Introduction
Colorectal cancer (CRC) is the sixth most common
malignancy and the fifth leading cause of cancer-related death in
China (1). Although a majority of
patients with CRC can be cured by surgery, approximately 50% of
these patients eventually develop metastasis and succumb to the
disease (2). Even at identical
stages, the incidence of metastasis varies among patients (3,4), which
demonstrates the heterogeneity of these tumors. The underlying
molecular mechanism of metastasis is an intricate process, which is
still unclear. Thus, defining new metastasis-related biomarkers is
an important goal towards prognostic evaluation and targeted
therapy.
Raf kinase inhibitor protein (RKIP) is a highly
evolutionarily conserved protein of the
phosphatidyletha-nolamine-binding protein family (5), which is ubiquitously expressed in
various tissues and organisms in many mammals including human
beings (6,7). Human RKIP is a 23 kDa protein that is
encoded by a 1,434 bp long mRNA transcribed from a gene located at
chromosome 12q24.23 (6,7). It was first designated as RKIP in 1999
due to its negative regulation of mitogen-activated protein kinase
(MAPK) signaling through Raf-1 binding (8). Recent data indicate that loss or
downregulation of RKIP expression could be associated with poor
prognosis and distant metastasis in certain types of human cancers
such as breast (9), prostate
(10), ovarian cancer (11) and others (9–16). In
CRC, Al-Mulla et al first indicated that loss of RKIP
expression may be involved in the metastatic process of CRC through
immunohistochemistry (IHC) in 269 patients (13). In our studies, the results
corroborated the potential prognostic value of RKIP in the distant
metastasis of CRC (17–19). However, most of these studies were
performed in Western cohorts by histological IHC detection. The
role of RKIP in CRC remains underdetermined. Furthermore, RKIP
serves as only a metastatic suppressor without any impact on the
tumorigenic phenotype in breast and prostate cancer (9,10);
however, it is both a metastatic and tumorigenesis suppressor in
ovarian cancer (11). To further
illuminate the role of RKIP in CRC, we explored the association
between RKIP expression with clinical characteristics and prognosis
of CRC, and we further investigated the effect of RKIP on the
metastatic and proliferative properties of human colon cancer cells
in vitro and in vivo.
Materials and methods
Patients and tissue specimens
Paraffin-embedded tissue samples were sectioned for
IHC from primary tumors and adjacent non-cancerous tissues. The
tissues were obtained from 129 randomly selected stage II CRC
patients who underwent surgery at the Sun Yat-sen University Cancer
Center (Guangzhou, China) from January 1998 to December 2002. The
CRC patients were histopathologically and clinically diagnosed with
CRC (T3/4N0 M0, stage II) according to the American Joint Committee
on Cancer (AJCC) TNM staging system. The clinical information was
collected from unprocessed medical files and pathological reports.
The study was carried out with the approval of the Ethics Committee
of the Sun Yat-sen University Cancer Institutional Board, and prior
written informed consent was obtained from all of the patients
involved.
IHC
The corresponding tissue blocks were cut into 5
µm thick sections. Hematoxylin and eosin (H&E) sections
were used for analyzing the tumor location. IHC was performed with
a rabbit-RKIP antibody (1:200; Santa Cruz Biotechnology, Santa
Cruz, CA, USA) according to standard procedures (20). Human benign prostate tissues were
used as a positive control. For the negative control, tissue slides
were incubated with PBST instead of the rabbit-RKIP antibody. In
the present study, a semi-quantitative estimation was made by both
the extent and intensity of immunoreactivity: the percentage of
staining was defined as follows: ≤5%=0, >5 to ≤25%=1, >25 to
≤50%=2, and >50%=3. The scores for intensity were evaluated as 0
for negative staining; 1 for weak staining; 2 for moderate
staining; and 3 for strong staining. Finally, the indexed sum was
acquired by the addition of the intensity grade and the percentage
of the staining area. Slides were scored by two independent
pathologists who were blinded to the patient data. Discrepancies
were resolved by consensus after reevaluation of the slides. A
score of 4.5 was suggested as a cut-off for dichotomizing RKIP
levels for the prognosis of TTP and overall survival (OS) according
to ROC curves. If the final score was <4.5, the tumor was
considered to have low expression; otherwise, the tumor was
considered to have high expression.
Cell culture and transfection
Human colon cancer cell lines (HT-29, HCT116, SW480
and LoVo) were obtained from the American Type Culture Collection
and were maintained in RPMI-1640 medium (Gibco, Carlsbad, CA, USA)
containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA)
in a humidified chamber with 5% CO2 at 37°C. For stable
overexpression of endogenous RKIP, the coding sequence of RKIP was
amplified and subcloned into the LV5 (pGCMV/GFP+Puro) vector, a
lentivirus purchased from GenePharma Biotech (Shanghai, China),
according to the manufacturer's instructions. HCT116 cells were
then transfected with the overexpressing RKIP lentivirus or
negative control lentiviral vectors and named HCT116/RKIP and
HCT116/vector cells, respectively. To generate stable
RKIP-knockdown cells, an annealed short interfering RNA (siRNA) for
RKIP was inserted into the pGPU6/GFP/Neo vector (GenePharma
Biotech) according to the manufacturer's instructions to obtain
pGPU6/GFP/Neo containing RKIP targeting short hairpin RNA
(pGPU6/GFP/Neo-shRKIP). The target sequence for effective knockdown
of RKIP expression was 5′-CCC ACC CAG GTT AAG AAT A-3′.
pGPU6/GFP/Neo-shRKIP or empty pGPU6/GFP/Neo vectors, which served
as negative controls, were transfected into SW480 cells named
SW480/sh-RKIP and SW480/vector, respectively. All stable clonal
cells generated were selected and cultured according to the
manufacturer's instructions for further studies. Western blot
assays were used to detect the expression of RKIP in all stable
cell lines as described below.
