Introduction
Ovarian cancer is the most lethal cancer of
gynecologic cancers even though it causes fewer deaths than breast
cancer and precursor lesions of the uterine cervix (1). New cases of ovarian cancer are
estimated to be 21,980 in the United States in 2014 and deaths due
to ovarian cancer totaled 14,270, which represent 5% of deaths due
to female malignancies (2). Most
patients with ovarian cancer are not diagnosed at early stages due
to lack of obvious symptoms, few effective diagnostic approaches
and no tumor markers (3). Ovarian
cancer is difficult to treat and patient prognosis is poor unless
diagnosis occurs early, conferring a survival rate of 90–95%
(4). Thus, earlier detection is
crucial. Ovarian cancer is influenced by many factors such as
physical and chemical exposures, biotic factors, and heredity
(5). Genetic variants in
AURKA, BRCA1, and CCNE1 genes are associated
with ovarian cancer risk (6). Women
with hereditary ovarian cancer syndrome, such as mutations in BRCA1
and BRCA2, are estimated to confer a 40% greater risk of ovarian
cancer, but these mutations are found in only 0.05% of the female
population (1). Increased frequency
of single-nucleotide polymorphisms (SNP) rs11954856 and rs351771 of
adenomatous polyposis coli (APC) can increase the risk of ovarian
cancer in Polish women (7).
Therefore, more studies are needed to identify potential markers to
predict ovarian cancer susceptibility and this may improve
treatment strategies and increase survival.
Calcium-sensing receptor (CaSR) is a
G-protein-coupled receptor initially cloned from the bovine
parathyroid gland in 1993 (8). Its
structure has three domains: extracellular (coded for by the first
6 exons of the CaSR gene), a membrane-spanning motif, and an
intracellular tail (coded for by the 7th exon) (9). CaSR has been characterized as a sensor
for calcium and parathyroid hormone regulation via the parathyroid
gland and kidney in response to blood calcium (8,10,11).
CaSR function is required for normal epidermal differentiation via
mediation of calcium signaling in vivo (12). Mutations in the CaSR gene
lead to loss or gain of function, but most cause alterations in
extracellular calcium (13).
Disruption of CaSR function contributes to alterations in the
physiology of neoplastic cells (14), which modifies tumor development and
progression. SNP rs17251221 is located in an intron of the
CaSR gene on chromosome 3, and it is significantly
associated with serum calcium regulation (15,16).
Evidence suggests a significant association between CaSR rs17251221
and stone multiplicity in nephrolithiasis patients (17). CaSR rs17251221 has also been
verified to be associated with hepatocellular carcinoma (HCC) risk,
and the rs17251221 G allele genotype offers a better prognosis for
HCC treated with transcatheter hepatic arterial chemoembolization
(18). Data also show that
rs17251221 was strongly associated with prostate cancer (15). Previously, we reported that genetic
variations in rs17251221 for the CaSR gene are associated
with breast cancer risk and may be prognostic indicators for
patient outcomes (19). In the
present study, we analyzed rs17251221 of the CaSR gene to
clarify any association between CaSR rs17251221 and ovarian cancer
susceptibility.
Materials and methods
Patients and samples
Study participants (n=290) (mean age, 52.58±13.08
years) were diagnosed with ovarian cancer at the Qilu Hospital of
Shandong University between September 2008 and December 2014.
Patient data are presented in Table
I. Subjects were age-matched with 312 cancer-free females (mean
age 51.7±12.58 years) who were recruited from women who had annual
physical examinations. Participants involved were Han Chinese
residents. The study was approved by the Ethics Committee of
Shandong University, and written informed consent was obtained from
all the participants in the present study.
 | Table Irs17251221 genotype and allele
distribution in ovarian cancer and control. |
Table I
rs17251221 genotype and allele
distribution in ovarian cancer and control.
| Genotype | Ovarian (%)a | Control (%)a | P-value | OR 95% CI |
|---|
| AA | 277 (95.52) | 275 (88.14) | | 1 (reference) |
| AG | 13 (4.48) | 36 (11.54) | 0.001 | 0.359
(0.186–0.691) |
| GG | 0 (0) | 1 (0.32) | 0.001 | |
| AA | 277 (95.52) | 275 (88.14) | | 1 (reference) |
| AG+GG | 13 (4.48) | 37 (11.86) | 0.001 | 0.349
(0.181–0.671) |
| AA | 277 (100) | 275 (99.64) | | |
| GG | 0 | 1 (0.36) | 0.499 | |
| A | 567 (97.76) | 586 (93.91) | | 1 (reference) |
| G | 13 (2.24) | 38 (6.09) | 0.001 | 0.354
(0.186–0.671) |
DNA extraction
Blood was obtained from each subject and DNA was
extracted from the samples according to the protocol of the TIANamp
Genomic DNA kit (Tiangen, Beijing, China). DNA concentration and
purity were measured by using an ultraviolet spectrophotometer (GE
Healthcare, Pittsburgh, PA, USA). DNA samples were stored at −80°C
as previously described (20,21).
