Introduction
Testicular tumors are common malignant solid tumors
of the urinary and reproductive system. Chemotherapy is the most
common method to treat testicular cancer (1,2).
However, low therapeutic efficiency is a crucial factor affecting
the therapeutic results (3). It has
been found that P-glycoprotein (P-gp), as a multidrug
resistance-associated protein, is highly expressed in capillary
endothelial cells of the testis to form a biological barrier,
blocking access of chemotherapy agents to tumor sites, playing an
important role in the drug resistance of testicular tumors
(4,5). P-gp, encoded by the MDR1 gene, is a
membrane protein that functions as an ATP-dependent efflux pump,
pumping out water insoluble toxic substances from cells, and
impeding the delivery of chemotherapeutic drugs to the testis
(6,7). Therefore, breaking through the
blood-testis barrier and the reversal of drug resistance are key
goals for the treatment of testicular tumors (8).
Thus, we aimed to use RNA interference technology to
silence the expression of the MDR1 gene and inhibit expression of
P-gp, to improve the responsiveness to chemotherapeutic drugs of
testicular tumors in vitro (9,10).
The first and most important problem of gene therapy
is development of a safe and effective gene therapy carrier, as
well as an effective way to delivery genes into target cells.
However, commonly used traditional transfection methods are not
satisfactorily translatable to in vivo conditions (11). Recent studies have shown that
microbubble contrast agents can carry and release genes under
ultrasonic action, promoting the in vitro and in vivo
transfection of target genes. Ultrasound microbubble-mediated gene
therapy is expected to be a safe, efficient and non-invasive gene
therapy (12,13).
In a previous study, we revealed that ultrasound
micro-bubble-mediated gene delivery effectively assisted the entry
of siMDR1 into L2RYC cells, inhibited the expression of the MDR1
gene and P-protein, as well as the efflux pump function of
P-protein, increasing the aggregation of chemotherapy drugs in
cells, thus resulting in a more effective antitumor effect
(9). In the present study, we
demonstrated that the siMDR gene-carrying polymer coated
microbubble contrast agent reached the testis through intravenous
injection, and destruction of microbubbles under the ultrasonic
action successfully transfected the siMDR1 gene into rat testicular
capillary endothelial cells. Silencing of MDR1 inhibited the
expression and function of P-gp, so that the anticancer drug
daunorubicin entered more easily into testicular tumors. Ultrasound
microbubble-mediated gene therapy combined with RNAi, has a good
application prospect in the treatment of testicular tumors.
Materials and methods
Cell culture and chemicals
The L2RYC cell line was obtained from ATCC
(Manassas, VA, USA), and maintained in complete Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS; HyClone, Logan, UT, USA), 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C in 5% CO2. Unless
indicated otherwise, all chemicals were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Ultrasound microbubble-mediated gene
transfection
Sprague-Dawley (SD) male rats, 4-weeks of age
(n=25), were randomly divided into five groups: group 1, plasmid
only; group 2, ultrasound + plasmid; group 3, microbubble +
plasmid; group 4, ultrasound + microbubbles + plasmid; and group 5,
untreated control. The siMDR1-loaded lipid micro-bubble was
prepared as described (ref?). Plasmid (50 µg) in 100
µl phosphate-buffered saline (PBS) or lipid microtubule was
injected into the tail vein of the experimental rats, and the right
testis was exposed to 300 kHz-ultrasound irradiation at the
acoustic intensity of 2 W/cm2 for 10 min. After 2 weeks,
frozen sections of the right testis were observed. Green
fluorescent protein (GFP) expression was considered as an indicator
of efficiency of gene delivery.
Real-time PCR analysis
As previously described (14), freshly prepared testis tissues were
minced and homogenized in TRIzol reagent and total RNA was
extracted by TRIzol method. cDNA templates were generated with 10
µg of total RNA and hexamer using SuperScript II reverse
transcriptase (Invitrogen). The PCR primers specific for detecting
MDR1 (sense, 5′-GAGAACATCGCCTACGG-3′ and antisense,
5′-GCTTCCTGGACGACCTT-3′); and GAPDH (sense,
5′-TGGATGGTCCCTCTGGAA-3′ and antisense, 5′-GTGAG CTTCCCGTTCAGC-3′)
were designed using the Primer 3.0 program. Real-time PCR reactions
were carried out using a Bio-Rad protocol as follows: 94°C for 20
sec, 55°C for 20 sec, 70°C for 20 sec for 40 cycles, reading plates
after each cycle. Data are reported as the fold-change with
endogenous GAPDH normalization.