Quantitative real-time RT-PCR
Total RNA was isolated from the tissues or cell
lines using TRIzol (Invitrogen). Then, 2 µg of total RNA was
reverse-transcribed into cDNA in a 10 µl reaction solution
for real-time PCR (RT-PCR) using GoTaq® qPCR Master Mix
(Promega, Madison, WI, USA) as directed by the manufacturer. The
programmed parameters for RT-PCR were as follows: heating at 95°C
for 10 min to activate AmpliTaq Gold polymerase, followed by 40
cycles of denaturation at 95°C for 15 sec, annealing and extension
at 60°C for 60 sec. The expression levels of β-actin were used as
an endogenous control to ensure equal loading of the samples.
Invitrogen synthesized both oligonucleotide primers for RKIP and
β-actin (Shanghai, China). The primer sequences used were as
follows: RKIP forward, 5′-CAA TGA CAT CAG TGG CAC AGT C-3′ and RKIP
reverse, 5′-CAC AAG TCA TCC CAC TCG GCC TG-3′; β-actin forward,
5′-TGG ATC AGC AAG CAG GAG TA-3′ and β-actin reverse, 5′-TCG GCC
ACA TTG TGA ACT TT-3′. The relative mRNA expression of RKIP was
normalized against the internal control and analyzed by the
2−ΔΔct method. The experiment was performed in
triplicate and repeated three times.
Western blot analysis
Western blot analyses were performed using a
standard protocol (21). Next, 24
µg of protein extracts was subjected to 12% SDS-PAGE gel
electrophoresis. A rabbit anti-RKIP antibody (1:50; Santa Cruz
Biotechnology) and a rabbit anti-GAPDH antibody (1:5,000; Cell
Signaling Technology, Danvers, MA, USA) were used for analysis
according to the manufacturer's instructions. RKIP protein levels
were normalized to the total GAPDH levels on the same membrane,
which were visualized using an enhanced chemiluminescence ECL
detection system (KeyGen Biotech, Nanjing, China).
MTT assay
Cell proliferation was evaluated using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. HCT116/vector and HCT116/RKIP cells were plated at a density
of 2,000 cells/well, and SW480/vector and SW480/sh-RKIP cells were
plated at 4,000 cells/well. The absorbance value of each sample was
read at 570 nm using a microplate reader. All experiments were
performed in octuplicate to calculate the average result.
Wound-healing assay
To determine the effect of RKIP on cell mobility, a
scratch test was performed. Monolayer cells were scrape-wounded
using standard micropipette tips. The vertical distance of the
inner face of the denuded zone was measured using a fluorescence
inverted microscope at 0, 12, 24 and 36 h. All experiments were
performed in triplicate.
Transwell migration and invasion
assays
To better evaluate the invasive and migratory
potentials of the cells, 24-well Transwell chambers (8 µm
pores) from BD Biosciences were used. For the migration assay, the
tumor cells were resuspended at a density of 6×105 cells
in 200 µl serum-free medium and transferred to the top
chamber of each insert without matrix gel. Then, 500 µl of
serum was added to the lower compartment as the chemotactic factor.
After incubation for 16 h, the non-migrating cells on the upper
side were removed, and the cells that migrated to the undersurface
were fixed and dyed with 0.1% crystal violet. In parallel, the
invasion Transwell assay was performed as mentioned above with the
inclusion of Matrigel mix pre-coated on the inserts and cultured
for 24 h. The number of migrating or invading cells was
microscopically quantified by counting five independent fields. The
final results were compared using the mean of triplicate assays
(22).
In vivo proliferation and metastasis
assays
Female BABL/c athymic nude mice aged 5 weeks were
purchased from the Animal Center of Guangdong Province (Guangzhou,
China) and maintained under specific pathogen-free conditions. For
the in vivo proliferation assays, a total of 106
cells of HCT116/RKIP or SW480/sh-RKIP were injected subcutaneously
into the left dorsal flanks of nude mice and the corresponding
negative control cells were injected into the right (n=6). An
algorithm, volume = length × width × length × 0.5236 (23), was used to calculate the tumor
volume every 4 days. Four weeks after injection, the animals were
sacrificed and the tumors were weighed. To investigate the effect
of RKIP on metastasis, a concentration of 2×107 cells/ml
of the HCT116/RKIP, HCT116/vector, SW480/sh-RKIP and SW480/vector
cells was injected into the tail veins of mice. Six weeks after
injection, the mice were sacrificed, and the lungs and livers were
dissected out and embedded in paraffin. The animal tissue blocks
were then cut into 4 µm sections consecutively for further
staining with H&E. The micrometastases in the lungs and livers
were examined and counted by pathologists who had no prior
knowledge of the mouse groups (24). All of the in vivo experiments
were conducted in strict accordance with the National Institutes of
Health guidelines. The protocol was approved by the Ethics
Committee of Animal Experiments of the Sun Yat-sen University
Cancer Center.
