immunopathology. J Virol. 79:11935–11942. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Valzasina B, Guiducci C, Dislich H,
Killeen N, Weinberg AD and Colombo MP: Triggering of OX40 (CD134)
on CD4(+)CD25+ T cells blocks their inhibitory activity:
A novel regulatory role for OX40 and its comparison with GITR.
Blood. 105:2845–2851. 2005. View Article : Google Scholar
|