Western blot analysis
Western blotting was performed as previously
described (14,15). Freshly prepared testis tissues were
homogenized in liquid nitrogen and lysed in RIPA buffer with
phenylmethanesulfonyl fluoride (PMSF). Approximately 20 µg
of total proteins/lane were subjected to 10% SDS-PAGE gel and then
electrically transferred to Immobilon-P membranes (Millipore). The
membranes were blocked with 5% fat-free skimmed milk in
Tris-buffered saline and Tween-20 (TBST) buffer at room temperature
for 1 h, followed by incubation with anti-MDR1 or anti-β-actin at
4°C overnight. After washing with TBST, the membranes were probed
with the appropriate secondary antibody conjugated to horseradish
peroxidase (Santa Cruz Biotechnology, USA) at room temperature for
1 h. The expression of the proteins was visualized using enhanced
chemiluminescent substrate (Kaiji Biotechnology, China) and exposed
under the Syngene G:BOX Imaging System.
Daunorubicin accumulation
Daunorubicin accumulation was detected at 2 days
after the SD rat treatment. Daunorubicin (2 ml) at a concentration
of 0.1 mg/ml was administered via tail vein injection. Testis
tissues were collected after 2 h and prepared into frozen sections.
Red fluorescence which was emitted by daunorubicin indicated the
accumulation/concentration of daunorubicin and was detected under a
fluorescence microscope.
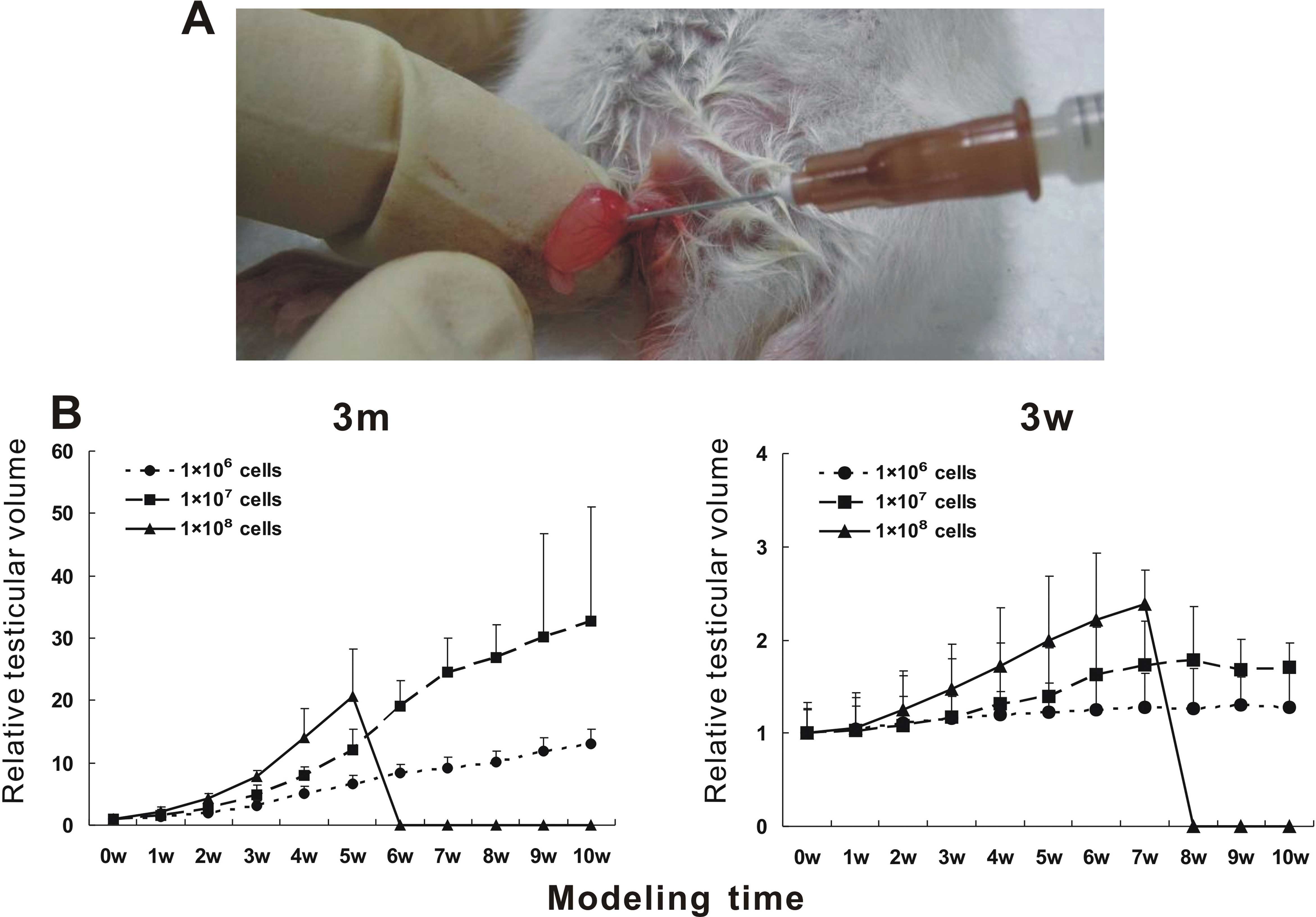
Establishment of the testicular tumor
model
Sixty SD male rats 3-weeks and 3-months of age were
collected; 10 rats in each group. After being anesthetized by
chloral hydrate, the right testis of the rats was exposed by
opening the skin, meat membrane and tunica vaginalis. A cell (10
µl) suspension containing 1×106, 1×107
or 1×108 cells was injected into the testis via the
connection part of the ductuli efferentes testis (16). Then the testis was put back into the
scrotum, the spermatic cord and meat membrane were fixed to prevent
retraction, and finally the scrotal incision was sutured. The
survival and general situation of the rats were dynamically
monitored. The testis volume was detected every 7 days to draw a
growth curve. Testis was scanned with Color Doppler Ultrasound
Diagnostic instrument to observe the change in testicular size,
blood supply and sonographic characteristics. Hematoxylin and eosin
(H&E) staining and immunohistochemistry were performed to
evaluate tumor progression.