Statistical analysis
The associations between RKIP expression levels and
clinical characteristics were evaluated using the Chi-square
analysis. Survival curves were drawn using the Kaplan-Meier method
and assessed by the log-rank test. Disease-free survival (DFS) was
defined as the interval between the operation date to the date of
metastasis or recurrence and OS was computed from the date of
surgery to the date of death, or the last follow-up. Univariate and
multivariate analyses were performed using the Cox proportional
hazards regression model. A Student's t-test was used to analyze
the single comparison between two means. All tests were two-sided
and considered to be significant with p-values of <0.05. Data
are expressed as the mean ± standard error mean (SEM) unless
otherwise stated. Statistical analyses were performed using the
Statistical Package for the Social Sciences software version
13.
Results
Loss of RKIP expression in CRC and its
correlation with prognosis
In order to investigate the expression pattern of
RKIP in human CRC, IHC was performed to detect RKIP in 129 cases of
primary CRC tumor samples as well as 127 cases of adjacent
non-cancerous tissues. RKIP staining had a predominantly
cytoplasmic and membrane-associated distribution and was rarely
nuclear (Fig. 1A and B). The
average IHC score in 129 primary CRC tumor samples was 3.95, which
was significantly lower than that in the 127 adjacent non-cancerous
tissues (average IHC score was 5.04, p<0.001) (Fig. 1C). Moreover, 74 of the 129 primary
CRC tumor samples (57.4%) had low RKIP protein expression. In
contrast, in the adjacent non-cancerous tissues, only 35 of 127
cases (27.7%) had low RKIP expression staining (p<0.001;
Table I). These results indicate
that RKIP expression was significantly downregulated in CRC in
comparison to the adjacent non-cancerous tissues.
 | Table IComparison of RKIP expression between
tumor and adjacent non-tumor tissues. |
Table I
Comparison of RKIP expression between
tumor and adjacent non-tumor tissues.
| Tissue type | N | RKIP expression
| P-value |
|---|
| Low, n (%) | High, n (%) |
|---|
| T | 129 | 74 (57.4) | 55 (42.6) | <0.001a |
| ANT | 127 | 35 (27.6) 92
(72.40 | | |
Among the 129 cases of primary stage II CRC, 74
cases of primary CRC tumor samples had low RKIP protein expression,
and reduced RKIP expression was found to have a significant
correlation with tubular adenocarcinoma (p=0.015; Table II). No statistically significant
association was found between RKIP expression and other
clinicopathological variables including gender, age, location of
primary mass, tumor size, depth of invasion and tumor
differentiation (Table II).
 | Table IICorrelation between the RKIP level