SNP genotyping analysis of CaSR
Cycling probes were synthesized by Takara
Biotechnology (Dalian, China), and an allelic discrimination assay
(Cycleave PCR® Core kit, CY505S, Takara) was performed
using an ABI 7900HT thermal cycler for SNP genotyping. Each
20-µl reaction contained: 1X Cycleave PCR reaction mixture,
0.2 µm PCR forward primer, 0.2 µm PCR reverse primer,
0.4 µm cycling probe, and 50 ng DNA template. PCR
amplification conditions were as follows: 95°C for 30 sec, 45
cycles of 95°C for 5 sec, 55°C for 10 sec, and 72°C for 25 sec.
Sequence Detection systems software version 2.4.1 was used for data
analysis. To confirm genotyping data, several DNA samples were
randomly selected for sequencing analysis.
Statistical analysis
Data analysis was performed as previously described
(21). The genotype and allele
frequency of CaSR were examined using a χ2 test for
Hardy-Weinberg equilibrium (HWE) (22). P>0.05 was set as a non-deviation
from HWE. The genotype and allele distribution were analyzed using
the χ2 test between ovarian cancer and control groups.
When 25% of analyzed cells had counts <5, the Fisher's exact
test was used. The association between ovarian cancer and the
CASR polymorphism was measured using the odds ratios (OR)
and clinicopathological characteristics of ovarian cancer were
analyzed using logistic regression models. The Kaplan-Meier method
was used to assess the association of the SNP rs17251221 genotype
with ovarian cancer patient survival. Data were considered
statistically significant at P<0.05. The data in our study were
analyzed with SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA)
software.
Results
Relationship between rs17251221 and
ovarian cancer susceptibility
Ovarian cancer patients and healthy controls were
all Mainland Chinese women. There was no significant difference
between the two groups with respect to matching characteristics. A