Ultrasound microbubble-mediated siMDR1
transfection combined with chemotherapy in the treatment of testis
tumors
Forty SD male rats 3-weeks of age were collected to
set up the testicular tumor model as described above. After 3
weeks, successful constructed testicular tumor-bearing rats were
randomly divided into 4 groups: group 1, chemotherapy only; group
2, blank microbubbles + ultrasound + chemo- therapy; group 3,
siMDR1 plasmid-loaded microbubbles + ultrasound + chemotherapy; and
group 4, saline control. Microbubbles or siMDR1 plasmid-loaded
microbubbles were constantly injected into the tail vein and the
right testis was exposed to an ultrasound wave field at 300 kHz, 2
W/cm2, irradiation for 10 min. At 1 day after
microbubble transfection, the rat models were treated with
vincristine chemotherapy by intravenous injection 1 time/week, for
2 weeks. Surface area was calculated on the basis of the
Meeh-Rubner formula: A (m3) = 9.1 × W
(g)2/3/10,000; rat and human dose conversion formula:
trial dose in rats (mg/kg) = dose in human (mg/kg) × 36/6 (human
dose conversion factor/rat dose conversion factor). The survival
and general situation of rats was dynamically monitored. The volume
of the testis was measured every week. Models were sacrificed at 1
week after chemotherapy for H&E staining, immunohistochemistry
and Fas/p53 gene detection.
H&E staining and
immunohistochemistry
The harvested tumor tissues were fixed with 10%
formalin, embedded in paraffin, and serially cut into 5-µm
sections. Sections of each specimen were stained with H&E. For
immunohistochemistry, the sections were dewaxed in xylene,
rehydrated in graded ethanol solutions, and denatured in water at
60°C for 1 h, followed by incubation with α-1-antitrypsin (ATT)
antibody at 4°C overnight. After washing in PBS 3 times, the
sections were incubated with anti-goat IgG HRP-conjugated secondary
antibody at 37°C for 1 h, colored with DAB and counterstained with
hematoxylin.
Statistical analysis
Quantitative data are presented as the mean ± SD.
Statistical analysis was performed using the two-tailed Student's
t-test or analysis of variance. The probability value of P<0.05
was considered to indicate a statistically significant result.
Results
Ultrasound-targeted microbubble
destruction promotes siMDR1 gene delivery in vivo
The MDR1 gene which is highly expressed in the
testicular capillary wall, hinders chemotherapy drugs into the
testis (4,17). Herein, we aimed to use
ultrasound-targeted microbubble destruction to deliver the siMDR1
gene into in vivo target testicular capillaries in
vivo, to explore whether the expression and function of the
MDR1 gene and P-gp were effectively suppressed.
Since the pSEB-siMDR1 plasmid contains the GFP gene
sequence, GFP is an indicator to measure the gene transfection
efficiency. As shown in Fig. 1A,
GFP expression in testicular interstitial capillary endothelial
cells, was only observed in the microbubbles + ultrasound group
(group 4), suggesting that the pSEB-siMDR1 plasmid was successfully
transfected. There was no GFP expression in the other 4 groups. We
further demonstrated that the mRNA expression of the MDR1 gene
(Fig. 1B) and protein expression
(Fig. 1C) of P-gp were reduced in
the microbubble + ultrasound group only. No difference was found
between the other 3 intervention and control groups. Daunorubicin
as a P-gp substrate, spontaneously emits red fluorescence. At 1 h
after daunorubicin injection via tail vein, red fluorescence
indicating increased daunorubicin accumulation was observed in the
frozen sections of testis in the microbubble + ultrasound group
(Fig. 1D), suggesting that the
function of P-gp was inhibited and the drug accessed the testis
easier. The above results indicated that the combination of
ultrasound and microbubbles is an effective method to mediate gene
transfection in vivo.
 | Figure 1Ultrasound microbubble-mediated gene
delivery promotes siMDR1 gene transfection in vivo.