and the clinicopathological features of stage II CRC patients. |
Table II
Correlation between the RKIP level
and the clinicopathological features of stage II CRC patients.
| Features | N | RKIP expression
| P-value |
|---|
| Low, n (%) | High, n (%) |
|---|
| Gender | | | | |
| Male | 75 | 43 (57.3) | 32 (42.7) | 0.993b |
| Female | 54 | 31 (57.4) | 23 (42.6) | |
| Age (years) | | | | |
| ≤60 | 73 | 45 (61.6) | 28 (38.4) | 0.262b |
| >60 | 56 | 29 (51.8) | 27 (48.2) | |
| Location | | | | |
| Colon | 69 | 36 (52.1) | 33 (47.8) | 0.136b |
| Rectum | 60 | 38 (63.3) | 22 (36.7) | |
| Tumor size
(cm) | | | | |
| ≤5 | 69 | 44 (63.8) | 25 (36.2) | 0.115b |
| >5 | 60 | 30 (50.0) | 30 (50.0) | |
| T
classification | | | | |
| T3 | 106 | 59 (55.7) | 47 (44.3) | 0.401b |
| T4 | 23 | 15 (65.2) | 8 (34.8) | |
| Histology | | | | |
| TA | 118 | 72 (32.2) | 46 (39.0) | 0.015a |
| MA | 11 | 2 (8.3) | 9 (75.0) | |
| Pathological
differentiation | | | | |
| Poor | 19 | 10 (61.0) | 9 (47.4) | 0.651b |
| Moderate/well | 110 | 64 (58.2) | 46 (41.8) | |
| Preoperative CEA
(ng/ml) | | | | |
| ≤5 | 102 | 58 (56.9) | 44 (43.1) | 0.981b |
| >5 | 21 | 12 (57.1) | 9 (40.9) | |
| Preoperative
LDH | | | | |
| Normal | 112 | 63 (56.3) | 49 (43.8) | 0.783b |
| Elevated | 15 | 9 (60.0) | 6 (40.0) | |
To further investigate the prognostic significance
of RKIP expression, DFS and OS analyses were performed in these 129
CRC cases using Kaplan-Meier analysis with a log-rank test
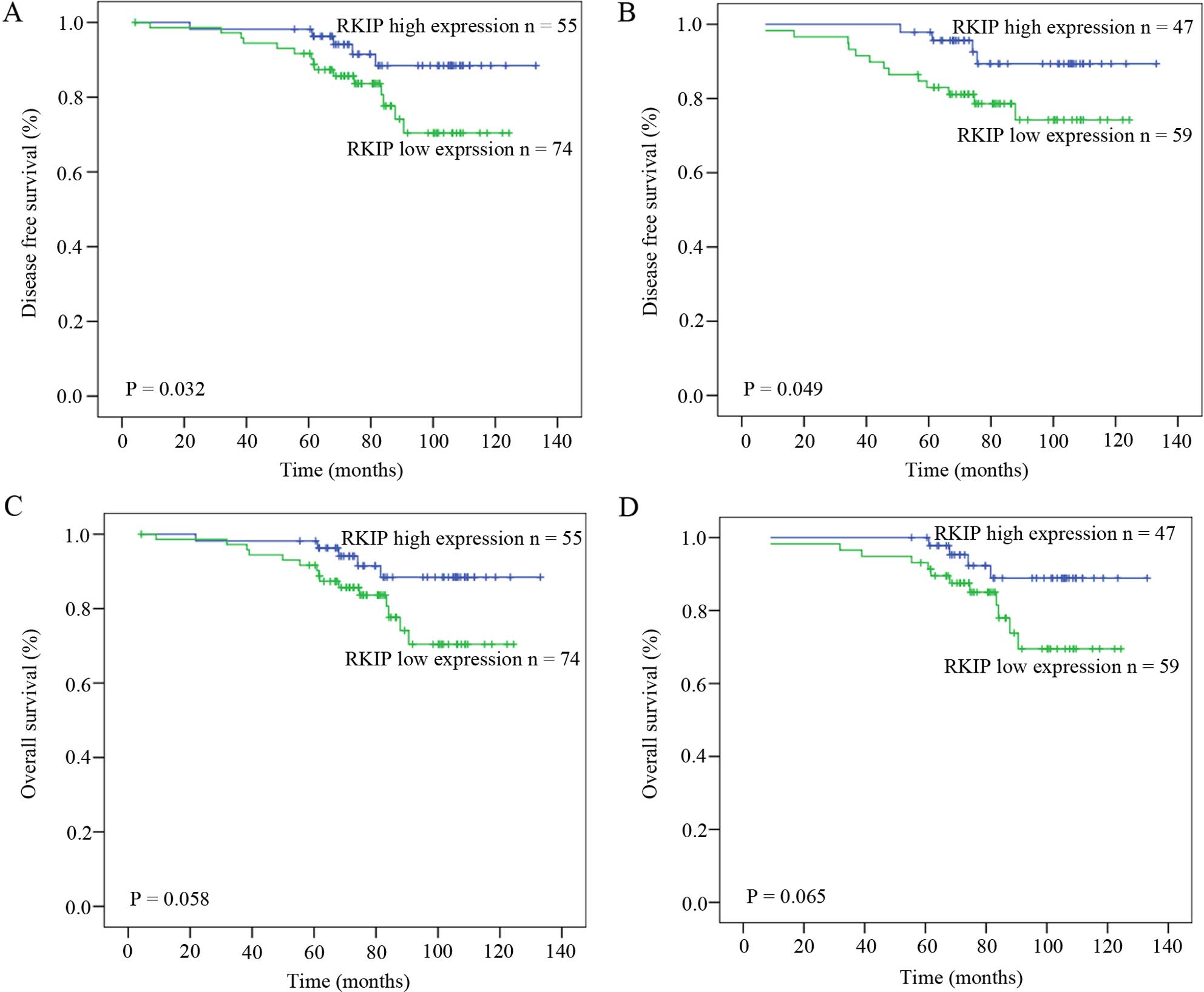
(Fig. 2). At the end of the
follow-up time, 17.8% of the cases (23/129) were observed to have
metastasis and/or disease recurrence. Patients with low RKIP
expression had a shorter DFS time than those with high RKIP
expression (median DFS 125 vs. 143 months; p=0.032; Fig. 2A). Additionally, the T3 stage
population (106 cases) was separately analyzed to evaluate the
prognostic effect of RKIP by excluding the 23 T4 stage cases. A
good correlation between the low RKIP expression and relapse
independent of T stage (median DFS 106 vs. 126 months; p=0.049;
Fig. 2B). Furthermore, univariate
and multivariate analyses indicated that RKIP expression was an
independent prognostic factor for CRC relapse (Table III). However the relationship
between RKIP expression and overall survival was not statistically
significant both in the entire studied population (median OS 125
vs. 107 months; p=0.058; Fig. 2C)
and in the T3 stage population (median OS 126 vs. 108 months;
p=0.065; Fig. 2D).
 | Table IIIUnivariate and multivariate analyses
of various prognostic parameters for metastasis or relapse in stage
II CRC patients. |
Table III
Univariate and multivariate analyses
of various prognostic parameters for metastasis or relapse in stage
II CRC patients.