χ2 test was used to confirm that subjects met HWE. The
values for χ2 for healthy control and ovarian cases were
0.02 and 0.15, respectively (P>0.05).
An allelic discrimination assay was used to analyze
the rs17251221 polymorphism distribution in the ovarian cases and
controls. Significant differences between ovarian cancer cases and
controls are shown in Table I. AG
and GG genotypes had fewer cancer cases than controls and the
χ2 results showed that the genotype distribution between
the two groups was statistically significant (AA vs. AG vs. GG,
P=0.001). The difference between the additive genetic model of AA
and AG genotypes was statistically significant (P=0.001).
Additionally, AG patients had a low risk for ovarian cancer
compared with AA patients. Few GG patients were included for the
analysis. Thus, AA patients of the genotype AG+GG were assessed and
the results showed that this genotype was significantly correlated
with lower risk of ovarian cancer risk [P=0.001, OR=0.349, 95% CI
(0.181–0.671)]. The G allele of the CaSR rs17251221 polymorphism
appears to be protective against ovarian cancer [P=0.001, OR=0.345,
95% CI (0.186–0.671)]. Random DNA samples were subsequently
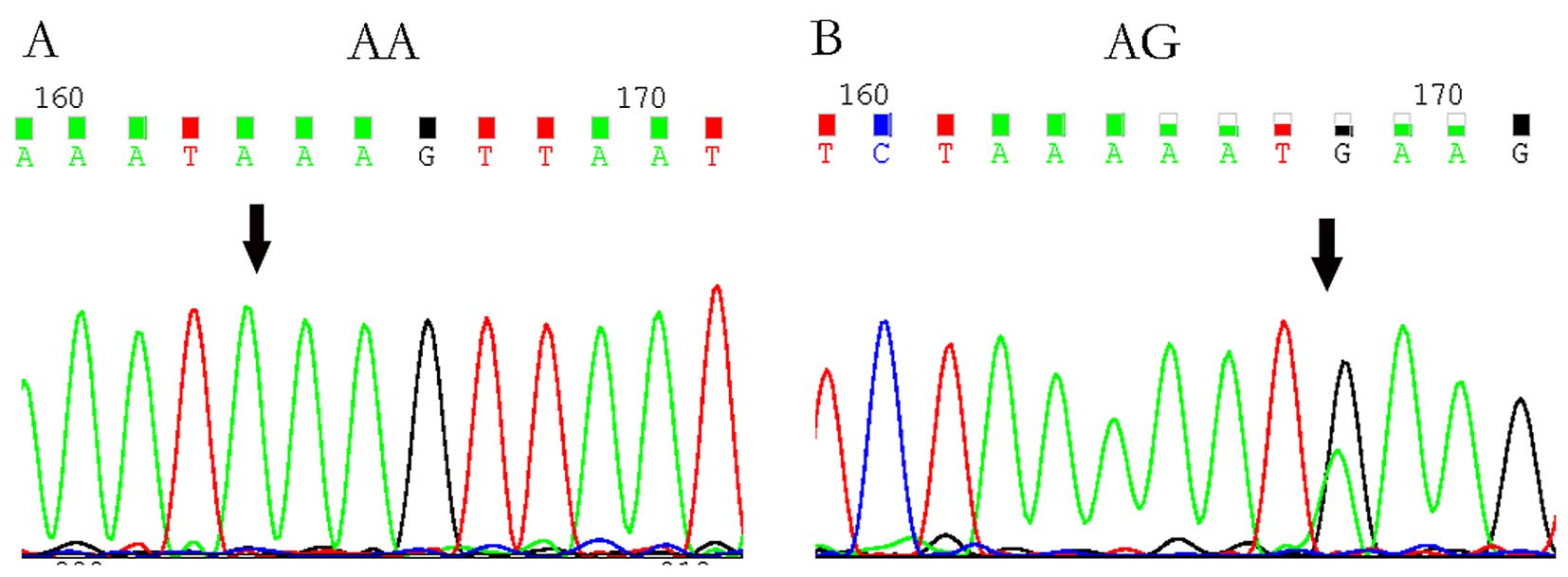
selected for sequencing to confirm genotyping results (Fig. 1).
Association analysis between rs17251221
and clinicopathological variables
To assess the association between rs17251221 and age
at diagnosis, tumor size, histologic type, pathological subtype,
and lymph node metastasis, serum CA-125 expression was measured
(Table II). The Kaplan-Meier
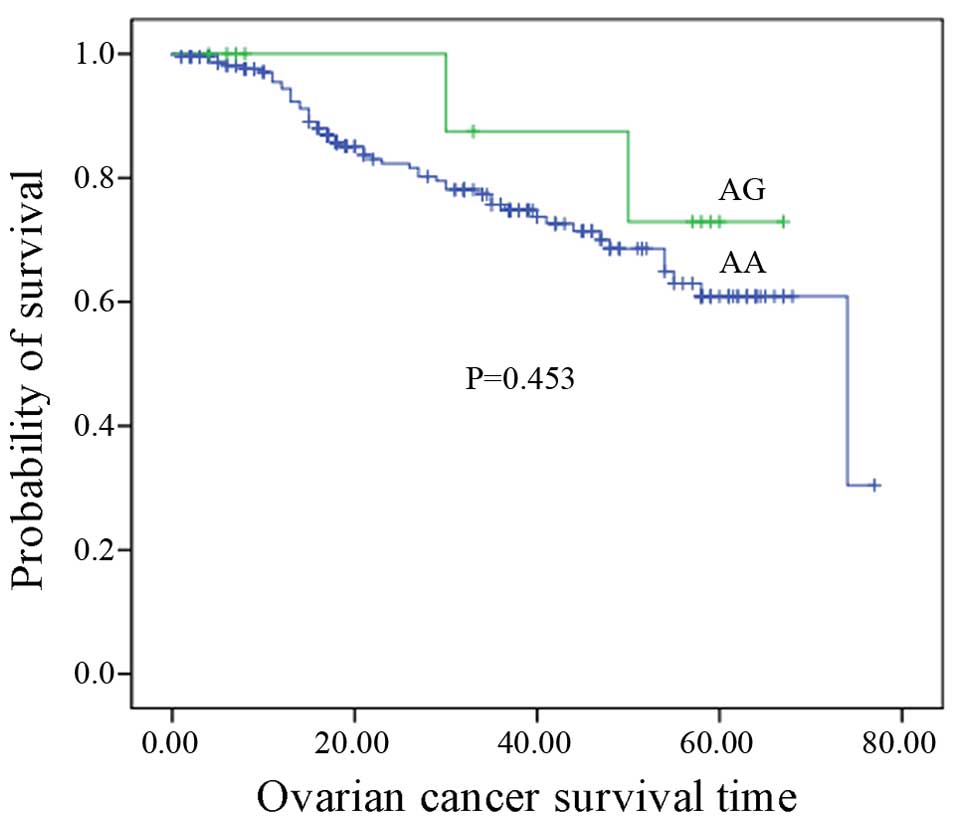
method was used to assess the association between rs17251221
genotypes and ovarian cancer survival. No correlation between
rs17251221 genotypes and ovarian cancer survival was identified
(P=0.453, P>0.05) (Fig. 2).