Twenty-five Sprague-Dawley male rats 4-weeks of age were randomly
divided into five groups: group 1, plasmid only; group 2,
ultrasound + plasmid; group 3, microbubble + plasmid; group 4,
ultrasound + micro-bubbles + plasmid; group 5, untreated control.
The pSEB-siMDR plasmid, microbubbles or siMDR1-loaded lipid
microbubbles were injected into the tail vein of the experimental
rats, and the right testis was exposed to ultrasound irradiation.
After 2 weeks of treatment, the tissues were harvested for
detection. (A) Ultrasound microbubble-mediated gene delivery
efficiently promoted the transfection of pSEB-siMDR1 into
testicular vascular endothelial cells. GFP expression indicates the
efficiency of gene delivery (scale bar, 200 µm). (B) The
mRNA expression of the MDR1 gene in testis tissues was detected by
real-time PCR. All samples were normalized to GAPDH in triplicate,
and the PCR results were confirmed in at least 3 sets of
independent experiments (*P<0.05 compared with group
1). (C) The protein expression of MDR1 in testis tissues was
detected by western blotting using the anti-MDR1 or β-actin
antibody. Proteins were collected and lysed at 2 weeks after
treatment and subjected to SDS-PAGE and western blotting using the
anti-MDR1 antibody. Equal loading of the samples was normalized by
β-actin detection. (D) Daunorubicin accumulation was increased in
the tissues treated with siMDR1-loaded lipid microbubble
transfection. Red fluorescent cells were observed under a
microscope. Cells in group 4 exhibited more red granular
fluorescence in the cytoplasm (indicated by a white arrow; scale
bar, 200 µm). GFP, green fluorescent protein. |
These data suggest that ultrasound
microbubble-mediated gene delivery effectively promoted the plasmid
DNA transfection in vivo.
Establishment of the testicular tumor
model
Rat yolk sac tumor L2 cells were injected into
testicular tissue of the SD rats system to establish the testicular
tumor model (Fig. 2A), and then we
evaluated the model feasibility and application value by observing
the biological characteristics of the tumors, growth rate and
morphology. In rats of different ages, stages and different
planting cell concentrations, the testicular tumor formation rate
and the survival time of the tumor-bearing rats were different
(Table I). In the group with
implanted cells at the concentration of 1×106/ml, no
tumors were present at the end of the experiment. At 3 weeks after
implantion of cells at the concentration of 1×107, the
tumor formation rate of the 3-week-old rats was 100%, and increased
gradually with time after planting, and reached a peak at the 6th
week with regions of ulceration and erosion; some rats began to
die. The tumor formation rate of the 3-month-old rats was only
12.5%, and tumors increased more slowly. When plantation of the
cells was at the concentration of 1×108, the tumor
formation rate of both 3-week- and 3-month-old rats were 100%, yet
the tumors grew very quickly with a high mortality rate.
 | Table ITesticular tumor formation rate and
the survival time of the tumor bearing rats. |
Table I
Testicular tumor formation rate and
the survival time of the tumor bearing rats.
| Injected cell
no. | No. of samples | Tumor formation/no.
of surviving rats after 1-week injection | Tumor formation/no.
of surviving rats after 3-week injection | Tumor formation/no.
of surviving rats after 6-week injection | Tumor formation/no.
of surviving rats after 10-week injection |
|---|
| 3-Week-old SD
rats |
1×106 | 10 | 0/10 | 0/10 | 0/9 | 0/9 |
|
1×107 | 10 | 6/9 | 9/9 | 7/7 | 2/2 |
|
1×108 | 10 | 8/8 | 4/4 | 0/0 | 0/0 |
| 3-Month-old SD
rats |
1×106 | 10 | 0/9 | 0/9 | 0/9 | 0/9 |
|
1×107 | 10 | 1/8 | 1/8 | 1/8 | 0/7 |
|
1×108 | 10 | 9/9 | 7/7 | 2/2 | 0/0 |
The growth curve of testicular tumors (Fig. 2B) showed that the testicular volume
of the rats injected with 1×106 cells was similar to
that of the control group. With injection of 1×107 tumor
cells, the relative volume of the testis was significantly larger
than that of the control group, particularly the 3-week-old rats.
With injection of 1×108 tumor cells, the testis volume
increased rapidly; all rats died after 5 weeks in the 3-week-old
rats and 7 weeks in the 3-month-old SD rats, respectively. Thus, we
chose to establish the testicular tumor model with the 3-week-old
rats and implanted cells at the concentration of 1×107.
Ultrasound imaging showed that compared with the control group, the
testicular volume of the model side increased significantly; the
internal echo was medium and uniform, with small punctuate and
cord-like high echo. Part of the testicular tissue exhibited
liquefied necrosis with no echo area (Fig. 3A). The affected testis was much
larger than the normal side; the testicular mass was suborbicular,
cystic and enveloped. Transverse section of the tumor was pale
yellow, soft fleshy with partial necrosis (Fig. 3B). Pathological sections under the
microscope exhibited loose reticulate structure, regions of adenoid
structure, eosinophilic granular and Schiler-Duval bodies by
H&E staining. Immunohistochemistry showed that AAT was
positively stained (Fig. 3C).