| Variables | Univariate analysis
| Multivariate
analysis
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years
(≤60/>60) | 1.024
(0.990–1.059) | 0.170 | 0.791
(0.286–2.185) | 0.650 |
| Location
(colon/rectum) | 1.568
(0.687–33.580) | 0.285 | 1.265
(0.454–3.520) | 0.653 |
| Tumor size
(≤5/>5 cm) | 0.873
(0.383–1.991) | 0.747 | 1.077
(0.397–2.920) | 0.884 |
| Histology
(TA/MA) | 0.458
(0.062–3.396) | 0.445 | 0.000 (0.000–) | 0.982 |
| Pathological
differentiation (P/M/W) | 1.061
(0.450–2.498) | 0.893 | 0.535
(0.188–1.518) | 0.240 |
| T classification
(T3/T4) | 1.600
(0.590–4.339) | 0.356 | 1.971
(0.575–6.749) | 0.280 |
| PBO (yes/no) | 1.074
(0.363–3.176) | 0.897 | 2.258
(0.626–8.139) | 0.213 |
| LN
(<12/≥12) | 0.335
(0.078–1.439) | 0.142 | 0.174
(0.035–0.876) | 0.034 |
| Adjuvant
chemotherapy (yes/no) | 0.935
(0.218–4.003) | 0.928 | 0.610
(0.105–3.537) | 0.581 |
| Preoperative CEA
(≤5/>5 ng/ml) | 2.789
(1.125–6.914) | 0.027 | 3.471
(1.258–9.580) | 0.016 |
| Preoperative LDH
(normal/elevated) | 1.861
(0.625–5.543) | 0.265 | 2.276
(0.607–8.540) | 0.223 |
| RKIP expression
(low/high) | 0.333
(0.123–0.8970 | 0.030 | 0.265
(0.082–0.861) | 0.027 |
Effects of RKIP expression on
proliferation and metastasis of CRC in vitro
We detected both RKIP mRNA and protein levels by
qPCR and western blotting in four CRC cell lines (HT29, Lovo, SW480
and HCT116) and in one normal colonic tissue. RKIP was found to be
reduced in all these CRC cell lines compared to the normal tissue
(Fig. 3A and B). To further
investigate the influence of RKIP on malignant phenotypes in CRC
in vitro, the CRC cell line HCT116 which had the relatively
lowest RKIP expression level, was chosen for reconstituting RKIP
expression. The CRC SW480 cells which had the relatively highest
RKIP expression level were chosen for RKIP shRNA knockdown
experiment. The effects of exogenous RKIP overexpression in HCT116
cells and RKIP knockdown in SW480 cells were confirmed by western
blotting (Fig. 3C).
Cell motility was determined using migration and
invasion assays. As shown in Fig. 4A
and B, the restoration of RKIP expression inhibited HCT116/RKIP
cells from penetrating the polycarbonate membrane and a collagen
matrix by an average of 48.5 and 45.6%, respectively, compared to
the HCT116/vector cells. Similar results were observed in the SW480
cell line in which RKIP knockdown significantly increased cell
migration and invasion (Fig. 4C and
D). Moreover, RKIP suppression of cell motility was confirmed
by a wound healing assay. At 32 h, wound closure in the HCT116/RKIP
cells was modest compared to the HCT116/vector cells (26.7±6.1 vs.
65.1±4.0%; p<0.001). Conversely, the distance closure of the
SW480/vector cells was much shorter than that of the SW480/sh-RKIP
cells (47.7±4.1 vs. 70.2±2.3%; p<0.001; Fig. 4E and F). Next, the effect of RKIP on
proliferation of cancer cells was evaluated using the MTT assay in
the transfected cells. The cell proliferation curve indicated that
neither exogenous overexpression nor reduction of RKIP had
generated statistically significant differences in cell
proliferative rates (p>0.05).
Effect of RKIP expression on CRC
proliferation and metastasis in vivo
To further verify the inhibitory effect of RKIP on
metastasis in vivo, the two paired transfected cell lines
(HCT116/RKIP and HCT116/vector, SW480/sh-RKIP and SW480/vector)
were injected into the tail veins of nude mice. After 6 weeks, the
nude mice were sacrificed to measure lung and liver metastases.
Only mice injected with the HCT116/vector cells had observed
macrometastases when compared to the HCT116/RKIP group, including
66.7% (4/6 mice) pulmonary, 16.7% (1/6 mice) hepatic, 50% (3/6
mice) subcutaneous and 16.7% (1/6 mice) muscle macrometastases,
indicating that the overexpression of RKIP markedly decreased the
incidence of metastasis. Furthermore, the lung and liver
micrometastases obtained from the four groups were examined and
counted under a microscope. The average number of lung