 | Table IIAssociation analysis between CaSR
rs17251221 and clinicopathological characteristics. |
Table II
Association analysis between CaSR
rs17251221 and clinicopathological characteristics.
| Clinicopathological
data | All (%) | Genotype (%)
| P-value | OR |
|---|
| AA | AG |
|---|
| Age (years) | | | | 0.153 | |
| ≤50 | 110 | 108 (98.18) | 2 (1.82) | | 1 (reference) |
| >50 | 179 | 168 (93.85) | 11 (6.15) | | 3.536 |
| Tumor size
(cm) | | | | 0.539 | |
| <10 | 177 | 170 (96.05) | 7 (3.95) | | 1 (reference) |
| ≥10 | 109 | 103 (94.495) | 6 (5.505) | | 1.415 |
| Tumor histologic
type | | | | 0.231 | |
| Epithelial | 252 | 241 (95.63) | 11 (4.37) | | 1 (reference) |
| Others | 29 | 28 (96.55) | 1 (3.45) | | 0.782 |
| Tumor pathological
subtype | | | | 0.054 | |
| Serous | 195 | 189 (96.92) | 6 (3.08) | | 1 (reference) |
| Others | 90 | 84 (93.33) | 6 (6.67) | | 2.25 |
| Positive lymph
node | | | | 0.393 | |
| Positive | 56 | 55 (98.21) | 1 (1.79) | | 1 (reference) |
| Negative | 226 | 215 (95.13) | 11 (4.87) | | 2.814 |
| CA-125 (U/ml) | | | | 0.067 | |
| <500 | 155 | 146 (94.19) | 9 (5.81) | | 1 (reference) |
| ≥500 | 127 | 123 (96.85) | 4 (3.15) | | 0.527 |
| Clinical stage | | | | 0.091 | |
| I, II | 100 | 94 (94) | 6 (6) | | 1 (reference) |
| III, IV | 174 | 168 (96.55) | 6 (3.45) | | 0.56 |
| Degree of
differentiation | | | | 0.099 | |
| Low | 189 | 183 (96.83) | 6 (3.17) | | 1 (reference) |
| Middle, high | 35 | 32 (91.43) | 3 (8.57) | | 2.859 |
Discussion
Ovarian cancer is the most deadly of the gynecologic
cancers (1). Largely due to
non-specific symptoms, late diagnosis, and few effective clinical
examination methods and indications during early stages of ovarian
cancer (23). Current screening
strategies are limited by sensitivity and specificity (24,25).
Thus, investigations are underway to identify ovarian cancer
biomarkers, especially genetic markers for assessing cancer risk
and monitoring therapeutic response (23).
CaSR is a G-protein-coupled receptor (8) that senses calcium and parathyroid
hormone regulation in response to blood calcium (8,10,11).
CaSR regulates homeostasis in response to the extracellular changes
of polycationic small molecules (14). In vivo, CaSR is required for
normal epidermal differentiation by mediating calcium signaling
required for keratinocyte differentiation (12). CaSR has been confirmed to act as a
tumor suppressor in colon and breast cancer (26,27).
If normal CaSR-induced responses to extracellular calcium are lost
or upregulated, neoplastic cell physiology can be altered,
contributing to neoplastic progression (14). rs17251221 is a CaSR genetic
variation confirmed to be a susceptibility marker of stone
multiplicity in nephrolithiasis and it is associated with coronary
heart disease, type 2 diabetes, HCC, and prostate and breast cancer
risk (15,17–19).