Therefore, the testis tumor model was successfully constructed.
Ultrasound microbubble-mediated siMDR1
gene therapy improves the effect of chemotherapy on the testicular
tumors
This experiment aimed to investigate the treatment
efficiency of chemotherapy drugs on testicular tumors following the
inhibition of P-gp. Following treatment with chemotherapy drugs,
tumor growth was inhibited. The survival rate was improved
obviously when compared with the control group at the same
time-point. At the end-point, the survival rate of group 3 was
increased to 60%, which was significantly different with the other
groups (P<0.05). Chemotherapy was reported to inhibit the growth
of tumors (18). Relative testis
volume was significantly smaller than that of the control group. In
our groups, the testicular volume of the ultrasound
microbubble-mediated siMDR1 therapy after chemotherapy was the
smallest (Fig. 4A and B),
indicating the best efficiency of tumor suppression.
H&E staining showed that the numbers of tumor
cells within the testicular tissues of group 1–3 were significantly
decreased compared with that of group 4, without tumor-specific
adenoid structure, and no eosinophilic bodies and Schiler-Duval
bodies. Particularly group 3 had visible lumen-like structure in
testis tissues. Immunohistochemistry results showed that positive
AAT expression of testicular tissues in group 1–3 was lower than
that in group 4 (Fig. 5).
Therefore, we further confirmed that ultrasound combined with
micro-bubble transfection of siMDR1 in the testicular capillary
wall provided easier access of vincristine to the testis tissues,
and thus improved the effect of chemotherapy on testicular
tumors.
Discussion
Testicular cancer is one of the most common types of
cancer of the urinary and reproductive system. The effect of
chemotherapy still requires improvement (1,2,19,20).
The blood-testis barrier hinders chemotherapy drugs into the testis
tissues, which is an important factor that affects treatment
outcome (21,22). A recent study has found that an
ATP-dependent drug efflux pump protein is expressed in the
capillary endothelium of the testis and is related to the formation
of the blood-testis barrier (23).
Previous studies have demonstrated that this protein associated
with tumor multidrug-resistance is P-glycoprotein (P-gp), which is
strongly expressed in testicular interstitial capillary endothelial
cells (24). P-gp is a glycoprotein
encoded by the MDR1 gene, is located on the cell membrane and is an
ATP-dependent efflux pump, which pumps the insoluble toxic
substances out of the cell, so that chemotherapy drugs cannot
easily enter into the testis (4,5,25). In
an MDR1-knockout rat model, the concentration of P-gp substrates in
the testis was significantly higher than that in the normal rat
(26). The structure of the
blood-brain barrier is similar to the blood-testis barrier. The
rate of positive P-gp expression was found to be 65.8% in the brain
tissues of children with intracranial tumors, while the positive
rate of P-gp in brain tissues of children with non-intracranial
tumors was only 10% (27).
Hendrikse et al (28) used
isotype-labeled P-gp reversal agents to block the P-gp function in
the blood-brain barrier, and found that the concentration of
intracranial drug increased by 13-fold, indicating that P-gp plays
an important role in the blood-brain barrier, and possible also in
the blood-testis barrier. P-gp-mediated reverse efflux is not only
a part of the biological function of the blood-testis barrier, yet
also may be associated with the application of chemotherapy drugs
in testicular tumors. Therefore, breaking through the barrier has a
great effect on the treatment of testicular cancer (5,8,29,30).
In the present study, we aimed to use RNA
interference technology to silence the mRNA expression of the
endogenous MDR1 gene, resulting in inhibition of P-gp expression
and resistance reversal in testicular tumors, providing a suitable
condition for chemotherapy drug treatment. The main concern of
in vivo gene therapy is how to successfully transfect and
express the objective gene in target cells (31). In recent studies, it has been
demonstrated that ultrasound-mediated gene transfer is a safe,
efficient and non-invasive gene therapy as a possible alternative
to viral gene transfer. It plays a significant role in gene
therapy-based approaches to the treatment of diseases (32–34).
We previously demonstrated that ultrasound microbubble-mediated
destruction could effectively delivery siRNA specific for the MDR1
gene into L2-RYC cells using ultrasound microbubble-mediated
destruction. We successfully inhibited MDR1 expression and function
of P-gp. L2-RYC cells with MDR1 silencing became more sensitive to
anticancer drugs, vincristine and dactinomycin (9). In the present study, we further
confirmed that ultrasound microbubble-mediated destruction led to
transfection of pSEB-siMDR1 into rat testis capillary endothelial
cells. The endogenous expression of the MDR1 gene and P-protein
decreased. P-gp function was also suppressed, altering the
high-resistance state of testicular tumors in response to
chemotherapy drugs.