micrometastases in mice injected with the HCT116/RKIP cells was
64.3% less than that in mice injected with the HCT116/vector cells
(p=0.010; Fig. 5A and B). The
average number of pulmonary micrometastases in mice injected with
the SW480/vector cells was 79.6% less than that in those injected
with the SW480/siRKIP cells (p=0.039; Fig. 5C and D), which confirmed the role of
RKIP in metastasis suppression in vivo in CRC. However,
there were no statistically significant differences in the
comparison of the average number of liver micrometastases between
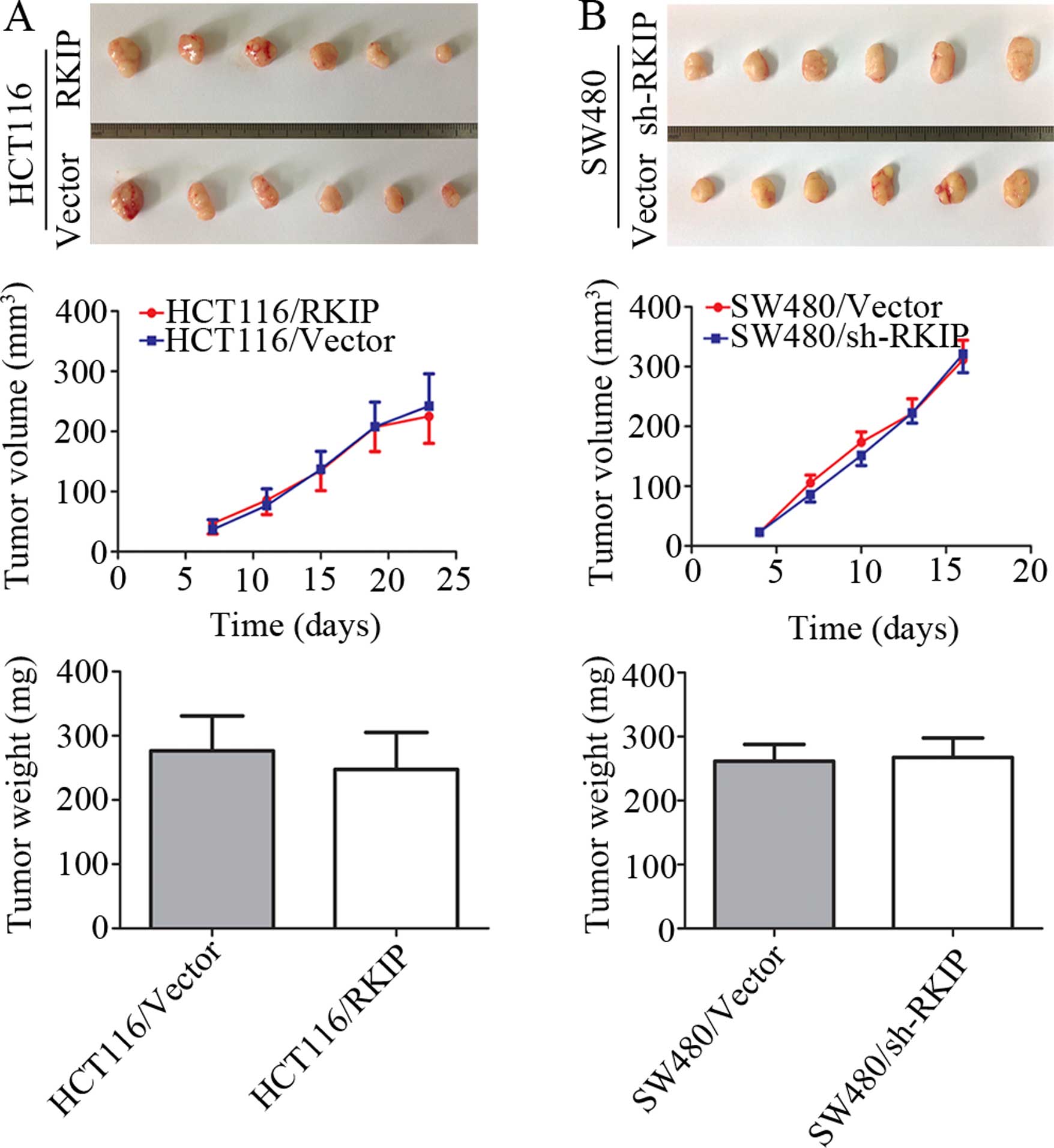
the two groups (p>0.05). To explore the effect of RKIP on
tumorigenesis in vivo, the reconstructed cells and their
control cells were injected into the left and right dorsal flanks
of nude mice, respectively, as described in Materials and methods.
The tumor growth curve was generated by measuring tumor volume over
time, and the data showed no statistically significant difference
between the reconstructed cells and their control cell groups. RKIP
neither suppressed nor promoted tumor growth (Fig. 6).
Discussion
Metastasis contributes to the majority of
CRC-related mortalities. Thus, a large number of genes have been
identified as metastasis-suppressor genes for the use of clinical
predication and treatment strategies. Previous studies suggest that
RKIP may be a crucial metastasis-suppressor gene in CRC, based on
Western patient cohorts (13,17–19).
Our results showed that low RKIP expression significantly predicted
the high risk of distant metastases in Chinese CRC stage II disease
patients and showed a trend towards poor overall survival, although
the results of overall survival were not statistically significant,
most likely due to the low sample size and data mining. We also
verified the independent negative prognostic value of RKIP in
T3N0M0 stage disease. These results are consistent with previous
studies. Doyle et al indicated that loss of RKIP predicted
poor prognosis in Western Dukes' B CRC patients (17). Zlobec et al also reported
that loss of RKIP endowed node-negative patients with a similar
probability of metastasis as node-positive patients with positive
RKIP expression (19).
Nevertheless, in 74 patients with low RKIP expression, 77.0% of
cases (57/74) were without metastasis or recurrence during the
follow-up period, suggesting that a number of factors are likely
involved and combined in assessing accurate risk-stratification. In
the present study, RKIP expression was found to have a significant
correlation with tubular adenocarcinoma; however, this conclusion
should be carefully drawn due to a small sample bias.
To the best of our knowledge, most of the previous
studies concerning RKIP in CRC were performed in Western cohorts by
histological detection (13,17–19).
Research focusing on the role of RKIP in CRC tumor biology in
vitro and in vivo is limited. To this end, we
overexpressed and knocked down RKIP expression in colon cancer cell
lines, demonstrating that the ectopic expression of RKIP inversely
affected cell migration and invasion abilities, which are two
prerequisite components of the metastasis cascade. To convincingly
confirm the inverse association between RKIP expression and
metastasis, orthotopic nude mice were sacrificed to set-up a
metastatic animal model. This revealed that knockdown of RKIP
enhanced in vivo metastasis, whereas restoration of RKIP
impaired metastasis. Additionally, we demonstrated that RKIP had
limited impact on the cell proliferation or the subcutaneous
transplanted model in nude mice. These results were in line with
previous studies in prostate (10),
breast (9) and gastric cancer
(15). However, our results were
not comparable to studies in epithelial ovarian cancer (11) and insulinoma (25) in which RKIP inhibited cell
proliferation. Thus, the regulation of cell proliferation by RKIP
is presumably under exquisite regulatory control through different
signal transduction pathways in specific types of cancer.