In the present study, we examined CaSR expression in
ovarian cancer patients and controls and identified that AG and GG
genotypes were correlated with low ovarian cancer risk compared to
the controls. The genotype distribution between the two groups was
statistically significant. Patients with AG genotypes had less risk
for ovarian cancer compared with homozygote AA. We also assessed
combined AG+GG genotypes in both groups and found a significant
correlation with low ovarian cancer risk. The G allele of the CaSR
polymorphism, rs17251221, appears to protect against ovarian
cancer. However, rs17251221 was not associated with
clinicopathological variables, and a prognosis analysis showed that
the genotype AG was not associated with ovarian cancer survival.
These results suggested that rs17251221 may be an independent
factor contributing to the progression of ovarian cancer in the Han
Chinese population, but it was not a prognostic indicator for
ovarian cancer survival. Enrolled subjects were from the Shandong
Province of China, but we suggest these results may be extrapolated
to larger samples. Our results can be used to suggest strategies
for ovarian cancer prediction. This represents the first variant
study of the CaSR polymorphism, rs17251221, and ovarian cancer risk
and, to the best of our knowledge, this is the first report to
suggest that the G allele of the CaSR polymorphism protects against
ovarian cancer, thus, the homozygous GG genotype may indicate lower
risk of ovarian cancer.
Acknowledgments
We thank LetPub for its linguistic assistance during
the preparation of this manuscript. This study was supported by the
Natural Science Foundation of Shandong Province (no. ZR2014HM070),
the Foundation of Shandong Medicine and Health Technology
Development Plan (no. 2014WS0134), the National High Technology
Research and Development Program ('863' Program) of China (no.
2014AA020605), the National Science and Technology Project (no.
2015BAI13B05), the National Natural Science Foundation of China
(no. 81101983), and the Science and Technology Development Project
of Shandong Province (no. 2014GSF118071).
References
|
1
|
Smith RA, Manassaram-Baptiste D, Brooks D,
Doroshenk M, Fedewa S, Saslow D, Brawley OW and Wender R: Cancer
screening in the United States, 2015: A review of current American
cancer society guidelines and current issues in cancer screening.
CA Cancer J Clin. 65:30–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang M, He Y, Shi L and Shi C:
Multivariate analysis by Cox proportional hazard model on prognosis
of patient with epithelial ovarian cancer. Eur J Gynaecol Oncol.
32:171–177. 2011.PubMed/NCBI
|
|
4
|
American College of Obstetricians and
Gynecologists Committee on Gynecologic Practice: Committee opinion
no. 477: The role of the obstetrician-gynecologist in the early
detection of epithelial ovarian cancer. Obstet Gynecol.
117:742–746. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sueblinvong T and Carney ME: Current
understanding of risk factors for ovarian cancer. Curr Treat
Options Oncol. 10:67–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng L, Song A, Ruan Y, Chen L, Liu D, Li
X, Guo H, Han J, Li Y, Tian X, et al: Genetic polymorphisms in
AURKA, BRCA1, CCNE1 and CDK2 are associated with ovarian cancer
susceptibility among Chinese Han women. Cancer Epidemiol.
37:639–646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mostowska A, Pawlik P, Sajdak S, Markowska
J, Pawałowska M, Lianeri M and Jagodzinski PP: An analysis of
polymorphisms within the Wnt signaling pathway in relation to
ovarian cancer risk in a Polish population. Mol Diagn Ther.
18:85–91. 2014. View Article : Google Scholar :
|
|
8
|
Brown EM, Gamba G, Riccardi D, Lombardi M,
Butters R, Kifor O, Sun A, Hediger MA, Lytton J and Hebert SC:
Cloning and characterization of an extracellular Ca(2+)-sensing
receptor from bovine parathyroid. Nature. 366:575–580. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vezzoli G, Terranegra A, Arcidiacono T,
Gambaro G, Milanesi L, Mosca E and Soldati L; GENIAL network
(Genetics and Environment in Nephrolithiasis Italian Alliance):
Calcium kidney stones are associated with a haplotype of the
calcium-sensing receptor gene regulatory region. Nephrol Dial
Transplant. 25:2245–2252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pearce SH and Thakker RV: The
calcium-sensing receptor: Insights into extracellular calcium
homeostasis in health and disease. J Endocrinol. 154:371–378. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brown EM and Hebert SC:
Calcium-receptor-regulated parathyroid and renal function. Bone.
20:303–309. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tu CL, Oda Y, Komuves L and Bikle DD: The
role of the calcium-sensing receptor in epidermal differentiation.