Since the origin and classification of testicular
tumors are markedly complex, the animal models are not, strictly
speaking, valid for the study of testicular tumors. Usually the
transplanted tumor model is established in nude rats; however, nude
rats have characteristics of immunodeficiency, therefore the
biological characteristics of testicular tumors are different
compared with normal immune rats (35). In this study, we implanted a rat
yolk sac tumor cell line.
L2RYC cells were injected into the testis tissues of
normal immune system SD rats to establish the animal model. Graft
rejective reaction is the most important problem of a cell
implanted animal model and the use of immunosuppressants is needed.
However, in tumor therapy, immunosuppressants are also used as
antitumor medicines. The efficiency of chemotherapeutics on tumors
may be affected using immunosuppressants (36–38).
Thus, in the present experiment, we used 3-week- and 3-month-old SD
rats to set-up the animal model. The establishment of a cell
transplantation tumor model prior to development of an adaptive
immune system may be feasible.
The number of inoculated cells is another important
factor affecting the establishment of a transplanted tumor model
(39). Usually in the nude rat
model, 1×106 inoculated cells form a tumor (40). However, no tumor formation was found
in the 3-week- or 3-month-old SD rats following 1×106
L2RYC cell transplantation. When 1×107 cells were
implanted into the testis, tumors were formed in the 3-week-old
rats but not in the 3-month-old rats. This result may be associated
with host immune graft rejection suggesting that more cells are
required to establish the xenografts in normal animals compared to
the nude rats. If the number of cells was excessive
(1×107), tumor cells were strongly invasive, leading to
the quick death of the host rat. The pathological manifestations
mainly exhibited testicular necrosis, which was different from the
clinical course and performance, and not suitable for tumor study.
At 4 weeks after 1×107 L2RYC cells were inoculated in
the 3-week-old SD rats, ultrasound imaging and histopathological
characteristics were similar to clinical testicular yolk sac
tumors, indicating that this method can successfully establish a
feasible model of testicular tumors.
Next, we assessed the feasibility of ultrasound
micro-bubble-mediated destruction in delivering chemotherapy drugs
in the tumor model. By intravenous injection of microbubbles, the
permeability of the cell membrane was enhanced under local
ultrasonic action, thus promoting the transfection of target genes
or drugs (41). Through the
observation of tumor growth, the survival rate of tumor-bearing
rats, and detection of pathological changes, we found that
ultrasound microbubble-mediated destruction followed by
chemotherapy treatment was most effective for the treatment of
testis tumors. Compared with the chemotherapy alone group,
ultrasound microbubble-mediated destruction combined with
chemotherapy had a better effect, probably since the permeability
of testicular capillary transiently increased under ultrasound
microbubble-mediated destruction. The drug concentration in the
testis was slightly higher than that in the chemotherapy alone
group. Expression of an exogenous gene mediated by transfection
sustains for 2–3 weeks (42). MDR1
expression was suppressed during this time window. The permeability
of the testicular vascular was selectively increased following
suppression of the P-gp substrate, and then drugs easily entered
into the testis tissues, thereby enhancing the effect of
chemotherapy.
In summary, the present study mainly focused on the
issue that chemotherapy drugs cannot easily enter testicular
tumors. We used ultrasound microbubble-mediated destruction method
to increase the permeability of the capillary endothelial cell
membrane, and achieved efficient in vivo transfection of the
siMDR1 gene and inhibited P-gp production. The reversal of
chemotherapy drug resistance in testicular tumors improves the
treatment effect, which is expected to provide an effective method
for the treatment of testicular tumors.
Acknowledgments
The present study was supported in part by a
research grant from the National Natural Science Foundation of
China (nos.81001030 to Y.H. and 81301300 to X.-J.J.). This study
was also supported in part by the Special Fund of Chongqing Key
Laboratory (CSTC) (to G.-H.W.), Program for Innovation Team
Building at Higher Education Institutions in Chongqing, China (to
G.-H.W.) and National Clinical Key Subject Construction Project
(AXA medical office letter [2013]544).
References
|
1
|
Nakamura T and Miki T: Recent strategy for
the management of advanced testicular cancer. Int J Urol.
17:148–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Allaway M, Nseyo UO and Kandzari SJ:
Primary testicular sarcoma. J Urol. 163:18712000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawai K and Akaza H: Current status of
chemotherapy in risk-adapted management for metastatic testicular
germ cell cancer. Cancer Sci. 101:22–28. 2010. View Article : Google Scholar
|
|
4
|
Schrader AJ, Seger M, Konrad L, Olbert P,
Hegele A, Hofmann R and Heidenreich A: Clinical impact of
MDR1-expression in testicular germ cell cancer. Exp Oncol.