In the present study, we clarified the role of RKIP
in metastatic suppression; however, the RKIP-mediated pathological
signal cascade that antagonizes CRC metastasis remains unclear. It
has been reported that RKIP mediates crosstalk between distinct
pathways, including the Raf/MERK/ERK (8), NF-κB (26,27)
and G-protein pathways (28,29),
and GSK3β signaling (30), which
are all involved in pro-metastatic signaling pathways. It is
possible that RKIP plays a pivotal role in coordinating more than
one metastatic regulatory pathway ultimately suppressing the
expression of metastasis-associated genes. To date, E-cadherin
(31), matrix metalloproteinases
(MMP-2 and MMP-9) (31), signal
transducer and activator of transcription 3 (STAT3) (32), basic leucine zipper transcription
factor 1 (BACH1) (33), and high
mobility group AT-hook 2 (HMGA2) (34) have been shown to be downstream in
the mechanism targeting metastatic suppression of RKIP in prostate
and breast cancer. Further investigation of the mechanism of RKIP
loss and its downstream signal transduction in CRC is
warranted.
In conclusion, the present study indicates that
reduced RKIP expression is correlated with metastasis and
recurrence of disease in stage II CRC patients, as well as
migration and invasion in colon cancer cell lines and animal
models. RKIP is an important metastasis-suppressor gene in CRC.
Re-expression of RKIP may be a potential therapeutic target for an
antimetastasis strategy in CRC.
Acknowledgments
We would like to thank Dr Zi-Ming Du (Postdoctoral
Research Fellow at the Department of Pathology, Brigham and Women's
Hospital, Harvard Medical School, Harvard University) for kindly
offering us precious suggestions for the present study. The present
study was supported by the Science and Technology Department of
Guangdong Province, China (no. 2010B080701075).
References
|
1
|
Zheng ZX, Zheng RS, Zhang SW and Chen WQ:
Colorectal cancer incidence and mortality in China, 2010. Asian Pac
J Cancer Prev. 15:845–460. 2014. View Article : Google Scholar
|
|
2
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:10–17.
2014. View Article : Google Scholar
|
|
3
|
Cascinu S, Georgoulias V, Kerr D, Maughan
T, Labianca R and Ychou M: Colorectal cancer in the adjuvant
setting: Perspectives on treatment and the role of prognostic
factors. Ann Oncol. 14(Suppl 2): ii25–ii29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ovaska J, Järvinen H, Kujari H, Perttilä I
and Mecklin JP: Follow-up of patients operated on for colorectal
carcinoma. Am J Surg. 159:593–596. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bernier I and Jollès P: Purification and
characterization of a basic 23 kDa cytosolic protein from bovine
brain. Biochim Biophys Acta. 790:174–181. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hori N, Chae KS, Murakawa K, Matoba R,
Fukushima A, Okubo K and Matsubara K: A human cDNA sequence
homologue of bovine phosphatidylethanolamine-binding protein. Gene.
140:293–294. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seddiqi N, Bollengier F, Alliel PM, Périn
JP, Bonnet F, Bucquoy S, Jollès P and Schoentgen F: Amino acid
sequence of the Homo sapiens brain 21–23-kDa protein
(neuropolypeptide h3), comparison with its counterparts from Rattus
norvegicus and Bos taurus species, and expression of its mRNA in
different tissues. J Mol Evol. 39:655–660. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yeung K, Seitz T, Li S, Janosch P,
McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, et
al: Suppression of Raf-1 kinase activity and MAP kinase signalling
by RKIP. Nature. 401:173–177. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li HZ, Gao Y, Zhao XL, Liu YX, Sun BC,
Yang J and Yao Z: Effects of raf kinase inhibitor protein
expression on metastasis and progression of human breast cancer.
Mol Cancer Res. 7:832–840. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu Z, Smith PC, Zhang L, Rubin MA, Dunn
RL, Yao Z and Keller ET: Effects of raf kinase inhibitor protein
expression on suppression of prostate cancer metastasis. J Natl
Cancer Inst. 95:878–889. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li HZ, Wang Y, Gao Y, Shao J, Zhao XL,
Deng WM, Liu YX, Yang J and Yao Z: Effects of raf kinase inhibitor
protein expression on metastasis and progression of human
epithelial ovarian cancer. Mol Cancer Res. 6:917–928. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu Z, Kitagawa Y, Shen R, Shah R, Mehra R,
Rhodes D, Keller PJ, Mizokami A, Dunn R, Chinnaiyan AM, et al:
Metastasis suppressor gene Raf kinase inhibitor protein (RKIP) is a
novel prognostic marker in prostate cancer. Prostate. 66:248–256.