Cell Calcium. 35:265–273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hendy GN, D'Souza-Li L, Yang B, Canaff L
and Cole DE: Mutations of the calcium-sensing receptor (CASR) in
familial hypocalciuric hypercalcemia, neonatal severe
hyperparathyroidism, and autosomal dominant hypocalcemia. Hum
Mutat. 16:281–296. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rodland KD: The role of the
calcium-sensing receptor in cancer. Cell Calcium. 35:291–295. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jorde R, Schirmer H, Njølstad I, Løchen
ML, Bøgeberg Mathiesen E, Kamycheva E, Figenschau Y and Grimnes G:
Serum calcium and the calcium-sensing receptor polymorphism
rs17251221 in relation to coronary heart disease, type 2 diabetes,
cancer and mortality: The Tromsø Study. Eur J Epidemiol.
28:569–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Seaghdha CM, Yang Q, Glazer NL, Leak TS,
Dehghan A, Smith AV, Kao WH, Lohman K, Hwang SJ, Johnson AD, et al
GEFOS Consortium: Common variants in the calcium-sensing receptor
gene are associated with total serum calcium levels. Hum Mol Genet.
19:4296–4303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chou YH, Woon PY, Chen WC, Hsu YW, Chang
JM, Hwang DY, Chiu YC, Kuo HC, Chang WP, Hou MF, et al: A genetic
polymorphism (rs17251221) in the calcium-sensing receptor gene
(CASR) is associated with stone multiplicity in calcium
nephrolithiasis. PLoS One. 6:e252272011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang Q, Zhao Y, Wang Y and Wei M: A
genetic variant (rs17251221) in the calcium-sensing receptor
relates to hepatocellular carcinoma susceptibility and clinical
outcome treated by transcatheter hepatic arterial chemoembolization
(TACE) therapy. Med Oncol. 31:2672014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Kong X, Jiang L, Ma T, Yan S, Yuan C
and Yang Q: A genetic polymorphism (rs17251221) in the
calcium-sensing receptor is associated with breast cancer
susceptibility and prognosis. Cell Physiol Biochem. 33:165–172.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X, Zhang N, Li X, Moran MS, Yuan C,
Yan S, Jiang L, Ma T, Haffty BG and Yang Q: Identification of novel
variants of metadherin in breast cancer. PLoS One. 6:e175822011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang N, Li X, Tao K, Jiang L, Ma T, Yan
S, Yuan C, Moran MS, Liang F, Haffty BG, et al: BCL-2 (−938C >
A) polymorphism is associated with breast cancer susceptibility.
BMC Med Genet. 12:482011. View Article : Google Scholar
|
|
22
|
Rodriguez S, Gaunt TR and Day IN:
Hardy-Weinberg equilibrium testing of biological ascertainment for
Mendelian randomization studies. Am J Epidemiol. 169:505–514. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meng QH, Xu E, Hildebrandt MA, Liang D, Lu
K, Ye Y, Wagar EA and Wu X: Genetic variants in the fibroblast
growth factor pathway as potential markers of ovarian cancer risk,
therapeutic response, and clinical outcome. Clin Chem. 60:222–232.
2014. View Article : Google Scholar
|
|
24
|
Johnson CC, Kessel B, Riley TL, Ragard LR,
Williams CR, Xu JL and Buys SS; Prostate, Lung Colorectal and
Ovarian Cancer Project Team: The epidemiology of CA-125 in women
without evidence of ovarian cancer in the Prostate, Lung,
Colorectal and Ovarian Cancer (PLCO) Screening Trial. Gynecol
Oncol. 110:383–389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duffy MJ, Bonfrer JM, Kulpa J, Rustin GJ,
Soletormos G, Torre GC, Tuxen MK and Zwirner M: CA125 in ovarian
cancer: European Group on Tumor Markers guidelines for clinical
use. Int J Gynecol Cancer. 15:679–691. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aggarwal A, Prinz-Wohlgenannt M, Tennakoon
S, Höbaus J, Boudot C, Mentaverri R, Brown EM, Baumgartner-Parzer S
and Kállay E: Calcium-sensing receptor: A promising target for
prevention of colorectal cancer. Biochim Biophys Acta. Feb
18–2015.Epub ahead of print. View Article : Google Scholar
|
|
27
|
Liu G, Hu X and Chakrabarty S: Calcium
sensing receptor down-regulates malignant cell behavior and
promotes chemosensitivity in human breast cancer cells. Cell
Calcium. 45:216–225. 2009. View Article : Google Scholar
|