29:212–216. 2007.PubMed/NCBI
|
|
5
|
Bart J, Hollema H, Groen HJ, de Vries EG,
Hendrikse NH, Sleijfer DT, Wegman TD, Vaalburg W and van der Graaf
WT: The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and
MRP2, in the normal blood-testis barrier and in primary testicular
tumours. Eur J Cancer. 40:2064–2070. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kimura Y, Matsuo M, Takahashi K, Saeki T,
Kioka N, Amachi T and Ueda K: ATP hydrolysis-dependent multidrug
efflux transporter: MDR1/P-glycoprotein. Curr Drug Metab. 5:1–10.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sivapackiam J, Harpstrite SE, Prior JL, Gu
H, Rath NP and Sharma V: Synthesis, molecular structure, and
validation of metalloprobes for assessment of MDR1
P-glycoprotein-mediated functional transport. Dalton Trans.
39:5842–5850. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bart J, Groen HJ, van der Graaf WT,
Hollema H, Hendrikse NH, Vaalburg W, Sleijfer DT and de Vries EG:
An oncological view on the blood-testis barrier. Lancet Oncol.
3:357–363. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He Y, Bi Y, Hua Y, Liu D, Wen S, Wang Q,
Li M, Zhu J, Lin T, He D, et al: Ultrasound microbubble-mediated
delivery of the siRNAs targeting MDR1 reduces drug resistance of
yolk sac carcinoma L2 cells. J Exp Clin Cancer Res. 30:1042011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi Z, Liang YJ, Chen ZS, Wang XW, Wang
XH, Ding Y, Chen LM, Yang XP and Fu LW: Reversal of
MDR1/P-glycoprotein-mediated multidrug resistance by vector-based
RNA interference in vitro and in vivo. Cancer Biol Ther. 5:39–47.
2006. View Article : Google Scholar
|
|
11
|
Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ,
Dorkin JR and Anderson DG: Non-viral vectors for gene-based
therapy. Nat Rev Genet. 15:541–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carson AR, McTiernan CF, Lavery L, Hodnick
A, Grata M, Leng X, Wang J, Chen X, Modzelewski RA and Villanueva
FS: Gene therapy of carcinoma using ultrasound-targeted
micro-bubble destruction. Ultrasound Med Biol. 37:393–402. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carson AR, McTiernan CF, Lavery L, Grata
M, Leng X, Wang J, Chen X and Villanueva FS: Ultrasound-targeted
microbubble destruction to deliver siRNA cancer therapy. Cancer
Res. 72:6191–6199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bi Y, He Y, Huang J, Su Y, Zhu GH, Wang Y,
Qiao M, Zhang BQ, Zhang H, Wang Z, et al: Functional
characteristics of reversibly immortalized hepatic progenitor cells
derived from mouse embryonic liver. Cell Physiol Biochem.
34:1318–1338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bi Y, Gong M, He Y, Zhang X, Zhou X, Zhang
Y, Nan G, Wei X, Liu Y, Chen J, et al: AP2α transcriptional
activity is essential for retinoid-induced neuronal differentiation
of mesenchymal stem cells. Int J Biochem Cell Biol. 46:148–160.
2014. View Article : Google Scholar
|
|
16
|
Nettersheim D, Westernströer B, Haas N,
Leinhaas A, Brüstle O, Schlatt S and Schorle H: Establishment of a
versatile seminoma model indicates cellular plasticity of germ cell
tumor cells. Genes Chromosomes Cancer. 51:717–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakata S, Fujiwara M, Ohtsuka K, Kamma H,
Nagane M, Sakamoto A and Fujioka Y: ATP-binding cassette
transporters in primary central nervous system lymphoma: Decreased
expression of MDR1 P-glycoprotein and breast cancer resistance
protein in tumor capillary endothelial cells. Oncol Rep.
25:333–339. 2011.
|
|
18
|
Jin H, Yang R, Ross J, Fong S, Carano R,
Totpal K, Lawrence D, Zheng Z, Koeppen H, Stern H, et al:
Cooperation of the agonistic DR5 antibody apomab with chemotherapy
to inhibit orthotopic lung tumor growth and improve survival. Clin
Cancer Res. 14:7733–7740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanna NH and Einhorn LH: Testicular cancer
- discoveries and updates. N Engl J Med. 371:2005–2016. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Reilly A, MacEneaney P, Mayer N,
O'Reilly SP and Power DG: Testicular cancer and platinum: A
double-edged sword. J Clin Oncol. 32:e46–e48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang XB: A molecular understanding of
ATP-dependent solute transport by multidrug resistance-associated
protein MRP1. Cancer Metastasis Rev. 26:15–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su L, Mruk DD and Cheng CY: Drug
transporters, the blood-testis barrier, and spermatogenesis. J
Endocrinol. 208:207–223. 2011.