2006. View Article : Google Scholar
|
|
13
|
Al-Mulla F, Hagan S, Behbehani AI, Bitar
MS, George SS, Going JJ, García JJ, Scott L, Fyfe N, Murray GI, et
al: Raf kinase inhibitor protein expression in a survival analysis
of colorectal cancer patients. J Clin Oncol. 24:5672–5679. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu YF, Yi Y, Qiu SJ, Gao Q, Li YW, Dai CX,
Cai MY, Ju MJ, Zhou J, Zhang BH, et al: PEBP1 downregulation is
associated to poor prognosis in HCC related to hepatitis B
infection. J Hepatol. 53:872–879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia B, Liu H, Kong Q and Li B: RKIP
expression associated with gastric cancer cell invasion and
metastasis. Tumour Biol. 33:919–925. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Wang Y, Song Y, Fu Z and Yu W:
miR-27a regulates cisplatin resistance and metastasis by targeting
RKIP in human lung adenocarcinoma cells. Mol Cancer. 13:1932014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doyle B, Hagan S, Al-Mulla F, Scott L,
Harden S, Paul J, Mulcahy H, Murray GI, Sheahan K, O'Sullivan J, et
al: Raf kinase inhibitor protein expression combined with
peritoneal involvement and lymphovascular invasion predicts
prognosis in Dukes' B colorectal cancer patients. Histopathology.
62:505–510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Minoo P, Zlobec I, Baker K, Tornillo L,
Terracciano L, Jass JR and Lugli A: Loss of raf-1 kinase inhibitor
protein expression is associated with tumor progression and
metastasis in colorectal cancer. Am J Clin Pathol. 127:820–827.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zlobec I, Baker K, Minoo P, Jass JR,
Terracciano L and Lugli A: Node-negative colorectal cancer at high
risk of distant metastasis identified by combined analysis of lymph
node status, vascular invasion, and Raf-1 kinase inhibitor protein
expression. Clin Cancer Res. 14:143–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun J, Luo Y, Tian Z, Gu L, Xia SC and Yu
Y: Expression of ERBB3 binding protein 1 (EBP1) in salivary adenoid
cystic carcinoma and its clinicopathological relevance. BMC Cancer.
12:4992012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu Y, Chen W, Zhang Y, Hamburger AW, Pan H
and Zhang Z: Suppression of salivary adenoid cystic carcinoma
growth and metastasis by ErbB3 binding protein Ebp1 gene transfer.
Int J Cancer. 120:1909–1913. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Albini A: Tumor and endothelial cell
invasion of basement membranes. The Matrigel chemoinvasion assay as
a tool for dissecting molecular mechanisms. Pathol Oncol Res.
4:230–241. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maeda H, Segawa T, Kamoto T, Yoshida H,
Kakizuka A, Ogawa O and Kakehi Y: Rapid detection of candidate
metastatic foci in the orthotopic inoculation model of
androgen-sensitive prostate cancer cells introduced with green
fluorescent protein. Prostate. 45:335–340. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang S, Jean D, Luca M, Tainsky MA and
Bar-Eli M: Loss of AP-2 results in downregulation of c-KIT and
enhancement of melanoma tumorigenicity and metastasis. EMBO J.
17:4358–4369. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Fu Z, Binkley C, Giordano T,
Burant CF, Logsdon CD and Simeone DM: Raf kinase inhibitory protein
inhibits beta-cell proliferation. Surgery. 136:708–715. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yeung KC, Rose DW, Dhillon AS, Yaros D,
Gustafsson M, Chatterjee D, McFerran B, Wyche J, Kolch W and Sedivy
JM: Raf kinase inhibitor protein interacts with NF-kappaB-inducing
kinase and TAK1 and inhibits NF-kappaB activation. Mol Cell Biol.
21:7207–7217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang H, Park S, Sun SC, Trumbly R, Ren G,
Tsung E and Yeung KC: RKIP inhibits NF-kappaB in cancer cells by
regulating upstream signaling components of the IkappaB kinase
complex. FEBS Lett. 584:662–668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Corbit KC, Trakul N, Eves EM, Diaz B,
Marshall M and Rosner MR: Activation of Raf-1 signaling by protein
kinase C through a mechanism involving Raf kinase inhibitory
protein. J Biol Chem. 278:13061–13068. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lorenz K, Lohse MJ and Quitterer U:
Protein kinase C switches the Raf kinase inhibitor from Raf-1 to
GRK-2. Nature. 426:574–579. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Mulla F, Bitar MS, Al-Maghrebi M,
Behbehani AI, Al-Ali W, Rath O, Doyle B, Tan KY, Pitt A and Kolch
W: Raf kinase inhibitor protein RKIP enhances signaling by glycogen
synthase kinase-3β. Cancer Res. 71:1334–1343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xinzhou H, Ning Y, Ou W, Xiaodan L, Fumin
Y, Huitu L and Wei Z: RKIp inhibits the migration and invasion of
human prostate cancer PC-3M cells through regulation of
extracellular matrix. Mol Biol. 45:1004–1011. 2011. View Article : Google Scholar
|
|
32
|
Yousuf S, Duan M, Moen EL, Cross-Knorr S,
Brilliant K, Bonavida B, LaValle T, Yeung KC, Al-Mulla F, Chin E,
et al: Raf kinase inhibitor protein (RKIP) blocks signal transducer
and activator of transcription 3 (STAT3) activation in breast and
prostate cancer. PLoS One. 9:e924782014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee J, Lee J, Farquhar KS, Yun J,
Frankenberger CA, Bevilacqua E, Yeung K, Kim EJ, Balázsi G and
Rosner MR: Network of mutually repressive metastasis regulators can
promote cell heterogeneity and metastatic transitions. Proc Natl
Acad Sci USA. 111:E364–E373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun M, Gomes S, Chen P, Frankenberger CA,
Sankarasharma D, Chung CH, Chada KK and Rosner MR: RKIP and HMGA2
regulate breast tumor survival and metastasis through lysyl oxidase
and syndecan-2. Oncogene. 33:3528–3537. 2014. View Article : Google Scholar :
|