|
|
23
|
Klein DM, Wright SH and Cherrington NJ:
Localization of multidrug resistance-associated proteins along the
blood-testis barrier in rat, macaque, and human testis. Drug Metab
Dispos. 42:89–93. 2014. View Article : Google Scholar :
|
|
24
|
Melaine N, Liénard MO, Dorval I, Le
Goascogne C, Lejeune H and Jégou B: Multidrug resistance genes and
P-glycoprotein in the testis of the rat, mouse, Guinea pig, and
human. Biol Reprod. 67:1699–1707. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo C and Jin X: Chemoprotection effect of
multidrug resistance 1 (MDR1) gene transfer to hematopoietic
progenitor cells and engrafted in mice with cancer allows
intensified chemotherapy. Cancer Invest. 24:659–668. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Uhr M, Steckler T, Yassouridis A and
Holsboer F: Penetration of amitriptyline, but not of fluoxetine,
into brain is enhanced in mice with blood-brain barrier deficiency
due to mdr1a P-glycoprotein gene disruption.
Neuropsychopharmacology. 22:380–387. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo Z, Zhu J, Zhao L, Luo Q and Jin X:
Expression and clinical significance of multidrug resistance
proteins in brain tumors. J Exp Clin Cancer Res. 29:1222010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hendrikse NH, de Vries EG, Eriks-Fluks L,
van der Graaf WT, Hospers GA, Willemsen AT, Vaalburg W and Franssen
EJ: A new in vivo method to study P-glycoprotein transport in
tumors and the blood-brain barrier. Cancer Res. 59:2411–2416.
1999.PubMed/NCBI
|
|
29
|
Dave DS, Leppert JT and Rajfer J: Is the
testis a chemo-privileged site? Is there a blood-testis barrier?
Rev Urol. 9:28–32. 2007.PubMed/NCBI
|
|
30
|
França LR, Auharek SA, Hess RA, Dufour JM
and Hinton BT: Blood-tissue barriers: Morphofunctional and
immunological aspects of the blood-testis and blood-epididymal
barriers. Adv Exp Med Biol. 763:237–259. 2012.
|
|
31
|
Guo X and Huang L: Recent advances in
nonviral vectors for gene delivery. Acc Chem Res. 45:971–979. 2012.
View Article : Google Scholar
|
|
32
|
Panje CM, Wang DS and Willmann JK:
Ultrasound and microbubble-mediated gene delivery in cancer:
Progress and perspectives. Invest Radiol. 48:755–769. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sirsi SR and Borden MA: Advances in
ultrasound mediated gene therapy using microbubble contrast agents.
Theranostics. 2:1208–1222. 2012. View Article : Google Scholar
|
|
34
|
Castle J, Butts M, Healey A, Kent K,
Marino M and Feinstein SB: Ultrasound-mediated targeted drug
delivery: Recent success and remaining challenges. Am J Physiol
Heart Circ Physiol. 304:H350–H357. 2013. View Article : Google Scholar
|
|
35
|
Ortiz RJ, Lizama C, Codelia VA and Moreno
RD: A molecular evaluation of germ cell death induced by etoposide
in pubertal rat testes. Mol Hum Reprod. 15:363–371. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jing H, Lin SZ and Yang X: Synergistic
effect of emodin and cyclosporine A on rejective reaction against
liver graft in rats. Zhongguo Zhong Xi Yi Jie He Za Zhi.
28:614–616. 2008.In Chinese. PubMed/NCBI
|
|
37
|
Lin SZ, Chen KJ, Tong HF, Jing H, Li H and
Zheng SS: Emodin attenuates acute rejection of liver allografts by
inhibiting hepatocellular apoptosis and modulating the Th1/Th2
balance in rats. Clin Exp Pharmacol Physiol. 37:790–794.
2010.PubMed/NCBI
|
|
38
|
Lawrance IC: Topical agents for idiopathic
distal colitis and proctitis. J Gastroenterol Hepatol. 26:36–43.
2011. View Article : Google Scholar
|
|
39
|
van den Engel NK, Rüttinger D, Rusan M,
Kammerer R, Zimmermann W, Hatz RA and Winter H: Combination
immunotherapy and active-specific tumor cell vaccination augments
anti-cancer immunity in a mouse model of gastric cancer. J Transl
Med. 9:1402011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He LF, Wang TT, Gao QY, Zhao GF, Huang YH,
Yu LK and Hou YY: Stanniocalcin-1 promotes tumor angiogenesis
through up-regulation of VEGF in gastric cancer cells. J Biomed
Sci. 18:392011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park K: Ultrasound-activatable drug-loaded
microbubbles for intracellular targeting. J Control Release.
132:1512008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Deng F, Chen X, Liao Z, Yan Z, Wang Z,
Deng Y, Zhang Q, Zhang Z, Ye J, Qiao M, et al: A simplified and
versatile system for the simultaneous expression of multiple siRNAs
in mammalian cells using Gibson DNA Assembly. PLoS One.
9:e1130642014. View Article : Google Scholar : PubMed/NCBI
|